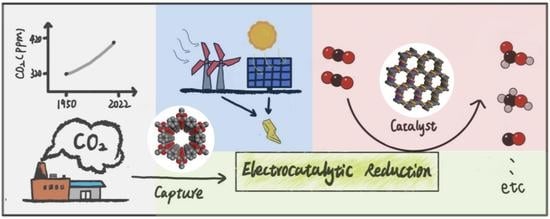
Porous Polymer Materials for CO2 Capture and Electrocatalytic Reduction
Abstract
:1. Introduction
2. Porous Polymers for CO2 Capture and Electrocatalytic Reduction
2.1. Porous Organic Polymers (POPs)
| POPs | SBET (m2 g−1) | Vtotal (cm3 g−1) | CO2 Uptake (mmol g−1) 273 K | CO2 Qst (kJ mol−1 ) | CO2/N2 Selectivity 273 K | Ref |
|---|---|---|---|---|---|---|
| isox-CTF-500 | 1683 | 0.70 | 4.92 | 29 | 29 | [43] |
| acac-CTF-500 | 1556 | 1.20 | 3.30 | 28 | 46 | [43] |
| cCTFs-400 | 744 | 0.36 | 2.86 | 49 | - | [45] |
| cCTFs-450 | 861 | 0.59 | 2.25 | 46 | - | [45] |
| cCTFs-500 | 1247 | 1.04 | 3.02 | 43 | - | [45] |
| LKK-CMP-1 | 467 | - | 2.24 | 35.0 | 44.2 | [46] |
| 6FA-PEPH-CL | 653 | 0.37 | 1.65 | - | 38.0 | [47] |
| 6FA-PE-CL | 698 | 0.46 | 2.02 | - | 58.0 | [47] |
| TTF-1 | 1234 | 0.59 | 2.86 | - | - | [48] |
2.2. Porous Coordination Polymers (PCPs)
| Catalysts | E/V vs. RHE | Major Products | FE/% | Structure | Ref. |
|---|---|---|---|---|---|
| Bi-MOF | −0.9 | HCOOH | 92.2 | microporous | [57] |
| NiPc-Ni(NH)4 | −0.7 | CO | 96.4 | microporous | [58] |
| 2D Ni(Im)2-5 nm | −0.9 | CO | 78.8 | mesoporous | [59] |
| Cu2(L)-e/Cu | −1.2 | HCOOH | 90.5 | micro- and mesoporous | [69] |
| PPy@MOF-545-Co | −0.8 | CO | 98 | micro- and mesoporous | [70] |
| MOF-NS-Cu | −0.6 | HCOOH | 83.1 | mesoporous | [71] |
| MOF-NS-Co | −0.6 | CO | 98.7 | mesoporous | [71] |
| PCN-222(Cu) | −0.7 | HCOOH | 44.3 | mesoporous | [72] |
| PCN-224(Cu) | −0.7 | HCOOH | 34.1 | microporous | [72] |
| Al2(OH)2TCPP-Co | −0.7 | CO | 76 | mesoporous | [73] |
| PcCu-O8-Zn | −0.7 | CO | 88 | microporous mesoporous | [74] |
| PCN-224-NH3 | −0.9 | CO | 60 | microporous | [75] |
| ZIF-A-LD | −1.1 | CO | 90.57 | microporous | [76] |
| D-P-CoPc | −0.6 | CO | 97 | macroporous | [77] |
| Cu30%ZIF-8 | −1.0 | CH4 | 35.21 | microporous mesoporous | [78] |
| p-CuL-4 | −0.37 | CH3COOH | 64 | microporous | [79] |
2.3. Covalent Organic Frameworks (COFs)
| Catalysts | E/V vs. RHE | Major Products | FE/% | Structure | Ref. |
|---|---|---|---|---|---|
| COF-366-Cu (HS) | −0.9 | CH4 | 52.4 | microporous | [86] |
| F-CTF-1-275 | −0.8 | CO | 95.7 | microporous | [87] |
| COF-366-Co | −0.67 | CO | 90 | microporous | [92] |
| CuPcF8-CoNPc-COF | −0.62 | CO | 97 | microporous | [93] |
| 2D-Co-COF500 | −0.8 | CO | 96.5 | mesoporous | [94] |
| MWCNT-PorCOF-Cu | −1.0 | CH4 | 71.2 | micro- and mesoporous | [95] |
| CoPc-2H2Por | −0.55 | CO | 95 | micro- and mesoporous | [96] |
| AAn-COF-Cu (NF) | −0.9 | CH4 | 77 | microporous | [97] |
| FN-CTF-400 | −0.8 | CH4 | 91.7 | microporous | [98] |
| TPE-CoPor-COF | −0.7 | CO | 95 | microporous | [99] |
| 3D-Por(Co/H)-COF | −0.6 | CO | 92.4 | microporous | [100] |
| COF@CoPor | −0.6 | CO | 94.3 | microporous | [101] |
| NiPc-TFPN COF | −0.9 | CO | 99.8 | microporous | [102] |
| CoPc-PI-COF-3 | −0.9 | CO | 96 | microporous | [103] |
| COF-366-(OMe)2-Co@CNT | −0.68 | CO | 93.6 | macroporous | [104] |
| CTF-Cu | −1.47 | C2H4 | 30.6 | micro- and mesoporous | [105] |
2.4. Nitrogen-Containing Polymers (N-Polymers)
3. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sebos, I. Fossil fraction of CO2 emissions of biofuels. Carbon Manag. 2022, 13, 154–163. [Google Scholar] [CrossRef]
- Kajla, S.; Kumari, R.; Nagi, G.K. Microbial CO2 fixation and biotechnology in reducing industrial CO2 emissions. Arch. Microbiol. 2022, 204, 149. [Google Scholar] [CrossRef] [PubMed]
- Burger, F.A.; John, J.G.; Frölicher, T.L. Increase in ocean acidity variability and extremes under increasing atmospheric CO2. Biogeosciences 2020, 17, 4633–4662. [Google Scholar] [CrossRef]
- Qu, Y.; Jevrejeva, S.; Jackson, L.P.; Moore, J.C. Coastal Sea level rise around the China Seas. Glob. Planet. Chang. 2019, 172, 454–463. [Google Scholar] [CrossRef]
- Malati, M.A. Mitigation of CO2 greenhouse effect. Combined disposal and utilisation by photocatalysis. Energy Convers. Manag. 1996, 37, 1345–1350. [Google Scholar] [CrossRef]
- Zhao, Y.; Su, Q.; Li, B.; Zhang, Y.; Wang, X.; Zhao, H.; Guo, S. Have those countries declaring “zero carbon” or “carbon neutral” climate goals achieved carbon emissions-economic growth decoupling? J. Clean. Prod. 2022, 363, 132450. [Google Scholar] [CrossRef]
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, H. High-purity and high-concentration liquid fuels through CO2 electroreduction. Nat. Catal. 2021, 4, 943–951. [Google Scholar] [CrossRef]
