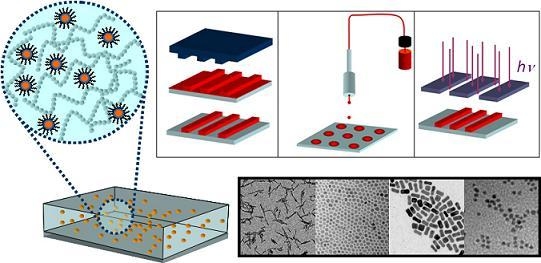
Colloidal Inorganic Nanocrystal Based Nanocomposites: Functional Materials for Micro and Nanofabrication
Abstract
:1. Introduction
2. Nanocomposite Materials


2.1. NC synthesis
2.2. NC functionalization

2.3. Synthetic approaches to nanocomposite materials

3. Nanocomposite Properties
3.1. Nanocomposite for optoelectronic applications
3.2. High refractive index nanocomposites
3.3. Nanocomposites for energy conversion
3.4. Nanocomposites with magnetic properties
3.5. Nanocomposites for sensing applications.
4. Patterning towards Devices: Conventional and Unconventional Methods
4.1. Lithographic techniques
4.1.1. Photolithographic techniques

4.1.2. Electron beam lithography
4.2. Block copolymer lithography
4.3. Direct-write techniques
4.3.1. Laser scanning
4.3.2. Ink-jet printing

4.3.3. Nano imprint lithography
5. Conclusions
References
- Bockstaller, M.R.; Mickiewicz, R.A.; Thomas, E.L. Block copolymer nanocomposites: Perspectives for tailored functional materials. Adv. Mater. 2005, 17, 1331–1349. [Google Scholar] [CrossRef]
- Colvin, V.L.; Schlamp, M.C.; Alivisatos, A.P. Light-emitting diodes made from cadmium selenide nanocrystals and a semiconducting polymer. Nature 1994, 370, 354–357. [Google Scholar] [CrossRef]
- Dabbousi, B.O.; Bawendi, M.G.; Onitsuka, O.; Rubner, M.F. Electroluminescence from CdSe quantum-dot/polymer composites. Appl. Phys. Lett. 1995, 66, 1316–1318. [Google Scholar] [CrossRef]
- Lu, C.; Guan, C.; Liu, Y.; Cheng, Y.; Yang, B. PbS/Polymer nanocomposite optical materials with high refractive index. Chem. Mater. 2005, 17, 2448–2454. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, Y.; Wang, C. Fabrication of PbS nanoparticles in polymer-fiber matrices by electrospinning. Adv. Mater. 2005, 17, 2485–2488. [Google Scholar] [CrossRef]
- Shenhar, R.; Norsten, T.B.; Rotello, V.M. Polymer-Mediated nanoparticle assembly: Structural control and applications. Adv. Mater. 2005, 17, 657–669. [Google Scholar] [CrossRef]
- Kickelbick, G. Introduction to Hybrid Materials in Hybrid Materials Synthesis, Characterization, and Applications. In Hybrid Materials: Synthesis, Characterization, and Applications; Kickelbick, G., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 1–48. [Google Scholar]
- Althues, H.; Henle, J.; Kaskel, S. Functional inorganic nanofillers for transparent polymers. Chem. Soc. Rev. 2007, 36, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Camargo, P.H.C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, structure, properties and new application opportunities. Mat. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- Hussain, F.; Hojjati, M.; Okamoto, M.; Gorga, R.E. Review article: Polymer-matrix nanocomposites, processing, manufacturing, and application: An overview. J. Compos. Mater. 2006, 40, 1511–1575. [Google Scholar] [CrossRef]
- Pyun, J. Nanocomposite materials from functional polymers and magnetic colloids. Polym. Rev. 2007, 47, 231–263. [Google Scholar] [CrossRef]
- Ramesh, G.V.; Porel, S.; Radhakrishnan, T.P. Polymer thin films embedded with in situ grown metal nanoparticles. Chem. Soc. Rev. 2009, 38, 2646–2656. [Google Scholar] [CrossRef] [PubMed]
- Sudeep, P.K.; Emrick, T. Polymer-Nanoparticle composites: Preparative methods and electronically active materials. Polym. Rev. 2007, 47, 155–163. [Google Scholar] [CrossRef]
- Caseri, W. Inorganic nanoparticles as optically effective additives for polymers. Chem. Eng. Commun. 2009, 196, 549–572. [Google Scholar] [CrossRef]
- Schmidt, A. Thermoresponsive magnetic colloids. Colloid Polym. Sci. 2007, 285, 953–966. [Google Scholar] [CrossRef]
- Lee, J.; Sundar, V.C.; Heine, J.R.; Bawendi, M.G.; Jensen, K.F. Full color emission from II-VI semiconductor quantum dot-polymer composites. Adv. Mater. 2000, 12, 1102–1105. [Google Scholar] [CrossRef]
- Nazzal, A.Y.; Qu, L.; Peng, X.; Xiao, M. Photoactivated CdSe nanocrystals as nanosensors for gases. Nano Lett. 2003, 3, 819–822. [Google Scholar] [CrossRef]
- Cozzoli, P.D.; Manna, L.; Curri, M.L.; Kudera, S.; Giannini, C.; Striccoli, M.; Agostiano, A. Shape and phase control of colloidal ZnSe nanocrystals. Chem. Mater. 2005, 17, 1296–1306. [Google Scholar] [CrossRef]
- Law, M.; Goldberger, J.; Yang, P. Semiconductor nanowires and nanotubes. Ann. Rev. Mater. Res. 2004, 34, 83–122. [Google Scholar] [CrossRef]
- Murray, C.B.; Kagan, C.R.; Bawendi, M.G. Synthesis and characterization of monodisperse nanocrystals and close-packed nanocrystal assemblies. Annu. Rev. Mater. Res. 2000, 30, 545–610. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Angelini, N.; Micali, N.; Villari, V.; Mineo, P.; Vitalini, D.; Scamporrino, E. Interactions between water soluble porphyrin-based star polymer and amino acids: Spectroscopic evidence of molecular binding. Phys. Rev. E 2005, 71, 021915. [Google Scholar] [CrossRef]
- Ng, C.H.B.; Yang, J.; Fan, W.Y. Synthesis and self-assembly of one-dimensional sub-10 nm Ag nanoparticles with cyclodextrin. J. Phys. Chem. C 2008, 112, 4141–4145. [Google Scholar] [CrossRef]
- Ahmadi, T.S.; Wang, Z.L.; Henglein, A.; El-Sayed, M.A. "Cubic" colloidal platinum nanoparticles. Chem. Mater. 1996, 8, 1161–1163. [Google Scholar] [CrossRef]
