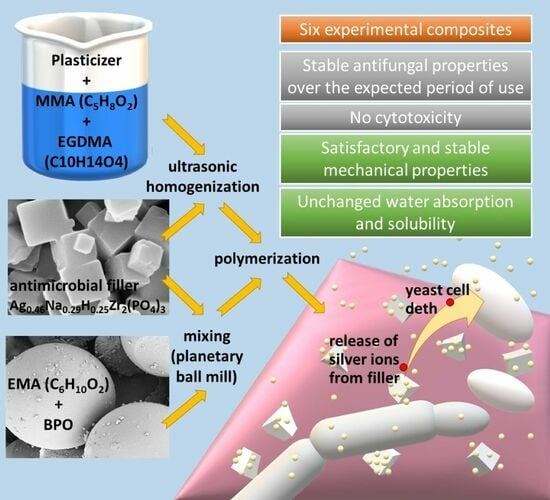
A Temporary Acrylic Soft Denture Lining Material Enriched with Silver-Releasing Filler-Cytotoxicity, Mechanical and Antifungal Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Methods
2.2.1. Scanning Electron Microscope Investigations
2.2.2. Antifungal Efficacy
2.2.3. Adherence of Candida albicans Cells
2.2.4. Cell Viability Assay (MTT Assay)
2.2.5. Shore A Hardness
2.2.6. Tensile Strength
2.2.7. Tensile Bond Strength
- Type A: debonding of the material from the denture base material (adhesive fracture) with the possible presence of remnants of the soft lining material invisible to the naked eye and not protruding from the plate.
- Type B—cohesive fracture: when only the soft lining was damaged (no zones indicating loss of bonding between the PMMA plate and the relining).
- Type A*—similar to type A: with the difference that there were single, visible with the naked eye, areas of cohesive destruction, where none of the dimensions exceeded 1 mm.
- Types A + B mixed fracture: when the fracture areas of types A and B were simultaneously represented.
2.2.8. Sorption and Solubility
2.2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anusavice, K.; Shen, C.; Rawls, H.R. Phillips’ Science of Dental Materials, 12th ed.; Saunders: St. Louis, MO, USA, 2013; ISBN 978-0-323-24205-9. [Google Scholar]
- Chladek, G.; Żmudzki, J.; Kasperski, J. Long-Term Soft Denture Lining Materials. Materials 2014, 7, 5816–5842. [Google Scholar] [CrossRef] [PubMed]
- EN ISO 10139-2:2016; Dentistry—Soft Lining Materials for Removable Dentures—Part 2: Materials for Long-Term Use. ISO International Organization for Standardization: Geneva, Switzerland, 2016.
- EN ISO 10139-1:2018; Dentistry—Soft Lining Materials for Removable Dentures—Part 1: Materials for Short-Term Use. ISO International Organization for Standardization: Geneva, Switzerland, 2018.
- Parker, S.; Braden, M. The Effect of Particle Size on the Gelation of Tissue Conditioners. Biomaterials 2001, 22, 2039–2042. [Google Scholar] [CrossRef] [PubMed]
- McCabe, J.F.; Walls, A.W.G. Applied Dental Materials, 9th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2008; ISBN 978-1-4051-3961-8. [Google Scholar]
- Goiato, M.C.; dos Santos, D.M.; de Medeiros, R.A.; Vechiato Filho, A.J.; Sinhoreti, M.A.C.; da Silva, E.V.F.; Moreno, A.; Goiato, M.C.; dos Santos, D.M.; de Medeiros, R.A.; et al. Tensile Bond Strength of a Soft Liner to an Acrylic Resin after Primer Application and Thermocycling. Mater. Res. 2015, 18, 1183–1187. [Google Scholar] [CrossRef]
- Białożyt-Bujak, E.; Wyszyńska, M.; Chladek, G.; Czelakowska, A.; Gala, A.; Orczykowska, M.; Białożyt, A.; Kasperski, J.; Skucha-Nowak, M. Analysis of the Hardness of Soft Relining Materials for Removable Dentures. Int. J. Env. Res. Public. Health 2021, 18, 9491. [Google Scholar] [CrossRef] [PubMed]
- Maciel, J.G.; Sugio, C.Y.C.; de Campos Chaves, G.; Procópio, A.L.F.; Urban, V.M.; Neppelenbroek, K.H. Determining Acceptable Limits for Water Sorption and Solubility of Interim Denture Resilient Liners. J. Prosthet. Dent. 2019, 121, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M.I. Advances in Soft Denture Liners: An Update. J. Contemp. Dent. Pract. 2015, 16, 314–318. [Google Scholar] [PubMed]