- Zang, G.; Sun, P.; Yoo, E.; Elgowainy, A.; Bafana, A.; Lee, U.; Wang, M.; Supekar, S. Synthetic Methanol/Fischer–Tropsch Fuel Production Capacity, Cost, and Carbon Intensity Utilizing CO2 from Industrial and Power Plants in the United States. Environ. Sci. Technol. 2021, 55, 7595–7604. [Google Scholar] [CrossRef]
- Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C.Y.; Zhu, X.; Wang, J.; et al. Industrial carbon dioxide capture and utilization: State of the art and future challenges. Chem. Soc. Rev. 2020, 49, 8584–8686. [Google Scholar] [CrossRef]
- Aminu, M.D.; Nabavi, S.A.; Rochelle, C.A.; Manovic, V. A review of developments in carbon dioxide storage. Appl. Energy 2017, 208, 1389–1419. [Google Scholar] [CrossRef] [Green Version]
- Bhanja, P.; Modak, A.; Bhaumik, A. Porous Organic Polymers for CO2 Storage and Conversion Reactions. ChemCatChem 2018, 11, 244–257. [Google Scholar] [CrossRef]
- Wang, S.; Yan, S.; Ma, X.; Gong, J. Recent advances in capture of carbon dioxide using alkali-metal-based oxides. Energy Environ. Sci. 2011, 4, 3805–3819. [Google Scholar] [CrossRef]
- Naguib, H.M.; Hou, G. Exploitation of Natural and Recycled Biomass Resources to Get Eco-friendly Polymer. J. Polym. Environ. 2023, 31, 533–540. [Google Scholar] [CrossRef]
- Hou, G.; Yan, Z.; Sun, J.; Naguib, H.M.; Lu, B.; Zhang, Z. Microstructure and mechanical properties of CO2-cured steel slag brick in pilot-scale. Constr. Build. Mater. 2021, 271, 121581. [Google Scholar] [CrossRef]
- Luo, Y.; Li, B.; Wang, W.; Wu, K.; Tan, B. Hypercrosslinked Aromatic Heterocyclic Microporous Polymers: A New Class of Highly Selective CO2Capturing Materials. Adv. Mater. 2012, 24, 5703–5707. [Google Scholar] [CrossRef]
- Singh, G.; Lakhi, K.S.; Kim, I.Y.; Kim, S.; Srivastava, P.; Naidu, R.; Vinu, A. Highly Efficient Method for the Synthesis of Activated Mesoporous Biocarbons with Extremely High Surface Area for High-Pressure CO2 Adsorption. ACS Appl. Mater. Interfaces 2017, 9, 29782–29793. [Google Scholar] [CrossRef]
- Cen, Q.; Fang, M.; Wang, T.; Majchrzak-Kucęba, I.; Wawrzyńczak, D.; Luo, Z. Thermodynamics and regeneration studies of CO2 adsorption on activated carbon. Greenh. Gases Sci. Technol. 2016, 6, 787–796. [Google Scholar] [CrossRef]
- Elfving, J.; Bajamundi, C.; Kauppinen, J.; Sainio, T. Modelling of equilibrium working capacity of PSA, TSA and TVSA processes for CO2 adsorption under direct air capture conditions. J. CO2 Util. 2017, 22, 270–277. [Google Scholar] [CrossRef]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef]
- Jia, S.; Ma, X.; Sun, X.; Han, B. Electrochemical Transformation of CO2 to Value-Added Chemicals and Fuels. CCS Chem. 2022, 4, 3213–3229. [Google Scholar] [CrossRef]
- Popovic, S.; Smiljanic, M.; Jovanovic, P.; Vavra, J.; Buonsanti, R.; Hodnik, N. Stability and Degradation Mechanisms of Copper-Based Catalysts for Electrochemical CO2 Reduction. Angew. Chem. 2020, 59, 14736–14746. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Cao, D.; Huang, L.; Shui, J.; Wang, M.; Dai, L. Nitrogen-Doped Holey Graphitic Carbon from 2D Covalent Organic Polymers for Oxygen Reduction. Adv. Mater. 2014, 26, 3315–3320. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Eisenberg, R. Catalysts made of earth-abundant elements (Co, Ni, Fe) for water splitting: Recent progress and future challenges. Energy Environ. Sci. 2012, 5, 6012–6021. [Google Scholar] [CrossRef]
- Wang, Y.-N.; Yang, Z.-J.; Yang, D.-H.; Zhao, L.; Shi, X.-R.; Yang, G.; Han, B.-H. FeCoP2 Nanoparticles Embedded in N and P Co-doped Hierarchically Porous Carbon for Efficient Electrocatalytic Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 8832–8843. [Google Scholar] [CrossRef]
- Cui, H.; Guo, Y.; Zhou, Z. Three-Dimensional Graphene-Based Macrostructures for Electrocatalysis. Small 2021, 17, e2005255. [Google Scholar] [CrossRef]
- Menga, D.; Ruiz-Zepeda, F.; Moriau, L.; Šala, M.; Wagner, F.; Koyutürk, B.; Bele, M.; Petek, U.; Hodnik, N.; Gaberšček, M.; et al. Active-Site Imprinting: Preparation of Fe–N–C Catalysts from Zinc Ion–Templated Ionothermal Nitrogen-Doped Carbons. Adv. Energy Mater. 2019, 9, 1902412. [Google Scholar] [CrossRef]
- Panda, D.; Kumar, E.A.; Singh, S.K. Introducing mesoporosity in zeolite 4A bodies for Rapid CO2 capture. J. CO2 Util. 2020, 40, 101223. [Google Scholar] [CrossRef]