- Kim, S.-W.; Park, J.; Jang, Y.; Chung, Y.; Hwang, S.; Hyeon, T.; Kim, Y.W. Synthesis of monodisperse palladium nanoparticles. Nano Lett. 2003, 3, 1289–1291. [Google Scholar] [CrossRef]
- Wang, H.; Jiao, X.; Chen, D. Monodispersed nickel nanoparticles with tunable phase and size: Synthesis, characterization, and magnetic properties. J. Phys. Chem. C 2008, 112, 18793–18797. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, G.; Wang, J.; Fan, M. Effects of ionic liquids on the characteristics of synthesized nano Fe(0) particles. Inorg. Chem. 2009, 48, 10435–10441. [Google Scholar] [CrossRef] [PubMed]
- Anyaogu, K.C.; Fedorov, A.V.; Neckers, D.C. Synthesis, characterization, and antifouling potential of functionalized copper nanoparticles. Langmuir 2008, 24, 4340–4346. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, B. Applications of nanomaterials inside cells. Nano Today 2009, in press. [Google Scholar]
- Pan, C.; Pelzer, K.; Philippot, K.; Chaudret, B.; Dassenoy, F.; Lecante, P.; Casanove, M.-J. Ligand-Stabilized ruthenium nanoparticles: Synthesis, organization, and dynamics. J. Am. Chem. Soc. 2001, 123, 7584–7593. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, E.V.; Talapin, D.V.; Schnablegger, H.; Kornowski, A.; Festin, O.; Svedlindh, P.; Haase, M.; Weller, H. Study of nucleation and growth in the organometallic synthesis of magnetic alloy nanocrystals: The role of nucleation rate in size control of CoPt3 nanocrystals. J. Am. Chem. Soc. 2003, 125, 9090–9101. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Anders, S.; Thomson, T.; Baglin, J.E.E.; Toney, M.F.; Hamann, H.F.; Murray, C.B.; Terris, B.D. Controlled synthesis and assembly of FePt nanoparticles. J. Phys. Chem. B 2003, 107, 5419–5425. [Google Scholar] [CrossRef]
- Cheon, J.; Kang, N.-J.; Lee, S.-M.; Lee, J.-H.; Yoon, J.-H.; Oh, S.J. Shape evolution of single-crystalline iron oxide nanocrystals. J. Am. Chem. Soc. 2004, 126, 1950–1951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, J.; Song, X.; Zhong, X. Controlling the synthesis of CoO nanocrystals with various morphologies. J. Phys. Chem. C 2008, 112, 5322–5327. [Google Scholar] [CrossRef]
- Cozzoli, P.D.; Kornowski, A.; Weller, H. Low-Temperature synthesis of soluble and processable organic-capped anatase TiO2 nanorods. J. Am. Chem. Soc. 2003, 125, 14539–14548. [Google Scholar] [CrossRef] [PubMed]
- Ba, J.; Polleux, J.; Antonietti, M.; Niederberger, M. Non-aqueous synthesis of tin oxide nanocrystals and their assembly into ordered porous mesostructures. Adv. Mater. 2005, 17, 2509–2512. [Google Scholar] [CrossRef]
- Halas, N.J. Nanoscience under Glass: The versatile chemistry of silica nanostructures. ACS Nano 2008, 2, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Zhu, H.; Hadjipanayis, G.C.; Xiao, J.Q. Low-temperature synthesis of hexagonal (Wurtzite) ZnS nanocrystals. J. Am. Chem. Soc. 2004, 126, 6874–6875. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.W.; Peng, X. Formation of High-Quality CdS and Other II-VI Semiconductor nanocrystals in noncoordinating solvents: Tunable reactivity of monomers13. Angew Chem. 2002, 114, 2474–2477. [Google Scholar] [CrossRef]
- Peng, Z.A.; Peng, X. Nearly monodisperse and shape-controlled cdse nanocrystals via alternative routes: Nucleation and growth. J. Am. Chem. Soc. 2002, 124, 3343–3353. [Google Scholar] [CrossRef] [PubMed]
- Shieh, F.; Saunders, A.E.; Korgel, B.A. General Shape Control of colloidal CdS, CdSe, CdTe ures. J. Phys. Chem. B 2005, 109, 8538–8542. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Beard, M.C.; Ellingson, R.J.; Ferrere, S.; Curtis, C.; Drexler, J.; Luiszer, F.; Nozik, A.J. Absorption cross-section and related optical properties of colloidal InAs quantum dots. J. Phys. Chem. B 2005, 109, 7084–7087. [Google Scholar] [CrossRef] [PubMed]
- Zeger, H.; Iwan, M.; Jose, C.M. In situ 1H NMR study on the trioctylphosphine oxide capping of colloidal InP nanocrystals. ChemPhysChem 2005, 6, 2578–2584. [Google Scholar] [CrossRef] [PubMed]
- Cademartiri, L.; Bertolotti, J.; Sapienza, R.; Wiersma, D.S.; von Freymann, G.; Ozin, G.A. Multigram scale, solventless, and diffusion-controlled route to highly monodisperse PbS nanocrystals. J. Phys. Chem. B 2005, 110, 671–673. [Google Scholar] [CrossRef]
- Moreels, I.; Lambert, K.; De Muynck, D.; Vanhaecke, F.; Poelman, D.; Martins, J.C.; Allan, G.; Hens, Z. Composition and size-dependent extinction coefficient of colloidal PbSe quantum dots. Chem. Mater. 2007, 19, 6101–6106. [Google Scholar] [CrossRef]
- Murphy, J.E.; Beard, M.C.; Norman, A.G.; Ahrenkiel, S.P.; Johnson, J.C.; Yu, P.; Micic, O.I.; Ellingson, R.J.; Nozik, A.J. PbTe Colloidal nanocrystals: Synthesis, characterization, and multiple exciton generation. J. Am. Chem. Soc. 2006, 128, 3241–3247. [Google Scholar] [CrossRef] [PubMed]
- Cozzoli, P.D.; Comparelli, R.; Fanizza, E.; Curri, M.L.; Agostiano, A.; Laub, D. Photocatalytic synthesis of silver nanoparticles stabilized by TiO2 nanorods: A semiconductor/metal nanocomposite in homogeneous nonpolar solution. J. Am. Chem. Soc. 2004, 126, 3868–3879. [Google Scholar] [CrossRef] [PubMed]