- ELsyad, M.A.; Shaheen, N.H.; Ashmawy, T.M. Long-Term Clinical and Prosthetic Outcomes of Soft Liner and Clip Attachments for Bar/Implant Overdentures: A Randomised Controlled Clinical Trial. J. Oral Rehabil. 2017, 44, 472–480. [Google Scholar] [CrossRef]
- Yadav, R.; Meena, A.; Lee, H.-H.; Lee, S.-Y.; Park, S.-J. Tribological Behavior of Dental Resin Composites: A Comprehensive Review. Tribol. Int. 2023, 190, 109017. [Google Scholar] [CrossRef]
- Yadav, R.; Singh, M.; Meena, A.; Lee, S.-Y.; Park, S.-J. Selection and Ranking of Dental Restorative Composite Materials Using Hybrid Entropy-VIKOR Method: An Application of MCDM Technique. J. Mech. Behav. Biomed. Mater. 2023, 147, 106103. [Google Scholar] [CrossRef]
- Spiechowicz, E.; Mierzwińska, N.E. Grzybice Jamy Ustnej; Wydawnictwo Medyczne Med Tour Press International: Warsaw, Poland, 1998. [Google Scholar]
- Bulad, K.; Taylor, R.L.; Verran, J.; McCord, J.F. Colonization and Penetration of Denture Soft Lining Materials by Candida albicans. Dent. Mater. 2004, 20, 167–175. [Google Scholar] [CrossRef]
- Kang, S.-H.; Lee, H.-J.; Hong, S.-H.; Kim, K.-H.; Kwon, T.-Y. Influence of Surface Characteristics on the Adhesion of Candida albicans to Various Denture Lining Materials. Acta Odontol. Scand. 2013, 71, 241–248. [Google Scholar] [CrossRef]
- Huh, J.-B.; Lim, Y.; Youn, H.-I.; Chang, B.M.; Lee, J.-Y.; Shin, S.-W. Effect of Denture Cleansers on Candida albicans Biofilm Formation over Resilient Liners. J. Adv. Prosthodont. 2014, 6, 109–114. [Google Scholar] [CrossRef]
- Dorocka-Bobkowska, B.; Medyński, D.; Pryliński, M. Recent Advances in Tissue Conditioners for Prosthetic Treatment: A Review. Adv. Clin. Exp. Med. 2017, 26, 723–728. [Google Scholar] [CrossRef]
- Iqbal, Z.; Zafar, M.S. Role of Antifungal Medicaments Added to Tissue Conditioners: A Systematic Review. J. Prosthodont. Res. 2016, 60, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, G.; Narayana, A.I.; Peralam, P.Y.; Balkrishanan, D. To Study the Effect of Cocos Nucifera Oil When Incorporated into Tissue Conditioner on Its Tensile Strength and Antifungal Activity: An in Vitro Study. J. Indian. Prosthodont. Soc. 2019, 19, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Vankadara, S.K.; Hallikerimath, R.B.; Patil, V.; Bhat, K.; Doddamani, M.H. Effect of Melaleuca Alternifolia Mixed with Tissue Conditioners in Varying Doses on Colonization and Inhibition of Candida albicans: An In Vitro Study. Contemp. Clin. Dent. 2017, 8, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.; Gujjari, A.K.; Gowda, V.; Angadi, S. Antifungal Response of Oral-Associated Candidal Reference Strains (American Type Culture Collection) by Supercritical Fluid Extract of Nutmeg Seeds for Geriatric Denture Wearers: An in Vitro Screening Study. J. Indian. Prosthodont. Soc. 2017, 17, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Kanathila, H.; Bhat, A.M.; Krishna, P.D. The Effectiveness of Magnesium Oxide Combined with Tissue Conditioners in Inhibiting the Growth of Candida albicans: An in Vitro Study. Indian J. Dent. Res. 2011, 22, 613. [Google Scholar] [CrossRef] [PubMed]