- Zhang, Z.; Cano, Z.P.; Luo, D.; Dou, H.; Yu, A.; Chen, Z. Rational design of tailored porous carbon-based materials for CO2 capture. J. Mater. Chem. A 2019, 7, 20985–21003. [Google Scholar] [CrossRef]
- Singh, G.; Lee, J.; Karakoti, A.; Bahadur, R.; Yi, J.; Zhao, D.; AlBahily, K.; Vinu, A. Emerging trends in porous materials for CO2 capture and conversion. Chem. Soc. Rev. 2020, 49, 4360–4404. [Google Scholar] [CrossRef]
- Yang, D.-H.; Tao, Y.; Ding, X.; Han, B.-H. Porous organic polymers for electrocatalysis. Chem. Soc. Rev. 2022, 51, 761–791. [Google Scholar] [CrossRef]
- Song, K.S.; Fritz, P.W.; Coskun, A. Porous organic polymers for CO2 capture, separation and conversion. Chem. Soc. Rev. 2022, 51, 9831–9852. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakravarty, C.; Mandal, B.; Sarkar, P. Multifunctionalities of an Azine-Linked Covalent Organic Framework: From Nanoelectronics to Nitroexplosive Detection and Conductance Switching. J. Phys. Chem. C 2018, 122, 3245–3255. [Google Scholar] [CrossRef]
- Sick, T.; Hufnagel, A.G.; Kampmann, J.; Kondofersky, I.; Calik, M.; Rotter, J.M.; Evans, A.; Döblinger, M.; Herbert, S.; Peters, K.; et al. Oriented Films of Conjugated 2D Covalent Organic Frameworks as Photocathodes for Water Splitting. J. Am. Chem. Soc. 2018, 140, 2085–2092. [Google Scholar] [CrossRef] [Green Version]
- Vardhan, H.; Nafady, A.; Al-Enizi, A.M.; Ma, S. Pore surface engineering of covalent organic frameworks: Structural diversity and applications. Nanoscale 2019, 11, 21679–21708. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Zhao, Y.; Liu, Z. Design of porous organic polymer catalysts for transformation of carbon dioxide. Green Chem. Eng. 2022, 3, 96–110. [Google Scholar] [CrossRef]
- Siegelman, R.L.; Kim, E.J.; Long, J.R. Porous materials for carbon dioxide separations. Nat. Mater. 2021, 20, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Clowes, R.; Sprick, R.S.; Cooper, A.I.; Cowan, A.J. Metal–organic conjugated microporous polymer containing a carbon dioxide reduction electrocatalyst. Sustain. Energy Fuels 2019, 3, 2990–2994. [Google Scholar] [CrossRef]
- Lu, C.; Yang, J.; Wei, S.; Bi, S.; Xia, Y.; Chen, M.; Hou, Y.; Qiu, M.; Yuan, C.; Su, Y.; et al. Atomic Ni Anchored Covalent Triazine Framework as High Efficient Electrocatalyst for Carbon Dioxide Conversion. Adv. Funct. Mater. 2019, 29, 1806884. [Google Scholar] [CrossRef]
- Eder, G.M.; Pyles, D.A.; Wolfson, E.R.; McGrier, P.L. A ruthenium porphyrin-based porous organic polymer for the hydrosilylative reduction of CO2 to formate. Chem. Commun. 2019, 55, 7195–7198. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, X.; Weng, W.; Yao, Y.; Kidkhunthod, P.; Wang, C.; Hou, Y.; Guo, J. Proton/Electron Donors Enhancing Electrocatalytic Activity of Supported Conjugated Microporous Polymers for CO2 Reduction. Angew. Chem. 2022, 61, e202115503. [Google Scholar] [CrossRef]
- Jena, H.S.; Krishnaraj, C.; Schmidt, J.; Leus, K.; Van Hecke, K.; Van Der Voort, P. Effect of Building Block Transformation in Covalent Triazine-Based Frameworks for Enhanced CO2 Uptake and Metal-Free Heterogeneous Catalysis. Chemistry 2020, 26, 1548–1557. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.-W.; Han, B.-H. Ionic porous organic polymers for CO2 capture and conversion. Curr. Opin. Green Sustain. Chem. 2019, 16, 20–25. [Google Scholar] [CrossRef]
- Buyukcakir, O.; Je, S.H.; Talapaneni, S.N.; Kim, D.; Coskun, A. Charged Covalent Triazine Frameworks for CO2 Capture and Conversion. ACS Appl. Mater. Interfaces 2017, 9, 7209–7216. [Google Scholar] [CrossRef]
- Ren, S.-B.; Li, P.-X.; Stephenson, A.; Chen, L.; Briggs, M.E.; Clowes, R.; Alahmed, A.; Li, K.-K.; Jia, W.-P.; Han, D.-M. 1,3-Diyne-Linked Conjugated Microporous Polymer for Selective CO2 Capture. Ind. Eng. Chem. Res. 2018, 57, 9254–9260. [Google Scholar] [CrossRef]
- Song, N.; Wang, T.; Ma, T.; Li, J.; Yao, H.; Guan, S. Microporous polyimide networks with tunable micropore size constructed through side-chain engineering of linear precursors. Polymer 2022, 255, 125161. [Google Scholar] [CrossRef]
- Zhu, X.; Tian, C.; Wu, H.; He, Y.; He, L.; Wang, H.; Zhuang, X.; Liu, H.; Xia, C.; Dai, S. Pyrolyzed Triazine-Based Nanoporous Frameworks Enable Electrochemical CO2 Reduction in Water. ACS Appl. Mater. Interfaces 2018, 10, 43588–43594. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.-K.; Zhu, C.-Y.; Jiang, T.-W.; Wei, L.; Wang, H.; Yu, K.; Yang, C.-L.; Zhang, Y.-B.; Chen, C.; Li, Z.-T.; et al. Anion exchange-induced single-molecule dispersion of cobalt porphyrins in a cationic porous organic polymer for enhanced electrochemical CO2 reduction via secondary-coordination sphere interactions. J. Mater. Chem. A 2020, 8, 18677–18686. [Google Scholar] [CrossRef]