- Cozzoli, P.D.; Curri, M.L.; Giannini, C.; Agostiano, A. Synthesis of TiO2-Au composites by titania-nanorod-assisted generation of gold nanoparticles at aqueous/nonpolar interfaces. Small 2006, 2, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Buonsanti, R.; Grillo, V.; Carlino, E.; Giannini, C.; Curri, M.L.; Innocenti, C.; Sangregorio, C.; Achterhold, K.; Parak, F.G.; Agostiano, A.; Cozzoli, P.D. Seeded growth of asymmetric binary nanocrystals made of a semiconductor TiO2 rodlike section and a magnetic γ-Fe2O3 spherical domain. J. Am. Chem. Soc. 2006, 128, 16953–16970. [Google Scholar] [CrossRef] [PubMed]
- Capek, R.K.; Lambert, K.; Dorfs, D.; Smet, P.F.; Poelman, D.; Eychmuller, A.; Hens, Z. Synthesis of extremely small CdSe and bright blue luminescent CdSe/ZnS nanoparticles by a prefocused hot-injection approach. Chem. Mater. 2009, 21, 1743–1749. [Google Scholar] [CrossRef]
- Sung, Y.-M.; Park, K.-S.; Lee, Y.-J.; Kim, T.-G. Ripening kinetics of CdSe/ZnSe core/shell nanocrystals. J. Phys. Chem. C 2006, 111, 1239–1242. [Google Scholar] [CrossRef]
- Gerion, D.; Pinaud, F.; Williams, S.C.; Parak, W.J.; Zanchet, D.; Weiss, S.; Alivisatos, A.P. Synthesis and properties of biocompatible water-soluble silica-coated CdSe/ZnS semiconductor quantum dots. J. Phys. Chem. B 2001, 105, 8861–8871. [Google Scholar] [CrossRef]
- Liz-Marzan, L.M.; Mulvaney, P. The assembly of coated nanocrystals. J. Phys. Chem. B 2003, 107, 7312–7326. [Google Scholar] [CrossRef]
- Manna, L.; Scher, E.C.; Li, L.-S.; Alivisatos, A.P. Epitaxial growth and photochemical annealing of graded CdS/ZnS shells on colloidal CdSe nanorods. J. Am. Chem. Soc. 2002, 124, 7136–7145. [Google Scholar] [CrossRef] [PubMed]
- Blackman, B.; Battaglia, D.; Peng, X. Bright and water-soluble near IR-emitting CdSe/CdTe/ZnSe Type-II/Type-I nanocrystals, tuning the efficiency and stability by growth. Chem. Mater. 2008, 20, 4847–4853. [Google Scholar] [CrossRef]
- Kwon, S.G.; Piao, Y.; Park, J.; Angappane, S.; Jo, Y.; Hwang, N.-M.; Park, J.-G.; Hyeon, T. Kinetics of monodisperse iron oxide nanocrystal formation by "heating-up" process. J. Am. Chem. Soc. 2007, 129, 12571–12584. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.J.; Jana, N.R. Controlling the aspect ratio of inorganic nanorods and nanowires. Adv. Mater. 2002, 14, 80–82. [Google Scholar] [CrossRef]
- Xue, C.; Me; x; traux, G.S.; Millstone, J.E.; Mirkin, C.A. Mechanistic study of photomediated triangular silver nanoprism growth. J. Am. Chem. Soc. 2008, 130, 8337–8344. [Google Scholar] [CrossRef] [PubMed]
- Borodko, Y.; Jones, L.; Lee, H.; Frei, H.; Somorjai, G. Spectroscopic study of tetradecyltrimethylammonium bromide Pt-C14TAB nanoparticles: Structure and stability. Langmuir 2009, 25, 6665–6671. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; He, F.; Gunn, T.M.; Zhao, D.; Roberts, C.B. Precise seed-mediated growth and size-controlled synthesis of palladium nanoparticles using a green chemistry approach. Langmuir 2009, 25, 7116–7128. [Google Scholar] [CrossRef] [PubMed]
- Rivas, L.; Sanchez-Cortes, S.; Garcia-Ramos, J.V.; Morcillo, G. Mixed Silver/Gold Colloids: A study of their formation, morphology, and surface-enhanced raman activity. Langmuir 2000, 16, 9722–9728. [Google Scholar] [CrossRef]
- Xia, H.; Bai, S.; Hartmann, J.; Wang, D. Synthesis of monodisperse quasi-spherical gold nanoparticles in water via silver(i)-assisted citrate reduction. Langmuir 2009. [Google Scholar] [CrossRef]
- Nair, P.S.; Fritz, Karolina P.; Scholes, Gregory D. Evolutionary shape control during colloidal quantum-dot growth. Small 2007, 3, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Giannici, F.; Placido, T.; Curri, M.L.; Striccoli, M.; Agostiano, A.; Comparelli, R. The fate of silver ions in the photochemical synthesis of gold nanorods: An Extended X-ray Absorption Fine Structure Analysis. Dalton Trans. 2009, 10367–10374. [Google Scholar] [CrossRef]
- Jun, Y.-w.; Lee, J.-H.; Choi, J.-s.; Cheon, J. Symmetry-controlled colloidal nanocrystals: Nonhydrolytic chemical synthesis and shape determining parameters. J. Phys. Chem. B 2005, 109, 14795–14806. [Google Scholar] [CrossRef] [PubMed]
- Buonsanti, R.; Grillo, V.; Carlino, E.; Giannini, C.; Kipp, T.; Cingolani, R.; Cozzoli, P.D. Nonhydrolytic synthesis of high-quality anisotropically shaped brookite TiO2 nanocrystals. J. Am. Chem. Soc. 2008, 130, 11223–11233. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.-S.; Talapin, D.V.; Gaschler, W.; Murray, C.B. Designing PbSe nanowires and nanorings through oriented attachment of nanoparticles. J. Am. Chem. Soc. 2005, 127, 7140–7147. [Google Scholar] [CrossRef] [PubMed]
- Manna, L.; Scher, E.C.; Alivisatos, A.P. Synthesis of soluble and processable rod-, arrow-, teardrop-, and tetrapod-shaped cdse nanocrystals. J. Am. Chem. Soc. 2000, 122, 12700–12706. [Google Scholar] [CrossRef]
- Liang, H.; Yang, H.; Wang, W.; Li, J.; Xu, H. High-yield uniform synthesis and microstructure-determination of rice-shaped silver nanocrystals. J. Am. Chem. Soc. 2009, 131, 6068–6069. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Hsin, C.F.; Liu, R.S.; Lee, J.F.; Jang, L.Y. Synthesis and characterization of multi-Pod-shaped Gold/silver nanostructures. J. Phys. Chem. C 2007, 111, 5909–5914. [Google Scholar] [CrossRef]