- Garner, S.J.; Nobbs, A.H.; McNally, L.M.; Barbour, M.E. An Antifungal Coating for Dental Silicones Composed of Chlorhexidine Nanoparticles. J. Dent. 2015, 43, 362–372. [Google Scholar] [CrossRef]
- Price, C.; Waters, M.G.J.; Williams, D.W.; Lewis, M.a.O.; Stickler, D. Surface Modification of an Experimental Silicone Rubber Aimed at Reducing Initial Candidal Adhesion. J. Biomed. Mater. Res. 2002, 63, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Habibzadeh, S.; Omidvaran, A.; Eskandarion, S.; Shamshiri, A.R. Effect of Incorporation of Silver Nanoparticles on the Tensile Bond Strength of a Long Term Soft Denture Liner. Eur. J. Dent. 2020, 14, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.-Y. In Vitro Antimicrobial Effect of the Tissue Conditioner Containing Silver Nanoparticles. J. Adv. Prosthodont. 2011, 3, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Chladek, G.; Kasperski, J.; Barszczewska-Rybarek, I.; Żmudzki, J. Sorption, Solubility, Bond Strength and Hardness of Denture Soft Lining Incorporated with Silver Nanoparticles. Int. J. Mol. Sci. 2012, 14, 563–574. [Google Scholar] [CrossRef]
- Ferreira, A.N.; D’Souza, K.; Aras, M.; Chitre, V.; Parsekar, S.; Pinto, M.J.W. Long Term Antifungal Efficacy of Silver-Zinc Zeolite Nanoparticles Incorporated in Two Soft Denture Liners—An in Vitro Assessment. Dent. Res. J. 2022, 19, 12. [Google Scholar] [CrossRef]
- Kreve, S.; Oliveira, V.C.; Bachmann, L.; Alves, O.L.; Reis, A.C.D. Influence of AgVO3 Incorporation on Antimicrobial Properties, Hardness, Roughness and Adhesion of a Soft Denture Liner. Sci. Rep. 2019, 9, 11889. [Google Scholar] [CrossRef] [PubMed]
- Silver Sodium Hydrogen Zirconium Phosphate. National Industrial Chemicals Notification and Assessment Scheme (Nicnas), Full Public Report, File No STD/1081, 11 March 2004. Available online: https://www.industrialchemicals.gov.au/sites/default/files/STD1081%20Public%20Report%20PDF.pdf (accessed on 14 February 2024).
- Chladek, G.; Basa, K.; Mertas, A.; Pakieła, W.; Żmudzki, J.; Bobela, E.; Król, W. Effect of Storage in Distilled Water for Three Months on the Antimicrobial Properties of Poly(Methyl Methacrylate) Denture Base Material Doped with Inorganic Filler. Materials 2016, 9, 328. [Google Scholar] [CrossRef]
- Jabłońska-Stencel, E.; Pakieła, W.; Mertas, A.; Bobela, E.; Kasperski, J.; Chladek, G. Effect of Silver-Emitting Filler on Antimicrobial and Mechanical Properties of Soft Denture Lining Material. Materials 2018, 11, 318. [Google Scholar] [CrossRef]
- Chladek, G.; Barszczewska-Rybarek, I.; Chrószcz-Porębska, M.; Mertas, A. The Effect of Quaternary Ammonium Polyethylenimine Nanoparticles on Bacterial Adherence, Cytotoxicity, and Physical and Mechanical Properties of Experimental Dental Composites. Sci. Rep. 2023, 13, 17497. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. ISO International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 7619-1:2010; Rubber, Vulcanized or Thermoplastic—Determination of Indentation Hardness—Part 1: Durometer Method (Shore Hardness). ISO International Organization for Standardization: Geneva, Switzerland, 2010.
