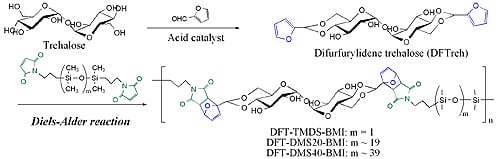
Synthesis and Properties of Trehalose-Based Flexible Polymers Prepared from Difurfurylidene Trehalose and Maleimide- Terminated Oligo(dimethylsiloxane) by Diels-Alder Reactions
Abstract
:1. Introduction

2. Experimental
2.1. Materials
2.2. Synthesis of 4,6,4’,6’-O-difurfurylidene-α,α-d-trehalose (DFTreh)
2.3. Synthesis of maleimide-terminated dimethylsiloxane oligomers (DMS-BMI)
2.4. Diels-Alder polymerization of DFTreh and DMS-BMI
2.5. Retro-Diels-Alder reaction of the polymer product from DFT-DMS20-BMI
2.6. Characterization
3. Results and Discussion
3.1. Synthesis of DFTreh and DMS-BMI

3.2. Synthesis of trehalose-based polymers by the Diels-Alder reaction
| Product | Reaction condition | Yield (%) | Molecular weight (GPC) | ||||
|---|---|---|---|---|---|---|---|
| Temperature (°C) | Time (h) | Mw | Mn | Mw/Mn | DP * | ||
| DFT-TMDS-BMI | 40 | 48 | 49 | 7,100 | 2,200 | 3.2 | 2.4 |
| DFT-TMDS-BMI | 55 | 48 | 47 | 11,000 | 2,900 | 3.8 | 3.2 |
| DFT-TMDS-BMI | 70 | 48 | 51 | 14,000 | 3,300 | 4.2 | 3.6 |
| DFT-DMS20-BMI | 40 | 48 | 69 | 8,900 | 4,000 | 2.2 | 1.8 |
| DFT-DMS20-BMI | 55 | 48 | 63 | 18,000 | 7,500 | 2.4 | 3.4 |
| DFT-DMS20-BMI | 70 | 48 | 60 | 19,000 | 6,800 | 2.8 | 3.0 |
| DFT-DMS40-BMI | 40 | 48 | 48 | 9,600 | 3,200 | 3.0 | 0.9† |
| DFT-DMS40-BMI | 55 | 48 | 82 | 13,000 | 9,000 | 1.4 | 2.4 |
| DFT-DMS40-BMI | 70 | 48 | 62 | 7,000 | 3,500 | 2.0 | 0.9† |
3.3. Solubility of polymers
| Solvent | DFT-TMDS-BMI | DFT-DMS20-BMI | DFT-DMS40-BMI |
|---|---|---|---|
| Water | i | i | i |
| Methanol | i | i | i |
| Ethanol | i | i | i |
| Dimethylsulfoxide | s | ps | i |
| N,N-dimethylacetamide | s | s | ps |
| DMF | s | s | s |
| Acetone | ps | s | s |
| Dioxane | s | s | s |
| Tetrahydrofuran | s | s | s |
| Chloroform | s | s | s |
| Ethyl acetate | i | s | s |
| Toluene | i | s | s |
| Diethylether | i | i | s |
| Hexane | i | i | i |

3.4. Thermal properties of polymers


| Product | Tg ( °C) | ΔCp at Tg (J/g•K) | Peak temperature at rDA (°C) | ΔH at rDA (J/g) |
|---|---|---|---|---|
| DFT-TMDS-BMI | -119 | 0.194 | 158 | 14.3 |
| DFT-DMS20-BMI | -128 | 0.306 | 136 | 7.48 |
| DFT-DMS40-BMI | -127 | 0.367 | 136 | 5.62 |