- Yen, C.W.; Mahmoud, M.A.; El-Sayed, M.A. Photocatalysis in gold nanocage nanoreactors. J. Phys. Chem. A 2009, 113, 4340–4345. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Pastoriza-Santos, I.; Rodriguez-Gonzalez, B.; Abajo, F.J.G.d.; Liz-Marzan, L.M. High-yield synthesis and optical response of gold nanostars. Nanotechnology 2008, 19, 015606. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shao, L.; Woo, K.C.; Ming, T.; Lin, H.-Q.; Wang, J. Shape-dependent refractive index sensitivities of gold nanocrystals with the same plasmon resonance wavelength. J. Phys. Chem. C 2009, 113, 17691–17697. [Google Scholar] [CrossRef]
- Huang, C.-J.; Chiu, P.-H.; Wang, Y.-H.; Chen, W.-R.; Meen, T.-H.; Yang, C.-F. Preparation and characterization of gold nanodumbbells. Nanotechnology 2006, 17, 5355–5362. [Google Scholar] [CrossRef]
- Kumar, S.; Nann, T. Shape control of II-VI semiconductor nanomaterials. Small 2006, 2, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.M.; Nie, S. Semiconductor nanocrystals: Structure, properties, and band gap engineering. Acc. Chem. Res. 2009. [Google Scholar]
- Murphy, C.J.; Sau, T.K.; Gole, A.M.; Orendorff, C.J.; Gao, J.; Gou, L.; Hunyadi, S.E.; Li, T. Anisotropic metal nanoparticles: Synthesis, assembly, and optical applications. J. Phys. Chem. B 2005, 109, 13857–13870. [Google Scholar] [CrossRef] [PubMed]
- Cozzoli, P.D.; Pellegrino, T.; Manna, L. Synthesis, properties and perspectives of hybrid nanocrystal structures. Chem. Soc. Rev. 2006, 35, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Grzelczak, M.; Perez-Juste, J.; Mulvaney, P.; Liz-Marzan, L.M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xiong, Y.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef]
- Corbierre, M.K.; Cameron, N.S.; Sutton, M.; Laaziri, K.; Lennox, R.B. Gold nanoparticle/polymer nanocomposites: Dispersion of nanoparticles as a function of capping agent molecular weight and grafting density. Langmuir 2005, 21, 6063–6072. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.; Achermann, M.; Nanda, J.; Ivanov, S.; Klimov, V.I.; Hollingsworth, J.A. Effect of the thiol-thiolate equilibrium on the photophysical properties of aqueous CdSe/ZnS nanocrystal quantum dots. J. Am. Chem. Soc. 2005, 127, 10126–10127. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Bawendi, M.G. Oligomeric ligands for luminescent and stable nanocrystal quantum dots. J. Am. Chem. Soc. 2003, 125, 14652–14653. [Google Scholar] [CrossRef] [PubMed]
- Bullen, C.; Mulvaney, P. The effects of chemisorption on the luminescence of CdSe quantum dots. Langmuir 2006, 22, 3007–3013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, C.; Li, M.; Ji, X.; Zhang, J.; Yang, B. Fluorescent nanocrystal-polymer composites from aqueous nanocrystals: Methods without ligand exchange. Chem. Mater. 2005, 17, 4783–4788. [Google Scholar] [CrossRef]
- Comparelli, R.; Zezza, F.; Striccoli, M.; Curri, M.L.; Tommasi, R.; Agostiano, A. Improved optical properties of CdS quantum dots by ligand exchange. Mater. Sci. Eng. C-Biomimetic Supramol. Syst. 2003, 23, 1083–1086. [Google Scholar] [CrossRef]
- Zezza, F.; Comparelli, R.; Striccoli, M.; Curri, M.L.; Tommasi, R.; Agostiano, A.; Della Monica, M. High quality CdS nanocrystals: Surface effects. Synth. Met. 2003, 139, 597–600. [Google Scholar] [CrossRef]
- Yang, J.; Wu, J.-C.; Wu, Y.-C.; Wang, J.-K.; Chen, C.-C. Organic solvent dependence of plasma resonance of gold nanorods: A simple relationship. Chem. Phys. Lett. 2005, 416, 215–219. [Google Scholar] [CrossRef]
- Reboud, V.; Kehagias, N.; Striccoli, M.; Placido, T.; Panniello, A.; Curri, M.L.; Zelsmann, M.; Reuther, F.; Gruetzner, G.; Torres, C.M.S. Photoluminescence enhancement in metallic nanocomposite printable polymer. J. Vac. Sci. Technol. B 2007, 25, 2642–2644. [Google Scholar] [CrossRef]
- Fanizza, E.; Cozzoli, P.D.; Curri, M.L.; Striccoli, M.; Sardella, E.; Agostiano, A. UV-light-driven immobilization of surface-functionalized oxide nanocrystals onto silicon. Adv. Funct. Mater. 2007, 17, 201–211. [Google Scholar] [CrossRef]
- Gravano, S.M.; Dumas, R.; Liu, K.; Patten, T.E. Methods for the surface functionalization of gamma-Fe2O3 nanoparticles with initiators for atom transfer radical polymerization and the formation of core-shell inorganic-polymer structures. J. Polym. Sci., A: Polym. Chem. 2005, 43, 3675–3688. [Google Scholar] [CrossRef]
- Potapova, I.; Mruk, R.; Prehl, S.; Zentel, R.; Basche, T.; Mews, A. Semiconductor nanocrystals with multifunctional polymer ligands. J. Am. Chem. Soc. 2003, 125, 320–321. [Google Scholar] [CrossRef] [PubMed]
- MacLachlan, M.J.; Manners, I.; Ozin, G.A. New (Inter)Faces: Polymers and Inorganic Materials. Adv. Mater. 2000, 12, 675–681. [Google Scholar] [CrossRef]
- Trinque, B.C.; Chiba, T.; Hung, R.J.; Chambers, C.R.; Pinnow, M.J.; Osborn, B.P.; Tran, H.V.; Wunderlich, J.; Hsieh, Y.-T.; Thomas, B.H.; Shafer, G.; DesMarteau, D.D.; Conley, W.; Willson, C.G. Recent advances in resists for 157 nm microlithography. J. Vac. Sci. Technol. B 2002, 20, 531–536. [Google Scholar] [CrossRef]