- Soliman, A.B.; Haikal, R.R.; Hassan, Y.S.; Alkordi, M.H. The potential of a graphene-supported porous-organic polymer (POP) for CO2 electrocatalytic reduction. Chem. Commun. 2016, 52, 12032–12035. [Google Scholar] [CrossRef]
- Haikal, R.R.; Soliman, A.B.; Amin, M.; Karakalos, S.G.; Hassan, Y.S.; Elmansi, A.M.; Hafez, I.H.; Berber, M.R.; Hassanien, A.; Alkordi, M.H. Synergism of carbon nanotubes and porous-organic polymers (POPs) in CO2 fixation: One-pot approach for bottom-up assembly of tunable heterogeneous catalyst. Appl. Catal. B Environ. 2017, 207, 347–357. [Google Scholar] [CrossRef]
- Wu, P.; Li, Y.; Zheng, J.-J.; Hosono, N.; Otake, K.-I.; Wang, J.; Liu, Y.; Xia, L.; Jiang, M.; Sakaki, S.; et al. Carbon dioxide capture and efficient fixation in a dynamic porous coordination polymer. Nat. Commun. 2019, 10, 4362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, M.-M.; Xi, J.-M.; Zhu, R.; Wang, L.-F.; Xu, Y.-K.; Liu, X.-G.; Zhang, R.; Lu, Z.-Z. Tubular porous coordination polymer for selective adsorption of CO2. Inorg. Chem. Commun. 2021, 132, 108798. [Google Scholar] [CrossRef]
- Inukai, M.; Tamura, M.; Horike, S.; Higuchi, M.; Kitagawa, S.; Nakamura, K. Storage of CO2 into Porous Coordination Polymer Controlled by Molecular Rotor Dynamics. Angew. Chem. 2018, 57, 8687–8690. [Google Scholar] [CrossRef]
- Kauffman, K.L.; Culp, J.T.; Allen, A.J.; Espinal, L.; Wong-Ng, W.; Brown, T.D.; Goodman, A.; Bernardo, M.P.; Pancoast, R.J.; Chirdon, D.; et al. Selective Adsorption of CO2 from Light Gas Mixtures by Using a Structurally Dynamic Porous Coordination Polymer. Angew. Chem. 2011, 50, 10888–10892. [Google Scholar] [CrossRef] [Green Version]
- Emerson, A.J.; Chahine, A.; Batten, S.R.; Turner, D.R. Synthetic approaches for the incorporation of free amine functionalities in porous coordination polymers for enhanced CO2 sorption. Coord. Chem. Rev. 2018, 365, 1–22. [Google Scholar] [CrossRef]
- Li, F.; Gu, G.H.; Choi, C.; Kolla, P.; Hong, S.; Wu, T.-S.; Soo, Y.-L.; Masa, J.; Mukerjee, S.; Jung, Y.; et al. Highly stable two-dimensional bismuth metal-organic frameworks for efficient electrochemical reduction of CO2. Appl. Catal. B Environ. 2020, 277, 119241. [Google Scholar] [CrossRef]
- Zhang, M.-D.; Si, D.-H.; Yi, J.-D.; Yin, Q.; Huang, Y.-B.; Cao, R. Conductive phthalocyanine-based metal-organic framework as a highly efficient electrocatalyst for carbon dioxide reduction reaction. Sci. China Chem. 2021, 64, 1332–1339. [Google Scholar] [CrossRef]
- Wu, J.-X.; Yuan, W.-W.; Xu, M.; Gu, Z.-Y. Ultrathin 2D nickel zeolitic imidazolate framework nanosheets for electrocatalytic reduction of CO2. Chem. Commun. 2019, 55, 11634–11637. [Google Scholar] [CrossRef]
- Zhao, X.; Pachfule, P.; Li, S.; Langenhahn, T.; Ye, M.; Schlesiger, C.; Praetz, S.; Schmidt, J.; Thomas, A. Macro/Microporous Covalent Organic Frameworks for Efficient Electrocatalysis. J. Am. Chem. Soc. 2019, 141, 6623–6630. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Qiao, Z.-A.; Fulvio, P.F.; Binder, A.J.; Tian, C.; Chen, J.; Nelson, K.M.; Zhu, X.; Dai, S. Template-Free Synthesis of Hierarchical Porous Metal–Organic Frameworks. J. Am. Chem. Soc. 2013, 135, 9572–9575. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J.; Ding, B.; Malgras, V.; Chang, Z.; Hao, X.; Wang, Y.; Dou, H.; Zhang, X.; Yamauchi, Y. Hierarchical porous carbons with layer-by-layer motif architectures from confined soft-template self-assembly in layered materials. Nat. Commun. 2017, 8, 15717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, M.W.; Holmes, S.M.; Hanif, N.; Cundy, C.S. Hierarchical Pore Structures through Diatom Zeolitization. Angew. Chem. 2000, 39, 2707–2710. [Google Scholar] [CrossRef]
- Li, K.; Lin, S.; Li, Y.; Zhuang, Q.; Gu, J. Aqueous-Phase Synthesis of Mesoporous Zr-Based MOFs Templated by Amphoteric Surfactants. Angew. Chem. 2018, 57, 3439–3443. [Google Scholar] [CrossRef]
- Cai, G.; Jiang, H.-L. A Modulator-Induced Defect-Formation Strategy to Hierarchically Porous Metal-Organic Frameworks with High Stability. Angew. Chem. 2016, 56, 563–567. [Google Scholar] [CrossRef]