3.5. Morphology of polymers

3.6. Mechanical properties of polymers

3.7. Retro-Diels-Alder reaction

4. Conclusions
Acknowledgements
References
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar]
- Shogren, R.L.; Fanta, G.F.; Doane, W.M. Development of starch based plastics—a reexamination of selected polymer systems in historical perspective. Starch 1993, 45, 276–280. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar]
- Teramoto, N.; Arai, Y.; Shibasaki, Y.; Shibata, M. A facile synthesis of a novel polyacetal containing trehalose residue in the main chain. Carbohydr. Polym. 2004, 56, 1–6. [Google Scholar] [CrossRef]
- Teramoto, N.; Abe, Y.; Enomoto, A.; Watanabe, D.; Shibata, M. Novel synthetic route of a trehalose-based linear polymer by ring opening of two epoxy groups with aliphatic diamine. Carbohydr. Polym. 2005, 59, 217–224. [Google Scholar] [CrossRef]
- Teramoto, N.; Arai, Y.; Shibata, M. Thermo-reversible Diels-Alder polymerization of difurfurylidene trehalose and bismaleimides. Carbohydr. Polym. 2006, 64, 78–84. [Google Scholar] [CrossRef]
- Teramoto, N.; Unosawa, M.; Matsushima, S.; Shibata, M. Synthesis and properties of thermoplastic alternating copolymers containing trehalose and siloxane units by hydrosilylation reaction. Polym. J. 2007, 39, 975–981. [Google Scholar] [CrossRef]
- Teramoto, N.; Sachinvala, N.D.; Shibata, M. Trehalose and trehalose-based polymers for environmentally benign, biocompatible and bioactive materials. Molecules 2008, 13, 1773–1816. [Google Scholar] [CrossRef] [PubMed]
- Newman, Y.M.; Ring, S.G.; Colaco, C. The role of trehalose and other carbohydrates in biopreservation. Biotechnol. Genet. Eng. Rev. 1993, 11, 263–294. [Google Scholar] [CrossRef] [PubMed]
- Elbein, A.D.; Pan, Y.T.; Pastuszak, I.; Carroll, D. New insights on trehalose: a multifunctional molecule. Glycobiology 2003, 13, 17R–27R. [Google Scholar] [CrossRef] [PubMed]
- Paiva, C.L.; Panek, A.D. Biotechnological applications of the disaccharide trehalose. Biotechnol Annu. Rev. 1996, 2, 293–314. [Google Scholar] [PubMed]
- Higashiyama, T. Novel functions and applications of trehalose. Pure Appl. Chem. 2002, 74, 1263–1270. [Google Scholar] [CrossRef]
- Stille, J.K.; Plummer, L. Polymerization by the Diels-Alder reaction. J. Org. Chem. 1961, 26, 4026–4029. [Google Scholar] [CrossRef]
- Engle, L.P.; Wagener, K.B. A review of thermally controlled covalent bond formation in polymer chemistry. J. Macromol. Sci., Rev. Macromol. Chem. 1993, C33, 239–257. [Google Scholar]
- Craven, J.M. Cross-linked thermally reversible polymers produced from condensation polymers with pendant furan groups cross-linked with maleimides. US Pat. 3,435,003, 1969. [Google Scholar]
- Kelen, T.; Iván, B.; Nagy, T.T.; Turcsányi, B.; Tűdős, F.; Kennedy, J.P. Reversible crosslinking during thermal degradation of PVC. Polym. Bull. 1978, 1, 79–84. [Google Scholar] [CrossRef]
- Kennedy, J.P.; Carlson, G.M. Synthesis, characterization, and Diels-Alder extension of cyclopentadiene telechelic polyisobutylene. IV. α,ω-Di(3-cyclopentadienylpropyldimethylsilyl)- polyisobutylene. J. Polym. Sci.: Polym. Chem. Ed. 1983, 21, 3551–3561. [Google Scholar] [CrossRef]
- Chujo, Y.; Sada, K.; Saegusa, T. Reversible gelation of polyoxazoline by means of Diels-Alder reaction. Macromolecules 1990, 23, 2636–2641. [Google Scholar] [CrossRef]
- Mikroyannidis, J.A. Synthesis and diels-alder polymerization of furfurylidene and fur-furyl-substituted maleamic acids. J. Polym. Sci. A: Polym. Chem. 1992, 30, 125–132. [Google Scholar] [CrossRef]
- Kuramoto, N.; Hayashi, K.; Nagai, K. Thermoreversible reaction of Diels-Alder polymer composed of difurufuryladipate with bismaleimidodiphenylmethane. J. Polym. Sci., Part A: Polym. Chem. 1994, 32, 2501–2504. [Google Scholar] [CrossRef]
- Goussé, C.; Gandini, A. Diels-Alder polymerization of difurans with bismaleimides. Polym. Int. 1999, 48, 723–731. [Google Scholar] [CrossRef]
- Gheneim, R.; Perez-Berumen, C.; Gandini, A. Diels-Alder reactions with novel polymeric dienes and dienophiles: Synthesis of reversibly cross-linked elastomers. Macromolecules 2002, 35, 7246–7253. [Google Scholar] [CrossRef]
- Watanabe, M.; Yoshie, N. Synthesis and properties of readily recyclable polymers from bisfuranic terminated poly(ethylene adipate) and multi-maleimide linkers. Polymer 2006, 47, 4946–4952. [Google Scholar] [CrossRef]
- Canary, S.A.; Stevens, M.P. Thermally reversible crosslinking of polystyrene via the furan-maleimide Diels-Alder reaction. J. Polym. Sci., Part A: Polym. Chem. 1992, 30, 1755–1760. [Google Scholar] [CrossRef]
- Kamahori, K.; Tada, S.; Ito, K.; Itsuno, S. Optically active polymer synthesis by Diels-Alder polymerization with chirally midified Lewis acid catalyst. Macromolecules 1999, 32, 541–547. [Google Scholar] [CrossRef]
- Walsh, R. Bond dissociation energy values in silicon-containing compounds and some of their implications. Acc. Chem. Res. 1981, 14, 246–252. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an-open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Teramoto, N.; Niwa, M.; Shibata, M. Synthesis and Properties of Trehalose-Based Flexible Polymers Prepared from Difurfurylidene Trehalose and Maleimide- Terminated Oligo(dimethylsiloxane) by Diels-Alder Reactions. Materials 2010, 3, 369-385. https://doi.org/10.3390/ma3010369
Teramoto N, Niwa M, Shibata M. Synthesis and Properties of Trehalose-Based Flexible Polymers Prepared from Difurfurylidene Trehalose and Maleimide- Terminated Oligo(dimethylsiloxane) by Diels-Alder Reactions. Materials. 2010; 3(1):369-385. https://doi.org/10.3390/ma3010369
Chicago/Turabian StyleTeramoto, Naozumi, Masashi Niwa, and Mitsuhiro Shibata. 2010. "Synthesis and Properties of Trehalose-Based Flexible Polymers Prepared from Difurfurylidene Trehalose and Maleimide- Terminated Oligo(dimethylsiloxane) by Diels-Alder Reactions" Materials 3, no. 1: 369-385. https://doi.org/10.3390/ma3010369
APA StyleTeramoto, N., Niwa, M., & Shibata, M. (2010). Synthesis and Properties of Trehalose-Based Flexible Polymers Prepared from Difurfurylidene Trehalose and Maleimide- Terminated Oligo(dimethylsiloxane) by Diels-Alder Reactions. Materials, 3(1), 369-385. https://doi.org/10.3390/ma3010369