- McClelland, G.M.; Hart, M.W.; Rettner, C.T.; Best, M.E.; Carter, K.R.; Terris, B.D. Nanoscale patterning of magnetic islands by imprint lithography using a flexible mold. Appl. Phys. Lett. 2002, 81, 1483–1485. [Google Scholar] [CrossRef]
- Fan, H.; Leve, E.; Gabaldon, J.; Wright, A.; Haddad, R.E.; Brinker, C.J. Ordered two- and three-dimensional arrays self-assembled from water-soluble nanocrystal-micelles. Adv. Mater. 2005, 17, 2587–2590. [Google Scholar] [CrossRef]
- Pang, L.; Shen, Y.; Tetz, K.; Fainman, Y. PMMA quantum dots composites fabricated via use of pre-polymerization. Opt. Express 2005, 13, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Tamborra, M.; Striccoli, M.; Comparelli, R.; Curri, M.L.; Petrella, A.; Agostiano, A. Optical properties of hybrid composites based on highly luminescent CdS nanocrystals in polymer. Nanotechnology 2004, 15, S240–S244. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, Z.; Wang, Y.; Zhang, K.; Ji, X.; C, L.; Yang, B.; Gao, M. From water-soluble CdTe nanocrystals to fluorescent nanocrystal-polymer transparent composites using polymerizable surfactants. Adv. Mater. 2003, 15, 777–780. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.; Zhang, H.; Liu, Y.; Wang, C.; Xu, X.; Tang, Y.; Yang, B. Electrospinning: A facile method to disperse fluorescent quantum dots in nanofibers without förster resonance energy transfer. Adv. Funct. Mater. 2007, 17, 3650–3656. [Google Scholar] [CrossRef]
- Dammer, O.; Vlcková, B.; Procházka, M.; Bondarev, D.; Vohlídal, J.; Pfleger, J. Effect of preparation procedure on the structure, morphology, and optical properties of nanocomposites of poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene] with gold nanoparticles. Mater. Chem. Phys. 2009, 115, 352–360. [Google Scholar] [CrossRef]
- Gao, S.-L.; Mäder, E. Characterisation of interphase nanoscale property variations in glass fibre reinforced polypropylene and epoxy resin composites. Compos. Pt. A-Appl. Sci. Manuf. 2002, 33, 559–576. [Google Scholar] [CrossRef]
- Strano, M.S. Nanocomposites: Polymer-wrapped nanotubes. Nat. Mater. 2006, 5, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Park, S.; Wei, S.; Pereira, T.; Moldovan, M.; Karki, A.B.; Young, D.P.; Hahn, H.T. Flexible high-loading particle-reinforced polyurethane magnetic nanocomposite fabrication through particle-surface-initiated polymerization. Nanotechnology 2007, 18, 335704. [Google Scholar] [CrossRef]
- Kalima, V.; Vartiainen, I.; Saastamoinen, T.; Suvanto, M.; Kuittinen, M.; Pakkanen, T.T. UV-curable ZnS/polymer nanocomposite for replication of micron and submicron features. Opt. Mater. 2009, 31, 1540–1546. [Google Scholar] [CrossRef]
- Ingrosso, C.; Fakhfouri, V.; Striccoli, M.; Agostiano, A.; Voigt, A.; Gruetmer, G.; Curri, M.L.; Brugger, J. An epoxy photoresist modified by luminescent nanocrystals for the fabrication of 3D high-aspect-ratio microstructures. Adv. Funct. Mater. 2007, 17, 2009–2017. [Google Scholar] [CrossRef]
- Tamborra, M.; Striccoli, M.; Curri, M.L.; Alducin, J.A.; Mecerreyes, D.; Pomposo, J.A.; Kehagias, N.; Reboud, V.; Torres, C.M.S.; Agostiano, A. Nanocrystal-based luminescent composites for nanoimprinting lithography. Small 2007, 3, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Sciancalepore, C.; Cassano, T.; Curri, M.L.; Mecerreyes, D.; Valentini, A.; Agostiano, A.; Tommasi, R.; Striccoli, M. TiO2 nanorods/PMMA copolymer-based nanocomposites: Highly homogeneous linear and nonlinear optical material. Nanotechnology 2008, 19, 205705. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, N.; Janczewski, D.; Han, M.; Vancso, G.J. Designer polymer-quantum dot architectures. Prog. Polym. Sci. 2009, 34, 393–430. [Google Scholar] [CrossRef]
- Lü, C.; Gao, J.; Fu, Y.; Du, Y.; Shi, Y.; Su, Z. A ligand exchange route to highly luminescent surface-functionalized ZnS nanoparticles and their transparent polymer nanocomposites. Adv. Funct. Mater. 2008, 18, 3070–3079. [Google Scholar] [CrossRef]
- Qi, X.-Y.; Pu, K.-Y.; Fang, C.; Wen, G.-A.; Zhang, H.; Boey, F.Y.C.; Fan, Q.-L.; Wang, L.-H.; Huang, W. Semiconductor nanocomposites of emissive flexible random copolymers and cdte nanocrystals: Preparation, characterization, and optoelectronic properties. Macromol. Chem. Phys. 2007, 208, 2007–2017. [Google Scholar] [CrossRef]
- Tessler, N.; Medvedev, V.; Kazes, M.; Kan, S.; Banin, U. Efficient near-infrared polymer nanocrystal light-emitting diodes. Science 2002, 295, 1506–1508. [Google Scholar] [CrossRef] [PubMed]
- Steckel, J.S.; Coe-Sullivan, S.; Bulovi, V.; cacute; Bawendi, M.G. 1.3 μm to 1.55 μm tunable electroluminescence from PbSe quantum dots embedded within an organic device. Adv. Mater. 2003, 15, 1862–1866. [Google Scholar] [CrossRef]
- Feng, M.; Chen, Y.; Gu, L.; He, N.; Bai, J.; Lin, Y.; Zhan, H. CdS nanoparticles chemically modified PAN functional materials: Preparation and nonlinear optical properties. Eur. Polym. J. 2009, 45, 1058–1064. [Google Scholar] [CrossRef]
- Lee, S.; Lee, B.; Kim, B.J.; Park, J.; Yoo, M.; Bae, W.K.; Char, K.; Hawker, C.J.; Bang, J.; Cho, J. Free-standing nanocomposite multilayers with various length scales, adjustable internal structures, and functionalities. J. Am. Chem. Soc. 2009, 131, 2579–2587. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Yang, B. High refractive index organic-inorganic nanocomposites: Design, synthesis and application. J. Mater. Chem. 2009, 19, 2884–2901. [Google Scholar] [CrossRef]