- Huang, H.; Li, J.-R.; Wang, K.; Han, T.; Tong, M.; Li, L.; Xie, Y.; Yang, Q.; Liu, D.; Zhong, C. An in situ self-assembly template strategy for the preparation of hierarchical-pore metal-organic frameworks. Nat. Commun. 2015, 6, 8847. [Google Scholar] [CrossRef] [Green Version]
- Shen, K.; Zhang, L.; Chen, X.; Liu, L.; Zhang, D.; Han, Y.; Chen, J.; Long, J.; Luque, R.; Li, Y.; et al. Ordered macro-microporous metal-organic framework single crystals. Science 2018, 359, 206–210. [Google Scholar] [CrossRef] [Green Version]
- Vattipalli, V.; Qi, X.; Dauenhauer, P.J.; Fan, W. Long Walks in Hierarchical Porous Materials due to Combined Surface and Configurational Diffusion. Chem. Mater. 2016, 28, 7852–7863. [Google Scholar] [CrossRef]
- Kang, X.; Li, L.; Sheveleva, A.; Han, X.; Li, J.; Liu, L.; Tuna, F.; McInnes, E.J.L.; Han, B.; Yang, S.; et al. Electro-reduction of carbon dioxide at low over-potential at a metal–organic framework decorated cathode. Nat. Commun. 2020, 11, 5464. [Google Scholar] [CrossRef] [PubMed]
- Xin, Z.; Liu, J.; Wang, X.; Shen, K.; Yuan, Z.; Chen, Y.; Lan, Y.-Q. Implanting Polypyrrole in Metal-Porphyrin MOFs: Enhanced Electrocatalytic Performance for CO2RR. ACS Appl. Mater. Interfaces 2021, 13, 54959–54966. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, L.; Yang, D.; Yang, H.; Lu, Q.; Zhang, Q.; Gu, L.; Wang, X. Enhancing CO2 Electrocatalysis on 2D Porphyrin-Based Metal–Organic Framework Nanosheets Coupled with Visible-Light. Small Methods 2021, 5, 2000991. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-J.; Cao, S.-M.; Feng, B.-Q.; Dong, B.-X.; Ding, Y.-X.; Zheng, Q.-H.; Teng, Y.-L.; Li, Z.-W.; Liu, W.-L.; Feng, L.-G. Revealing the structure–activity relationship of two Cu-porphyrin-based metal–organic frameworks for the electrochemical CO2-to-HCOOH transformation. Dalton Trans. 2020, 49, 14995–15001. [Google Scholar] [CrossRef]
- Kornienko, N.; Zhao, Y.; Kley, C.S.; Zhu, C.; Kim, D.; Lin, S.; Chang, C.J.; Yaghi, O.M.; Yang, P. Metal–Organic Frameworks for Electrocatalytic Reduction of Carbon Dioxide. J. Am. Chem. Soc. 2015, 137, 14129–14135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, H.; Ghorbani-Asl, M.; Ly, K.H.; Zhang, J.; Ge, J.; Wang, M.; Liao, Z.; Makarov, D.; Zschech, E.; Brunner, E.; et al. Synergistic electroreduction of carbon dioxide to carbon monoxide on bimetallic layered conjugated metal-organic frameworks. Nat. Commun. 2020, 11, 1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Y.; Hu, X.-J.; Tong, H.-Y.; Huang, Y.-B.; Cao, R. Unraveling the relationship of the pore structures between the metal-organic frameworks and their derived carbon materials. Inorg. Chem. Commun. 2020, 114, 107825. [Google Scholar] [CrossRef]
- Dou, S.; Song, J.; Xi, S.; Du, Y.; Wang, J.; Huang, Z.-F.; Xu, Z.J.; Wang, X. Boosting Electrochemical CO2 Reduction on Metal–Organic Frameworks via Ligand Doping. Angew. Chem. 2019, 58, 4041–4045. [Google Scholar] [CrossRef]
- Wu, H.; Zeng, M.; Zhu, X.; Tian, C.; Mei, B.; Song, Y.; Du, X.-L.; Jiang, Z.; He, L.; Xia, C.; et al. Defect Engineering in Polymeric Cobalt Phthalocyanine Networks for Enhanced Electrochemical CO2 Reduction. ChemElectroChem 2018, 5, 2717–2721. [Google Scholar] [CrossRef]
- Ahmad, A.; Iqbal, N.; Noor, T.; Hassan, A.; Khan, U.A.; Wahab, A.; Raza, M.A.; Ashraf, S. Cu-doped zeolite imidazole framework (ZIF-8) for effective electrocatalytic CO2 reduction. J. CO2 Util. 2021, 48, 101523. [Google Scholar] [CrossRef]
- Guo, F.; Liu, B.; Liu, M.; Xia, Y.; Wang, T.; Hu, W.; Fyffe, P.; Tian, L.; Chen, X. Selective electrocatalytic CO2 reduction to acetate on polymeric Cu–L (L = pyridinic N and carbonyl group) complex core–shell microspheres. Green Chem. 2021, 23, 5129–5137. [Google Scholar] [CrossRef]
- Iqbal, R.; Yasin, G.; Hamza, M.; Ibraheem, S.; Ullah, B.; Saleem, A.; Ali, S.; Hussain, S.; Nguyen, T.A.; Slimani, Y.; et al. State of the art two-dimensional covalent organic frameworks: Prospects from rational design and reactions to applications for advanced energy storage technologies. Coord. Chem. Rev. 2021, 447, 214152. [Google Scholar] [CrossRef]
- Li, H.; Feng, X.; Shao, P.; Chen, J.; Li, C.; Jayakumar, S.; Yang, Q. Synthesis of covalent organic frameworks via in situ salen skeleton formation for catalytic applications. J. Mater. Chem. A 2019, 7, 5482–5492. [Google Scholar] [CrossRef]