- EN ISO 37:2017; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. ISO International Organization for Standardization: Geneva, Switzerland, 2017.
- Chladek, G.; Pakieła, K.; Pakieła, W.; Żmudzki, J.; Adamiak, M.; Krawczyk, C. Effect of Antibacterial Silver-Releasing Filler on the Physicochemical Properties of Poly(Methyl Methacrylate) Denture Base Material. Materials 2019, 12, 4146. [Google Scholar] [CrossRef]
- Jagini, A.S.; Marri, T.; Jayyarapu, D.; Kumari, R. Effect of Long-Term Immersion in Water and Artificial Saliva on the Flexural Strength of Two Heat Cure Denture Base Resins. J. Contemp. Dent. Pract. 2019, 20, 341–346. [Google Scholar] [CrossRef]
- Gratzl, G.; Paulik, C.; Hild, S.; Guggenbichler, J.P.; Lackner, M. Antimicrobial Activity of Poly(Acrylic Acid) Block Copolymers. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 38, 94–100. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernández-García, M. Polymeric Materials with Antimicrobial Activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Nikawa, H.; Jin, C.; Makihira, S.; Egusa, H.; Hamada, T.; Kumagai, H. Biofilm Formation of Candida albicans on the Surfaces of Deteriorated Soft Denture Lining Materials Caused by Denture Cleansers in Vitro. J. Oral Rehabil. 2003, 30, 243–250. [Google Scholar] [CrossRef]
- Ma, C.; He, N.; Zhao, Y.; Xia, D.; Wei, J.; Kang, W. Antimicrobial Mechanism of Hydroquinone. Appl. Biochem. Biotechnol. 2019, 189, 1291–1303. [Google Scholar] [CrossRef]
- Tan, S.; Zhang, L.; Liu, Y.; Shi, Q.; Ouyang, Y.; Chen, Y. Antibacterial Activity of Silver-Carried Sodium Zirconium Phosphate Prepared by Ion-Exchange Reaction. J. Ceram. Soc. Jpn. 2008, 116, 767–770. [Google Scholar] [CrossRef]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial Silver in Medicinal and Consumer Applications: A Patent Review of the Past Decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Łysik, D.; Niemirowicz-Laskowska, K.; Bucki, R.; Tokajuk, G.; Mystkowska, J. Artificial Saliva: Challenges and Future Perspectives for the Treatment of Xerostomia. Int. J. Mol. Sci. 2019, 20, 3199. [Google Scholar] [CrossRef] [PubMed]
- Baygar, T.; Ugur, A.; Sarac, N.; Balci, U.; Ergun, G. Functional Denture Soft Liner with Antimicrobial and Antibiofilm Properties. J. Dent. Sci. 2018, 13, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A.; Komasa, S.; Hashimoto, Y.; Komasa, Y.; Okazaki, J. Adsorption of Saliva Related Protein on Denture Materials: An X-Ray Photoelectron Spectroscopy and Quartz Crystal Microbalance Study. Adv. Mater. Sci. Eng. 2016, 2016, e5478326. [Google Scholar] [CrossRef]
- Müller, R.; Eidt, A.; Hiller, K.-A.; Katzur, V.; Subat, M.; Schweikl, H.; Imazato, S.; Ruhl, S.; Schmalz, G. Influences of Protein Films on Antibacterial or Bacteria-Repellent Surface Coatings in a Model System Using Silicon Wafers. Biomaterials 2009, 30, 4921–4929. [Google Scholar] [CrossRef] [PubMed]
- Moraes, G.S.; Cachoeira, V.S.; Alves, F.M.C.; Kiratcz, F.; Albach, T.; Bueno, M.G.; Neppelenbroek, K.H.; Urban, V.M. Is There an Optimal Method to Detach Candida albicans Biofilm from Dental Materials? J. Med. Microbiol. 2021, 70, 001436. [Google Scholar] [CrossRef]