- Koziej, D.; Fischer, F.; Kränzlin, N.; Caseri, W.R.; Niederberger, M. Nonaqueous TiO2 Nanoparticle synthesis: A versatile basis for the fabrication of self-supporting, transparent, and uv-absorbing composite films. ACS Appl. Mater. Interfaces 2009, 1, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Di Gianni, A.; Trabelsi, S.; Rizza, G.; Sangermano, M.; Althues, H.; Kaskel, S.; Voit, B. Hyperbranched polymer/TiO2 hybrid nanoparticles synthesized via an in situ sol-gel process. Macromol. Chem. Phys. 2007, 208, 76–86. [Google Scholar] [CrossRef]
- Cheng, Y.; Lu, C.; Lin, Z.; Liu, Y.; Guan, C.; Lu, H.; Yang, B. Preparation and properties of transparent bulk polymer nanocomposites with high nanophase contents. J. Mater. Chem. 2008, 18, 4062–4068. [Google Scholar] [CrossRef]
- Boucle, J.; Ravirajan, P.; Nelson, J. Hybrid polymer-metal oxide thin films for photovoltaic applications. J. Mater. Chem. 2007, 17, 3141–3153. [Google Scholar] [CrossRef]
- Guan, C.; Lu, C.; Cheng, Y.; Song, S.; Yang, B. A facile one-pot route to transparent polymer nanocomposites with high ZnS nanophase contents via in situ bulk polymerization. J. Mater. Chem. 2009, 19, 617–621. [Google Scholar] [CrossRef]
- Huynh, W.U.; Dittmer, J.J.; Alivisatos, A.P. Hybrid nanorod-polymer solar cells. Science 2002, 295, 2425–2427. [Google Scholar] [CrossRef] [PubMed]
- Bouclé, J.; Chyla, S.; Shaffer, M.S.P.; Durrant, J.R.; Bradley, D.D.C.; Nelson, J. Hybrid solar cells from a blend of poly(3-hexylthiophene) and ligand-capped TiO2 nanorods. Adv. Funct. Mater. 2008, 18, 622–633. [Google Scholar] [CrossRef]
- De Girolamo, J.; Reiss, P.; Pron, A. Supramolecularly Assembled hybrid materials via molecular recognition between diaminopyrimidine-functionalized poly(hexylthiophene) and thymine-capped CdSe nanocrystals. J. Phys. Chem. C 2007, 111, 14681–14688. [Google Scholar] [CrossRef]
- De Girolamo, J.; Reiss, P.; Zagorska, M.; Bettignies, R.D.; Bailly, S.; Mevellec, J.-Y.; Lefrant, S.; Travers, J.-P.; Pron, A. Layer-by-layer assembled composite films of side-functionalized poly(3-hexylthiophene) and CdSe nanocrystals: Electrochemical, spectroelectrochemical and photovoltaic properties. Phys. Chem. Chem. Phys. 2008, 10, 4027–4035. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Word, R.C.; Nadarajah, A.; Konenkamp, R. Unipolar transport and interface charge transfer in nanostructured CdTe/polymer hybrid films. Phys. Rev. B: Condens. Matter 2008, 77, 195314–195316. [Google Scholar] [CrossRef]
- Guo, Z.; Park, S.; Hahn, H.T.; Wei, S.; Moldovan, M.; Karki, A.B.; Young, D.P. Giant magnetoresistance behavior of an iron/carbonized polyurethane nanocomposite. Appl. Phys. Lett. 2007, 90, 053111. [Google Scholar] [CrossRef]
- Mallouki, M.; Tran-Van, F.; Sarrazin, C.; Simon, P.; Daffos, B.; De, A.; Chevrot, C.; Fauvarque, J. Polypyrrole-Fe2O3 nanohybrid materials for electrochemical storage. J. Solid State Electrochem. 2007, 11, 398–406. [Google Scholar] [CrossRef] [Green Version]
- Dallas, P.; Georgakilas, V.; Niarchos, D.; Komninou, P.; Kehagias, T.; Petridis, D. Synthesis, characterization and thermal properties of polymer/magnetite nanocomposites. Nanotechnology 2006, 2046. [Google Scholar]
- Guo, Z.; Lin, H.; Karki, A.B.; Wei, S.; Young, D.P.; Park, S.; Willis, J.; Hahn, T.H. Facile monomer stabilization approach to fabricate iron/vinyl ester resin nanocomposites. Compos. Sci. Technol. 2008, 68, 2551–2556. [Google Scholar] [CrossRef]
- Liu, Y.; Mills, E.N.; Composto, R.J. Tuning optical properties of gold nanorods in polymer films through thermal reshaping. J. Mater. Chem. 2009, 19, 2704–2709. [Google Scholar] [CrossRef]
- Vitale, F.; Mirenghi, L.; Piscopiello, E.; Pellegrini, G.; Trave, E.; Mattei, G.; Fratoddi, I.; Russo, M.V.; Tapfer, L.; Mazzoldi, P. Gold nanoclusters-organometallic polymer nanocomposites: Synthesis and characterization. Mater. Sci. Eng. C-Biomimetic Supramol. Syst. 2007, 27, 1300–1304. [Google Scholar] [CrossRef]
- Namboothiry, M.A.G.; Zimmerman, T.; Coldren, F.M.; Liu, J.; Kim, K.; Carroll, D.L. Electrochromic properties of conducting polymer metal nanoparticles composites. Synth. Met. 2007, 157, 580–584. [Google Scholar] [CrossRef]
- Mukherjee, P.; Nandi, A.K. Electronic properties of poly(o-methoxy aniline)-silver nanocomposite thin films: Influence of nanoparticle size and density. J. Mater. Chem. 2009, 19, 781–786. [Google Scholar]
- Vodnik, V.; Vuković, J.; Nedeljković, J. Synthesis and characterization of silver—poly(methylmethacrylate) nanocomposites. Colloid Polym. Sci. 2009, 287, 847–851. [Google Scholar] [CrossRef]
- del Campo, A.; Arzt, E. Fabrication approaches for generating complex micro- and nanopatterns on polymeric surfaces. Chem. Rev. 2008, 108, 911–945. [Google Scholar] [CrossRef] [PubMed]
- Vaia, R.A.; Maguire, J.F. Polymer nanocomposites with prescribed morphology: Going beyond nanoparticle-filled polymers. Chem. Mater. 2007, 19, 2736–2751. [Google Scholar] [CrossRef]