- Shah, R.; Ali, S.; Raziq, F.; Ali, S.; Ismail, P.M.; Shah, S.; Iqbal, R.; Wu, X.; He, W.; Zu, X.; et al. Exploration of metal organic frameworks and covalent organic frameworks for energy-related applications. Coord. Chem. Rev. 2023, 477, 214968. [Google Scholar] [CrossRef]
- Lee, M.K.; Shokouhimehr, M.; Kim, S.Y.; Jang, H.W. Two-Dimensional Metal–Organic Frameworks and Covalent–Organic Frameworks for Electrocatalysis: Distinct Merits by the Reduced Dimension. Adv. Energy Mater. 2022, 12, 2003990. [Google Scholar] [CrossRef]
- Wang, Y.-R.; Ding, H.-M.; Ma, X.-Y.; Liu, M.; Yang, Y.-L.; Chen, Y.; Li, S.-L.; Lan, Y.-Q. Imparting CO2 Electroreduction Auxiliary for Integrated Morphology Tuning and Performance Boosting in a Porphyrin-based Covalent Organic Framework. Angew. Chem. 2022, 61, e202114648. [Google Scholar] [CrossRef]
- Zhu, H.-J.; Lu, M.; Wang, Y.-R.; Yao, S.-J.; Zhang, M.; Kan, Y.-H.; Liu, J.; Chen, Y.; Li, S.-L.; Lan, Y.-Q. Efficient electron transmission in covalent organic framework nanosheets for highly active electrocatalytic carbon dioxide reduction. Nat. Commun. 2020, 11, 497. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.-L.; Wang, Y.-R.; Gao, G.-K.; Liu, M.; Miao, C.; Li, L.-Y.; Cheng, W.; Zhao, Z.-Y.; Chen, Y.; Xin, Z.; et al. Self-assembly of single metal sites embedded covalent organic frameworks into multi-dimensional nanostructures for efficient CO2 electroreduction. Chin. Chem. Lett. 2022, 33, 1439–1444. [Google Scholar] [CrossRef]
- Zhou, T.; Zhao, Y.; Choi, J.W.; Coskun, A. Lithium-Salt Mediated Synthesis of a Covalent Triazine Framework for Highly Stable Lithium Metal Batteries. Angew. Chem. 2019, 58, 16795–16799. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Guo, L.; Jin, S.; Tan, B. Covalent triazine frameworks: Synthesis and applications. J. Mater. Chem. A 2019, 7, 5153–5172. [Google Scholar] [CrossRef]
- Ghosh, I.; Khamrai, J.; Savateev, A.; Shlapakov, N.; Antonietti, M.; König, B. Organic semiconductor photocatalyst can bifunctionalize arenes and heteroarenes. Science 2019, 365, 360–366. [Google Scholar] [CrossRef] [Green Version]
- Bhadra, M.; Kandambeth, S.; Sahoo, M.K.; Addicoat, M.; Balaraman, E.; Banerjee, R. Triazine Functionalized Porous Covalent Organic Framework for Photo-organocatalytic E–Z Isomerization of Olefins. J. Am. Chem. Soc. 2019, 141, 6152–6156. [Google Scholar] [CrossRef]
- Suo, X.; Zhang, F.; Yang, Z.; Chen, H.; Wang, T.; Wang, Z.; Kobayashi, T.; Do-Thanh, C.-L.; Maltsev, D.; Liu, Z.; et al. Highly Perfluorinated Covalent Triazine Frameworks Derived from a Low-Temperature Ionothermal Approach Towards Enhanced CO2 Electroreduction. Angew. Chem. 2021, 60, 25688–25694. [Google Scholar] [CrossRef]
- Lin, S.; Diercks, C.S.; Zhang, Y.-B.; Kornienko, N.; Nichols, E.M.; Zhao, Y.; Paris, A.R.; Kim, D.; Yang, P.; Yaghi, O.M.; et al. Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water. Science 2015, 349, 1208–1213. [Google Scholar] [CrossRef] [Green Version]
- Yue, Y.; Cai, P.; Xu, K.; Li, H.; Chen, H.; Zhou, H.-C.; Huang, N. Stable Bimetallic Polyphthalocyanine Covalent Organic Frameworks as Superior Electrocatalysts. J. Am. Chem. Soc. 2021, 143, 18052–18060. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Lu, C.; Xu, Q.; Yang, S.; Liu, M.; Liu, S.; Yu, C.; Zhuang, X.; Jiang, Z.; Zeng, G. CoN2O2 sites in carbon nanosheets by template-pyrolysis of COFs for CO2RR. Chem. Eng. J. 2022, 450, 138427. [Google Scholar] [CrossRef]
- Dong, H.; Lu, M.; Wang, Y.; Tang, H.-L.; Wu, D.; Sun, X.; Zhang, F.-M. Covalently anchoring covalent organic framework on carbon nanotubes for highly efficient electrocatalytic CO2 reduction. Appl. Catal. B Environ. 2021, 303, 120897. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, S.; Zhang, Y.; Li, R.; Zhang, J.; Peng, T. Structural Regulation of Coupled Phthalocyanine–Porphyrin Covalent Organic Frameworks to Highly Active and Selective Electrocatalytic CO2 Reduction. Adv. Mater. 2022, 34, 2203139. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.-R.; Ding, H.-M.; Lu, M.; Gao, G.-K.; Dong, L.-Z.; Li, Q.; Chen, Y.; Li, S.-L.; Lan, Y.-Q. Self-assembly of anthraquinone covalent organic frameworks as 1D superstructures for highly efficient CO2 electroreduction to CH4. Sci. Bull. 2021, 66, 1659–1668. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Wang, G.; Li, Y.; Wen, Z. Perfluorinated Covalent Triazine Framework Derived Hybrids for the Highly Selective Electroconversion of Carbon Dioxide into Methane. Angew. Chem. 2018, 57, 13120–13124. [Google Scholar] [CrossRef]