- Valentini, F.; Luz, M.S.; Boscato, N.; Pereira-Cenci, T. Biofilm Formation on Denture Liners in a Randomised Controlled in Situ Trial. J. Dent. 2013, 41, 420–427. [Google Scholar] [CrossRef]
- Kim, H.-E.; Liu, Y.; Dhall, A.; Bawazir, M.; Koo, H.; Hwang, G. Synergism of Streptococcus Mutans and Candida albicans Reinforces Biofilm Maturation and Acidogenicity in Saliva: An In Vitro Study. Front. Cell. Infect. Microbiol. 2020, 10, 623980. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A Systematic Review on Silver Nanoparticles-Induced Cytotoxicity: Physicochemical Properties and Perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Lima Chaves, C.; de Souza Costa, C.A.; Vergani, C.E.; Chaves de Souza, P.P.; Machado, A.L. Effects of Soft Denture Liners on L929 Fibroblasts, HaCaT Keratinocytes, and RAW 264.7 Macrophages. Biomed. Res. Int. 2014, 2014, 840613. [Google Scholar] [CrossRef]
- Naarala, J.; Korpi, A. Cell Death and Production of Reactive Oxygen Species by Murine Macrophages after Short Term Exposure to Phthalates. Toxicol. Lett. 2009, 188, 157–160. [Google Scholar] [CrossRef]
- Song, Y.H.; Song, H.J.; Han, M.K.; Yang, H.S.; Park, Y.J. Cytotoxicity of Soft Denture Lining Materials Depending on Their Component Types. Int. J. Prosthodont. 2014, 27, 229–235. [Google Scholar] [CrossRef]
- Babich, H.; Zuckerbraun, H.L.; Wurzburger, B.J.; Rubin, Y.L.; Borenfreund, E.; Blau, L. Benzoyl Peroxide Cytotoxicity Evaluated in Vitro with the Human Keratinocyte Cell Line, RHEK-1. Toxicology 1996, 106, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Gu, J.T.; Zare, E.N.; Ashtari, B.; Moeini, A.; Tay, F.R.; Niu, L.-N. Polymeric and Inorganic Nanoscopical Antimicrobial Fillers in Dentistry. Acta Biomater. 2020, 101, 69–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Baras, B.; Lynch, C.D.; Weir, M.D.; Melo, M.A.S.; Li, Y.; Reynolds, M.A.; Bai, Y.; Wang, L.; Wang, S.; et al. Developing a New Generation of Therapeutic Dental Polymers to Inhibit Oral Biofilms and Protect Teeth. Materials 2018, 11, 1747. [Google Scholar] [CrossRef]
- Agnihotri, R.; Gaur, S.; Albin, S. Nanometals in Dentistry: Applications and Toxicological Implications-a Systematic Review. Biol. Trace Elem. Res. 2019, 197, 70–88. [Google Scholar] [CrossRef]
- Pisani, M.X.; de Malheiros-Segundo, A.L.; Balbino, K.L.; de Souza, R.F.; de Paranhos, H.F.O.; Lovato da Silva, C.H. Oral Health Related Quality of Life of Edentulous Patients after Denture Relining with a Silicone-Based Soft Liner. Gerodontology 2012, 29, e474–e480. [Google Scholar] [CrossRef] [PubMed]
- Safari, A.; Vojdani, M.; Mogharrabi, S.; Iraji Nasrabadi, N.; Derafshi, R. Effect of Beverages on the Hardness and Tensile Bond Strength of Temporary Acrylic Soft Liners to Acrylic Resin Denture Base. J. Dent. 2013, 14, 178–183. [Google Scholar]