- Li, F.; Zhou, S.; You, B.; Wu, L. Kinetic study on the UV-induced photopolymerization of epoxy acrylate/TiO2 nanocomposites by FTIR spectroscopy. J. Appl. Polym. Sci. 2006, 99, 3281–3287. [Google Scholar] [CrossRef]
- Sangermano, M.; Priola, A.; Kortaberria, G.; Jimeno, A.; Garcia, I.; Mondragon, I.; Rizza, G. Photopolymerization of epoxy coatings containing iron-oxide nanoparticles. Macromol. Mater. Eng. 2007, 292, 956–961. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Dutoit, B.M.; Besse, P.A.; Blanchard, H.; Guérin, L.; Popovic, R.S. High performance micromachined Sm2Co17 polymer bonded magnets. Sens. Actuators, A 1999, 77, 178–182. [Google Scholar] [CrossRef]
- Cho, J.-D.; Ju, H.-T.; Park, Y.-S.; Hong, J.-W. Kinetics of cationic photopolymerizations of UV-curable epoxy-based su8-negative photoresists with and without silica nanoparticles. Macromol. Mater. Eng. 2006, 291, 1155–1163. [Google Scholar] [CrossRef]
- Jiguet, S.; Bertsch, A.; Hofmann, H.; Renaud, P. Conductive SU8 photoresist for microfabrication. Adv. Funct. Mater. 2005, 15, 1511–1516. [Google Scholar] [CrossRef]
- Lillemose, M.; Gammelgaard, L.; Richter, J.; Thomsen, E.V.; Boisen, A. Epoxy based photoresist/carbon nanoparticle composites. Compos. Sci. Technol. 2008, 68, 1831–1836. [Google Scholar] [CrossRef]
- Damean, N.; Parviz, B.A.; Lee, J.N.; Odom, T.; Whitesides, G.M. Composite ferromagnetic photoresist for the fabrication of microelectromechanical systems. J. Micromech. Microeng. 2005, 29. [Google Scholar]
- Jianwen, X.; Wong, C.P. High dielectric constant SU8 composite photoresist for embedded capacitors. J. Appl. Polym. Sci. 2007, 103, 1523–1528. [Google Scholar] [CrossRef]
- Dawan, F.; Jin, Y.; Goettert, J.; Ibekwe, S. High functionality of a polymer nanocomposite material for MEMS applications. Microsyst. Technol. 2008, 14, 1451–1459. [Google Scholar] [CrossRef]
- Schmidt, G.; Malwitz, M.M. Properties of polymer-nanoparticle composites. Curr. Opin. Colloid Interface Sci. 2003, 8, 103–108. [Google Scholar] [CrossRef]
- Voigt, A.; Heinrich, M.; Martin, C.; Llobera, A.; Gruetzner, G.; Pérez-Murano, F. Improved properties of epoxy nanocomposites for specific applications in the field of MEMS/NEMS. Microelectron. Eng. 84, 1075–1079.
- Convertino, A.; Leo, G.; Tamborra, M.; Sciancalepore, C.; Striccoli, M.; Curri, M.L.; Agostiano, A. TiO2 colloidal nanocrystals functionalization of PMMA: A tailoring of optical properties and chemical adsorption. Sens. Actuator B-Chem. 2007, 126, 138–143. [Google Scholar] [CrossRef]
- Qualtieri, A.; Martiradonna, L.; Cingolani, R.; De Vittorio, M. Colloidal nanocrystals air bridge fabricated by direct lithography. Microelectron. Eng. 2007, 84, 1488–1490. [Google Scholar] [CrossRef]
- Martiradonna, L.; Carbone, L.; Tandaechanurat, A.; Kitamura, M.; Iwamoto, S.; Manna, L.; De Vittorio, M.; Cingolani, R.; Arakawa, Y. Two-dimensional photonic crystal resist membrane nanocavity embedding colloidal dot-in-a-rod nanocrystals. Nano Lett. 2007, 8, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Martiradonna, L.; Qualtieri, A.; Stomeo, T.; Carbone, L.; Cingolani, R.; De Vittorio, M. Lithographic nano-patterning of colloidal nanocrystal emitters for the fabrication of waveguide photonic devices. Sens. Actuators, B-Chem. 2007, 126, 116–119. [Google Scholar] [CrossRef]
- Pompa, P.P.; Martiradonna, L.; Torre, A.D.; Carbone, L.; del Mercato, L.L.; Manna, L.; De Vittorio, M.; Calabi, F.; Cingolani, R.; Rinaldi, R. Fluorescence enhancement in colloidal semiconductor nanocrystals by metallic nanopatterns. Sens. Actuators, B 2007, 126, 187–192. [Google Scholar] [CrossRef]
- Qualtieri, A.; Martiradonna, L.; Stomeo, T.; Todaro, M.T.; Cingolani, R.; Vittorio, M.D. Multicolored devices fabricated by direct lithography of colloidal nanocrystals. Microelectron. Eng. 2009, 86, 1127–1130. [Google Scholar] [CrossRef]
- Bhuvana, T.; Subramaniam, C.; Pradeep, T.; Kulkarni, G.U. Conducting nanocrystal patterns using a silver organic complex blended with polystyrene as e-beam resist. J. Phys. Chem. C 2009, 113, 7038–7043. [Google Scholar] [CrossRef]
- Bates, F.S.; Fredrickson, G.H. Block copolymers-designer soft materials. Phys. Today 1999, 52, 32–38. [Google Scholar] [CrossRef]
- Hawker, C.J.; Russell, T.P. Block copolymer lithography: Merging "bottom-up" with "top-down" processes. MRS Bull. 2005, 30, 952–966. [Google Scholar] [CrossRef]
- Cheng, J.Y.; Ross, C.A.; Smith, H.I.; Thomas, E.L. Templated self-assembly of block copolymers: Top-down helps bottom-up. Adv. Mater. 2006, 18, 2505–2521. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Wang, B.; Li, C.Y. Alternating patterns on single-walled carbon nanotubes. Nat Nano 2009, 4, 358–362. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, W.J.; Kim, S.O. Highly efficient vertical growth of wall-number-selected, N-doped carbon nanotube arrays. Nano Lett. 2009, 9, 1427–1432. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, C.; Gindy, N.; Gutmann, J.S.; Frömsdorf, A.; Förster, S.; Fahmi, A. In situ synthesis and alignment of au nanoparticles within hexagonally packed cylindrical domains of diblock copolymers in bulk. Langmuir 2009, 25, 9571–9578. [Google Scholar] [CrossRef] [PubMed]