- Gong, L.; Gao, Y.; Wang, Y.; Chen, B.; Yu, B.; Liu, W.; Han, B.; Lin, C.; Bian, Y.; Qi, D.; et al. Efficient electrocatalytic carbon dioxide reduction with tetraphenylethylene- and porphyrin-based covalent organic frameworks. Catal. Sci. Technol. 2022, 12, 6566–6571. [Google Scholar] [CrossRef]
- Chi, S.-Y.; Chen, Q.; Zhao, S.-S.; Si, D.-H.; Wu, Q.-J.; Huang, Y.-B.; Cao, R. Three-dimensional porphyrinic covalent organic frameworks for highly efficient electroreduction of carbon dioxide. J. Mater. Chem. A 2022, 10, 4653–4659. [Google Scholar] [CrossRef]
- Zhai, L.; Yang, S.; Lu, C.; Cui, C.-X.; Xu, Q.; Liu, J.; Yang, X.; Meng, X.; Lu, S.; Zhuang, X.; et al. CoN5 Sites Constructed by Anchoring Co Porphyrins on Vinylene-Linked Covalent Organic Frameworks for Electroreduction of Carbon Dioxide. Small 2022, 18, 2200736. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, M.; Liu, C.-G.; Liu, J.; Shang, L.-J.; Wang, M.; Chang, J.-N.; Li, S.-L.; Lan, Y.-Q. Stable Dioxin-Linked Metallophthalocyanine Covalent Organic Frameworks (COFs) as Photo-Coupled Electrocatalysts for CO2 Reduction. Angew. Chem. 2021, 60, 4864–4871. [Google Scholar] [CrossRef]
- Han, B.; Jin, Y.; Chen, B.; Zhou, W.; Yu, B.; Wei, C.; Wang, H.; Wang, K.; Chen, Y.; Chen, B.; et al. Maximizing Electroactive Sites in a Three-Dimensional Covalent Organic Framework for Significantly Improved Carbon Dioxide Reduction Electrocatalysis. Angew. Chem. 2022, 61, e202114244. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, J.; Wei, W.; Ma, D.-D.; Wu, X.-T.; Zhu, Q.-L. Efficient Carbon Dioxide Electroreduction over Ultrathin Covalent Organic Framework Nanolayers with Isolated Cobalt Porphyrin Units. ACS Appl. Mater. Interfaces 2020, 12, 37986–37992. [Google Scholar] [CrossRef]
- Ma, L.; Hu, W.; Mei, B.; Liu, H.; Yuan, B.; Zang, J.; Chen, T.; Zou, L.; Zou, Z.; Yang, B.; et al. Covalent Triazine Framework Confined Copper Catalysts for Selective Electrochemical CO2 Reduction: Operando Diagnosis of Active Sites. ACS Catal. 2020, 10, 4534–4542. [Google Scholar] [CrossRef]
- Stalder, C.J.; Chao, S.; Wrighton, M.S. Electrochemical reduction of aqueous bicarbonate to formate with high current efficiency near the thermodynamic potential at chemically derivatized electrodes. J. Am. Chem. Soc. 1984, 106, 3673–3675. [Google Scholar] [CrossRef]
- O’Toole, T.R.; Margerum, L.D.; Westmoreland, T.D.; Vining, W.J.; Murray, R.W.; Meyer, T.J. Electrocatalytic reduction of CO2 at a chemically modified electrode. J. Chem. Soc. Chem. Commun. 1985, 1416–1417. [Google Scholar] [CrossRef]
- Yu, J.; Xie, L.-H.; Li, J.-R.; Ma, Y.; Seminario, J.M.; Balbuena, P.B. CO2 Capture and Separations Using MOFs: Computational and Experimental Studies. Chem. Rev. 2017, 117, 9674–9754. [Google Scholar] [CrossRef]
- Pardakhti, M.; Jafari, T.; Tobin, Z.; Dutta, B.; Moharreri, E.; Shemshaki, N.S.; Suib, S.; Srivastava, R. Trends in Solid Adsorbent Materials Development for CO2 Capture. ACS Appl. Mater. Interfaces 2019, 11, 34533–34559. [Google Scholar] [CrossRef]
- Peng, A.-Z.; Qi, S.-C.; Liu, X.; Xue, D.-M.; Peng, S.-S.; Yu, G.-X.; Liu, X.-Q.; Sun, L.-B. N-doped porous carbons derived from a polymer precursor with a record-high N content: Efficient adsorbents for CO2 capture. Chem. Eng. J. 2019, 372, 656–664. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, X.-F.; Li, L.; Jin, L.; Zhang, Y.-G.; Song, S.-L.; Liu, R.-P. Nitrogen-Enriched Porous Carbon Fiber as a CO2 Adsorbent with Superior CO2 Selectivity by Air Activation. Energy Fuels 2019, 33, 12558–12567. [Google Scholar] [CrossRef]
- Yoo, D.K.; An, H.J.; Khan, N.A.; Hwang, G.T.; Jhung, S.H. Record-high adsorption capacities of polyaniline-derived porous carbons for the removal of personal care products from water. Chem. Eng. J. 2018, 352, 71–78. [Google Scholar] [CrossRef]
- Qezelsefloo, E.; Khalili, S.; Jahanshahi, M.; Peyravi, M. Adsorptive removal of CO2 on Nitrogen-doped porous carbon derived from polyaniline: Effect of chemical activation. Mater. Chem. Phys. 2020, 239, 122304. [Google Scholar] [CrossRef]
- He, J.; To, J.W.F.; Psarras, P.C.; Yan, H.; Atkinson, T.; Holmes, R.T.; Nordlund, D.; Bao, Z.; Wilcox, J. Tunable Polyaniline-Based Porous Carbon with Ultrahigh Surface Area for CO2 Capture at Elevated Pressure. Adv. Energy Mater. 2016, 6, 1502491. [Google Scholar] [CrossRef]