- Mese, A.; Guzel, K.G. Effect of Storage Duration on the Hardness and Tensile Bond Strength of Silicone- and Acrylic Resin-Based Resilient Denture Liners to a Processed Denture Base Acrylic Resin. J. Prosthet. Dent. 2008, 99, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-J.; Yang, H.-S.; Chun, M.-G.; Park, Y.-J. Shore Hardness and Tensile Bond Strength of Long-Term Soft Denture Lining Materials. J. Prosthet. Dent. 2014, 112, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Kiat-amnuay, S.; Powers, J.M.; Zhao, Y. Effect of Nano-Oxide Concentration on the Mechanical Properties of a Maxillofacial Silicone Elastomer. J. Prosthet. Dent. 2008, 100, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Zidan, S.; Silikas, N.; Alhotan, A.; Haider, J.; Yates, J. Investigating the Mechanical Properties of ZrO2-Impregnated PMMA Nanocomposite for Denture-Based Applications. Materials 2019, 12, 1344. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Zhang, X.-J.; Huang, Z.-L.; Zhu, B.-S.; Chen, R.-R. Hybrid Effects of Zirconia Nanoparticles with Aluminum Borate Whiskers on Mechanical Properties of Denture Base Resin PMMA. Dent. Mater. J. 2014, 33, 141–146. [Google Scholar] [CrossRef]
- Ergun, G.; Sahin, Z.; Ataol, A.S. The Effects of Adding Various Ratios of Zirconium Oxide Nanoparticles to Poly(Methyl Methacrylate) on Physical and Mechanical Properties. J. Oral Sci. 2018, 60, 304–315. [Google Scholar] [CrossRef]
- El-Hadary, A.; Drummond, J.L. Comparative Study of Water Sorption, Solubility, and Tensile Bond Strength of Two Soft Lining Materials. J. Prosthet. Dent. 2000, 83, 356–361. [Google Scholar] [CrossRef]
- Dutta, A.; Sharma, M.; Kumar, S.; Chowdhary, Z.; Bumb, P.; Kumar, C. Effect of Water Sorption and Solubility on Two Soft Denture Lining Materials Stored in Three Different Mediums. Int. J. Prosthodont. Restor. Dent. 2023, 13, 129–136. [Google Scholar] [CrossRef]
- Inoue, M.; Nakajima, H.; Akiba, N.; Hibino, Y.; Nagasawa, Y.; Sumi, Y.; Minakuchi, S. Influence of Monomer Content on the Viscoelasticity, Water Sorption and Solubility of Experimental Fluorinated Soft Lining Materials. Dent. Mater. J. 2015, 34, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Kasraei, S.; Sami, L.; Hendi, S.; AliKhani, M.-Y.; Rezaei-Soufi, L.; Khamverdi, Z. Antibacterial Properties of Composite Resins Incorporating Silver and Zinc Oxide Nanoparticles on Streptococcus Mutans and Lactobacillus. Restor. Dent. Endod. 2014, 39, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Stencel, R.; Kasperski, J.; Pakieła, W.; Mertas, A.; Bobela, E.; Barszczewska-Rybarek, I.; Chladek, G. Properties of Experimental Dental Composites Containing Antibacterial Silver-Releasing Filler. Materials 2018, 11, 1031. [Google Scholar] [CrossRef]














| Material Code | Final Mass Concentration of AF in Material, % | Mass Concentration of AF in VSP, % | Mass Concentration of AF in VSL, % |
|---|---|---|---|
| CO | 0 | 0 | 0 |
| C1 | 1 | 1 | 1 |
| C2 | 2 | 1 | 3.3 |
| C4 | 4 | 1 | 7.6 |
| C6 | 6 | 1 | 11.7 |
| C8 | 8 | 1 | 15.6 |
| C10 | 10 | 1 | 19.4 |
| Time | Vt, × 102 CFU/mL | AFE, % | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CO @ | C1 # | C2 # | C4 # | C6 # | C8 # | C10 # | CO | C1 | C2 | C4 | C6 | C8 | C10 | ||
| 24 h @ | Med | 4.3 | 0 | 0 | 0 | 0.3 | 0 | 0 | 95.1 | 100 | 100 | 100 | 99.7 | 100 | 100 |