- Morin, S.A.; La, Y.-H.; Liu, C.-C.; Streifer, J.A.; Hamers, R.J.; Nealey, P.F.; Jin, S. Assembly of nanocrystal arrays by block-copolymer-directed nucleation13. Angew Chem. Int. Ed. 2009, 48, 2135–2139. [Google Scholar] [CrossRef]
- Bockstaller, M.; Kolb, R.; Thomas, E.L. Metallodielectric photonic crystals based on diblock copolymers. Adv. Mater. 2001, 13, 1783–1786. [Google Scholar] [CrossRef]
- Niu, S.; Saraf, R.F. Selective assembly of nanoparticles on block copolymer by surface modification. Nanotechnology 2007, 18, 125607. [Google Scholar] [CrossRef]
- Cumpston, B.H.; Ananthavel, S.P.; Barlow, S.; Dyer, D.L.; Ehrlich, J.E.; Erskine, L.L.; Heikal, A.A.; Kuebler, S.M.; Lee, I.Y.S.; McCord-Maughon, D.; Qin, J.Q.; Rockel, H.; Rumi, M.; Wu, X.L.; Marder, S.R.; Perry, J.W. Two-photon polymerization initiators for three-dimensional optical data storage and microfabrication. Nature 1999, 398, 51–54. [Google Scholar] [CrossRef]
- Das, R.N.; Egitto, F.D.; Lauffer, J.M.; Markovich, V.R. Laser micromachining of barium titanate (BaTiO3)-epoxy nanocomposite-based flexible/rollable capacitors: New approach for making library of capacitors. IEEE Trans. Electron. Packag. Manuf. 2008, 31, 97–103. [Google Scholar] [CrossRef]
- Stellacci, F.; Bauer, C.A.; Meyer-Friedrichsen, T.; Wenseleers, W.; Alain, V.; Kuebler, S.M.; Pond, S.J.K.; Zhang, Y.; Marder, S.R.; Perry, J.W. Laser and electron-beam induced growth of nanoparticles for 2D and 3D metal patterning. Adv. Mater. 2002, 14, 194–198. [Google Scholar] [CrossRef]
- Sirringhaus, H.; Kawase, T.; Friend, R.H.; Shimoda, T.; Inbasekaran, M.; Wu, W.; Woo, E.P. High-resolution inkjet printing of all-polymer transistor circuits. Science 2000, 290, 2123–2126. [Google Scholar] [CrossRef] [PubMed]
- De Gans, B.J.; Duineveld, P.C.; Schubert, U.S. Inkjet printing of polymers: State of the art and future developments. Adv. Mater. 2004, 16, 203–213. [Google Scholar] [CrossRef]
- Leveder, T.; Landis, S.; Davoust, L. Reflow dynamics of thin patterned viscous films. Appl. Phys. Lett. 2008, 92, 013107. [Google Scholar] [CrossRef]
- Tekin, E.; Smith, P.J.; Hoeppener, S.; van den Berg, A.M.J.; Susha, A.S.; Rogach, A.L.; Feldmann, J.; Schubert, U.S. Inkjet printing of luminescent CdTe nanocrystal-polymer composites. Adv. Funct. Mater. 2007, 17, 23–28. [Google Scholar] [CrossRef]
- Ko, S.H.; Pan, H.; Grigoropoulos, C.P.; Luscombe, C.K.; Frechet, J.M.J.; Poulikakos, D. All-inkjet-printed flexible electronics fabrication on a polymer substrate by low-temperature high-resolution selective laser sintering of metal nanoparticles. Nanotechnology 2007, 18, 345202. [Google Scholar] [CrossRef]
- Loffredo, F.; Mauro, A.D.G.D.; Burrasca, G.; La Ferrara, V.; Quercia, L.; Massera, E.; Di Francia, G.; Sala, D.D. Ink-jet printing technique in polymer/carbon black sensing device fabrication. Sens. Actuators, B 2009, 143, 421–429. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ingrosso, C.; Fakhfouri, V.; Striccoli, M.; Agostiano, A.; Curri, M.L.; Brugger, J. Inkjet-printed multicolor arrays of highly luminescent nanocrystal-based nanocomposites. Small 2009, 5, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Lee, S.-W.; Jeong, J.-H.; Choi, D.-G.; Lee, J.-H.; Lee, E.-S. Direct imprinted conductive patterns using nanosilver colloid-applied UV curable resist. Jpn. J. Appl. Phys. 2009, 48, 06FH02. [Google Scholar]
- Katayama, J.; Yamaki, S.; Mitsuyama, M.; Hanabata, M. Organic-inorganic hybrid materials for UV-Nanoimprint lithography. J. Photopolym. Sci. Technol. 2006, 19, 397–402. [Google Scholar] [CrossRef]
- Reboud, V.; Kehagias, N.; Sotomayor Torres, C.M.; Zelsmann, M.; Striccoli, M.; Curri, M.L.; Agostiano, A.; Tamborra, M.; Fink, M.; Reuther, F.; Gruetzner, G. Spontaneous emission control of colloidal nanocrystals using nanoimprinted photonic crystals. Appl. Phys. Lett. 2007, 90, 011115. [Google Scholar] [CrossRef]
- Reboud, V.; Kehagias, N.; Zelsmann, M.; Striccoli, M.; Tamborra, M.; Curri, M.L.; Agostiano, A.; Mecerreyes, D.; Alduncin, J.A.; Sotomayor Torres, C.M. Nanoimprinted photonic crystals for the modification of the (CdSe)ZnS nanocrystals light emission. Microelectron. Eng. 2007, 84, 1574–1577. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ingrosso, C.; Panniello, A.; Comparelli, R.; Curri, M.L.; Striccoli, M. Colloidal Inorganic Nanocrystal Based Nanocomposites: Functional Materials for Micro and Nanofabrication. Materials 2010, 3, 1316-1352. https://doi.org/10.3390/ma3021316
Ingrosso C, Panniello A, Comparelli R, Curri ML, Striccoli M. Colloidal Inorganic Nanocrystal Based Nanocomposites: Functional Materials for Micro and Nanofabrication. Materials. 2010; 3(2):1316-1352. https://doi.org/10.3390/ma3021316
Chicago/Turabian StyleIngrosso, Chiara, AnnaMaria Panniello, Roberto Comparelli, Maria Lucia Curri, and Marinella Striccoli. 2010. "Colloidal Inorganic Nanocrystal Based Nanocomposites: Functional Materials for Micro and Nanofabrication" Materials 3, no. 2: 1316-1352. https://doi.org/10.3390/ma3021316
APA StyleIngrosso, C., Panniello, A., Comparelli, R., Curri, M. L., & Striccoli, M. (2010). Colloidal Inorganic Nanocrystal Based Nanocomposites: Functional Materials for Micro and Nanofabrication. Materials, 3(2), 1316-1352. https://doi.org/10.3390/ma3021316