- Park, J.M.; Woo, H.C.; Jhung, S.H. Effective CO2 adsorption at low pressure over nitrogen-enriched porous carbons, derived from melamine-loaded polyaniline. Chem. Eng. J. 2021, 412, 128641. [Google Scholar] [CrossRef]
- Groppo, E.; Uddin, M.J.; Zavorotynska, O.; Damin, A.; Vitillo, J.G.; Spoto, G.; Zecchina, A. Exploring the Chemistry of Electron-Accepting Molecules in the Cavities of the Basic Microporous P4VP Polymer by in situ FTIR Spectroscopy. J. Phys. Chem. C 2008, 112, 19493–19500. [Google Scholar] [CrossRef]
- Shieh, J.-J.; Chung, T.S. Gas permeability, diffusivity, and solubility of poly(4-vinylpyridine) film. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2851–2861. [Google Scholar] [CrossRef]
- Ye, C.; Raaijman, S.J.; Chen, X.; Koper, M.T.M. Enhanced Electrochemical CO2 Reduction to Formate on Poly(4-vinylpyridine)-Modified Copper and Gold Electrodes. ACS Appl. Mater. Interfaces 2022, 14, 45263–45271. [Google Scholar] [CrossRef]
- Qian, X.; Meng, X.; Sun, J.; Jiang, L.; Wang, Y.; Zhang, J.; Hu, X.; Shalom, M.; Zhu, J. Salt-Assisted Synthesis of 3D Porous g-C3N4 as a Bifunctional Photo- and Electrocatalyst. ACS Appl. Mater. Interfaces 2019, 11, 27226–27232. [Google Scholar] [CrossRef]
- Ren, Y.; Han, Q.; Zhao, Y.; Wen, H.; Jiang, Z. The exploration of metal-free catalyst g-C3N4 for NO degradation. J. Hazard. Mater. 2021, 404, 124153. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Xue, J.; Wang, R.; Yin, Z.; Ding, L.; Wang, H. Graphene oxide-modified g-C3N4 nanosheet membranes for efficient hydrogen purification. Chem. Eng. J. 2021, 420, 129574. [Google Scholar] [CrossRef]
- Wu, J.; Liu, M.; Sharma, P.P.; Yadav, R.M.; Ma, L.; Yang, Y.; Zou, X.; Zhou, X.-D.; Vajtai, R.; Yakobson, B.I.; et al. Incorporation of Nitrogen Defects for Efficient Reduction of CO2 via Two-Electron Pathway on Three-Dimensional Graphene Foam. Nano Lett. 2016, 16, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Tang, Z.; Guo, S.; Han, M.; Zhu, C.; Zhou, Y.; Bai, L.; Gao, J.; Huang, H.; Li, Y.; et al. Enhanced Activity for CO2 Electroreduction on a Highly Active and Stable Ternary Au-CDots-C3N4 Electrocatalyst. ACS Catal. 2018, 8, 188–197. [Google Scholar] [CrossRef]
- Hu, C.; Liu, M.-T.; Sakai, A.; Yoshida, M.; Lin, K.-Y.A.; Huang, C.-C. Synergistic effect of Cu and Ru decoration on g-C3N4 for electrocatalytic CO2 reduction. J. Ind. Eng. Chem. 2022, 115, 329–338. [Google Scholar] [CrossRef]















| Cathodic Reaction | Products | E°/V |
|---|---|---|
| CO2 + 2H+ + 2e−→HCOOH | HCOOH | −0.61 |
| CO2 + 2H+ + 2e−→CO + H2O | CO | −0.53 |
| CO2 + 6H+ + 6e−→CH3OH + H2O | CH3OH | −0.38 |
| CO2 + 8H+ + 8e−→CH4 + 2H2O | CH4 | −0.24 |
| 2CO2 + 8H+ + 8e−→CH3COOH + 2H2O | CH3COOH | −0.30 |
| 2CO2 + 12H+ + 12e−→C2H4 + 4H2O | C2H4 | −0.34 |
| 2CO2 + 12H+ + 12e−→CH3CH2OH + 3H2O | CH3CH2OH | −0.33 |
| 2CO2 + 14H+ + 14e−→C2H6 + 4H2O | C2H6 | −0.27 |
| 2H+ + 2e−→H2 | H2 | −0.42 |
| Materials | Structural Features | Advantages | Disadvantages |
|---|---|---|---|
| POPs | A 2D or 3D network structure expanded by organic groups or fragments generated by chemical polymerization |
|
|
| PCPs | A solid crystal material formed by connecting metal ions or metal clusters with organic complexes. |
|
|
| COFs | A kind of organic building blocks connected together by covalent bonds to form a porous skeleton with periodic structure. |
|
|
| N-polymers | A kind of material with nitrogen functional groups in the main chain or side chain of polymer, which can complex or anchor metal particles. |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, G.; Hu, L.; Ge, B.; Yu, X.; Deng, J. Porous Polymer Materials for CO2 Capture and Electrocatalytic Reduction. Materials 2023, 16, 1630. https://doi.org/10.3390/ma16041630
Wang H, Wang G, Hu L, Ge B, Yu X, Deng J. Porous Polymer Materials for CO2 Capture and Electrocatalytic Reduction. Materials. 2023; 16(4):1630. https://doi.org/10.3390/ma16041630
Chicago/Turabian StyleWang, Hui, Genyuan Wang, Liang Hu, Bingcheng Ge, Xiaoliang Yu, and Jiaojiao Deng. 2023. "Porous Polymer Materials for CO2 Capture and Electrocatalytic Reduction" Materials 16, no. 4: 1630. https://doi.org/10.3390/ma16041630
APA StyleWang, H., Wang, G., Hu, L., Ge, B., Yu, X., & Deng, J. (2023). Porous Polymer Materials for CO2 Capture and Electrocatalytic Reduction. Materials, 16(4), 1630. https://doi.org/10.3390/ma16041630