| Max | 8 | 0 | 1 | 0 | 0.5 | 0.5 | 0.5 | 96.5 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Min | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 90.7 | 100 | 98.8 | 100 | 99.4 | 99.4 | 99.4 | |
| 7 d @ | Med | 4.8 | 1 | 0 | 0 | 0 | 0.5 | 0 | 94.5 | 98.8 | 100 | 100 | 100 | 99.4 | 100 |
| Max | 11.5 | 2 | 0.5 | 0 | 0 | 1 | 0.5 | 97.1 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Min | 2.5 | 0 | 0 | 0 | 0 | 0 | 0 | 86.6 | 97.7 | 99.4 | 100 | 100 | 98.8 | 99.4 | |
| 30 d @ | Med | 64 | 0 | 0.5 | 0.5 | 0.3 | 0 | 0 | 25.6 | 100 | 99.4 | 99.4 | 99.7 | 100 | 100 |
| Max | 107 | 0.5 | 1 | 1 | 2 | 0 | 0 | 52.9 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Min | 40.5 | 0 | 0 | 0 | 0 | 0 | 0 | −24.4 | 99.4 | 98.8 | 98.8 | 97.7 | 100 | 100 | |
| Material Code | Storing Time | ||
|---|---|---|---|
| 24 h | 7 Days | 30 Days | |
| (p < 0.0001) | (p < 0.0001) | (p < 0.0001) | |
| C0 (p = 0.05) | A; a | A; a,b | A; b |
| C1 (p = 0.0059) | B; a | B; a,b | B; b |
| C2 (p = 0.1577) | B; - | B; - | B; - |
| C4 (p = 0.1505) | C; - | C; - | C; - |
| C6 (p = 0.0447) | C; a | C; a,b | C; b |
| C8 (p = 0.0221) | D; a | D; a,b | D; b |
| C10 (p = 0.0156) | D; a | E; b | D; b |
| Material Code | Storing Time | ||
|---|---|---|---|
| 24 h (p = 0.1646) | 7 Days (p = 0.0252) | 30 Days (p = 0.0002) | |
| C0 (p = 0.4161) | - | A | A |
| C1 (p = 0.072) | - | A,B | A |
| C2 (p = 0.1703) | - | A | A |
| C4 (p = 0.1252) | - | A,B | A,B |
| C6 (p = 0.2738) | - | A,B | A,B |
| C8 (p = 0.9173) | - | B | B |
| C10 (p = 0.7359) | - | A,B | A,B |
| Material Code | Storing Time | ||
|---|---|---|---|
| 24 h | 7 Days | 30 Days | |
| (p = 0.169) | (p = 0.1799) | (p = 0.0038) | |
| C0 (p = 0.281) | - | - | A,B; - |
| C1 (p = 0.7639) | - | - | A,B; - |
| C2 (p = 0.4551) | - | - | B; - |
| C4 (p = 0.0777) | - | - | A,B; - |
| C6 (p = 0.0581) | - | - | A,B; - |
| C8 (p = 0.0381) | a | a,b | A; b |
| C10 (p < 0.0001) | a | b | A; c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chladek, G.; Kalamarz, I.; Pakieła, W.; Barszczewska-Rybarek, I.; Czuba, Z.; Mertas, A. A Temporary Acrylic Soft Denture Lining Material Enriched with Silver-Releasing Filler-Cytotoxicity, Mechanical and Antifungal Properties. Materials 2024, 17, 902. https://doi.org/10.3390/ma17040902
Chladek G, Kalamarz I, Pakieła W, Barszczewska-Rybarek I, Czuba Z, Mertas A. A Temporary Acrylic Soft Denture Lining Material Enriched with Silver-Releasing Filler-Cytotoxicity, Mechanical and Antifungal Properties. Materials. 2024; 17(4):902. https://doi.org/10.3390/ma17040902
Chicago/Turabian StyleChladek, Grzegorz, Igor Kalamarz, Wojciech Pakieła, Izabela Barszczewska-Rybarek, Zenon Czuba, and Anna Mertas. 2024. "A Temporary Acrylic Soft Denture Lining Material Enriched with Silver-Releasing Filler-Cytotoxicity, Mechanical and Antifungal Properties" Materials 17, no. 4: 902. https://doi.org/10.3390/ma17040902
APA StyleChladek, G., Kalamarz, I., Pakieła, W., Barszczewska-Rybarek, I., Czuba, Z., & Mertas, A. (2024). A Temporary Acrylic Soft Denture Lining Material Enriched with Silver-Releasing Filler-Cytotoxicity, Mechanical and Antifungal Properties. Materials, 17(4), 902. https://doi.org/10.3390/ma17040902