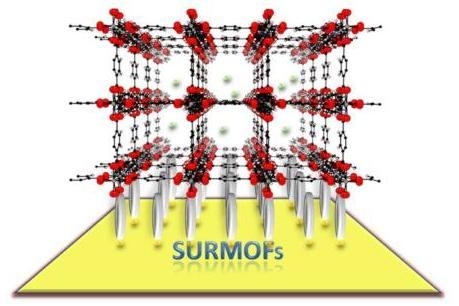
Layer-by-Layer Method for the Synthesis and Growth of Surface Mounted Metal-Organic Frameworks (SURMOFs)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and growth of [Cu3(btc)2.n(H2O)] (HKUST-1) SURMOF




2.2. Synthesis and growth of layer based MOFs (LBMOFs)







3. Experimental
3.1. SURMOFs preparation

3.2. SURMOFs characterization
4. Conclusion
| Inorganic coupling unit | Organic ligand | MOF | SAM termination |
| Cu(Ac)2 | H3btc | Cu3(btc)2. xH2O | COOH, OH |
| Cu(Ac)2 | H2bdc | Cux(bdc)y | COOH |
| Cu(Ac)2 | H2bdc+dabco | Cu2(bdc)2(dabco) | COOH, Pyridine |
| Cu(Ac)2 | H2ndc+dabco | Cu2(ndc)2(dabco) | COOH, Pyridine |
| Zn(Ac)2 | H2bdc | Znx(bdc)y | COOH |
| Zn(Ac)2 | H2ndc+dabco | Zn2(ndc)2(dabco) | COOH, Pyridine |
| Zn(Ac)2 | H2bdc+4,4′-Bipy | Zn(bdc)(4,4′-Bipy)0.5 | Pyridine |
Acknowledgment
References
- Ferey, G. Microporous solids: From organically templated inorganic skeletons to hybrid frameworks ecumenism in chemistry. Chem. Mater. 2001, 13, 3084–3098. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Rowsell, J.; Yaghi, O. Metal organic frameworks, a new class of porous materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Maji, T.; Uemura, K.; Chang, H.; Matsuda, R; Kitagawa, S. Expanding and shrinking porous modulation based on pillared-layer coordination polymers showing selective guest adsorption. Angew Chem. Int. Ed. 2004, 43, 3269–3272. [Google Scholar] [CrossRef]
- Hoskins, B.F; Robson, R. Design and construction of a new class of scaffolding-like materials comprising infinite polymeric frameworks of 3-D-linked molecular rods—a reappraisal of the Zn(CN)2 and Cd(CN)2 structures and the synthesis and structure of the diamond-related frameworks [N(CH3)4][CuiZnii(CN)4] and Cui[4,4',4'',4'''-tetracyanotetraphenylmethane]-BF4.xC6H5NO2. J. Am. Chem. Soc. 1990, 112, 1546–1554. [Google Scholar] [CrossRef]
- Zhao, X.S. Novel porous materials for emerging applications. J. Mater. Chem. 2006, 16, 623–625. [Google Scholar] [CrossRef]
- Mueller, U.; Schubert, M.; Teich, F.; Puetter, H.; Schierle-Arndt, K; Pastre, J. Metal-organic frameworks—prospective industrial applications. J. Mater. Chem. 2006, 16, 626–636. [Google Scholar] [CrossRef]
- Seo, J.; Whang, D.; Lee, H.; Jun, S.; Oh, J.; Jeon, Y.; Kim, K. A homochiral metal-organic porous material for enantioselective separation and catalysis. Nature 2000, 404, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Snurr, R.; Hupp, J.; Nguyen, S. Prospects for nanoporous metal-organic materials in advanced separations processes. AlChE J. 2004, 50, 1090–1095. [Google Scholar] [CrossRef]
- Hermes, S.; Schroder, F.; Amirjalayer, S.; Schmid, R.; Fischer, R.A. Loading of porous metal-organic open frameworks with organometallic CVD precursors: Inclusion compounds of the type [LnM](a)@MOF-5. J. Mater. Chem. 2006, 16, 2464–2472. [Google Scholar] [CrossRef]
- Hermes, S.; Schröder, F.; Schmid, R.; Khodeir, L.; Muhler, M.; Tissler, A.; Fischer, R.M; Fischer, R.A. Metal@MOF: Loading of highly porous coordination polymers host lattices by metal organic chemical vapor deposition. Angew Chem. Int. Ed. 2005, 44, 6237–6241. [Google Scholar] [CrossRef]
- Bauer, S.; Stock, N. MOFs—Metallorganische Gerüststrukturen. Chem. Unserer Zei 2008, 42, 12–19. [Google Scholar] [CrossRef]
- Zacher, D.; Shekhah, O.; Wöll, C.; Fischer, R.A. Thin films of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1418–1429. [Google Scholar] [CrossRef]
- Allendorf, M.; Houk, R.; Andruszkiewicz, L.; Talin, A.; Pikarsky, J.; Choudhury, A.; Gall, K.A; Hesketh, P. Stress–induced chemical detection using flexible metal-organic frameworks. J. Am. Chem. Soc. 2008, 130, 14404–14405. [Google Scholar] [CrossRef]
- Hermes, S.; Schröder, F.; Chelmowski, R.; Wöll, C.; Fischer, R.A. Selective nucleation and growth of metal-organic open framework thin films on patterned COOH/CF3-terminated self-assembled monolayers on Au(111). J. Am. Chem. Soc. 2005, 127, 13744–13745. [Google Scholar] [CrossRef] [PubMed]
- Biemmi, E.; Scherb, C.; Bein, T. Oriented growth of the metal organic framework Cu3(BTC)2(H2O)3·xH2O tunable with functionalized self-assembled monolayers. J. Am. Chem. Soc. 2007, 129, 8054–8055. [Google Scholar] [CrossRef] [PubMed]
- Shekhah, O.; Wang, H.; Kowarik, S.; Schreiber, F.; Paulus, M.; Tolan, M.; Sternemann, C.; Evers, F.; Zacher, D.; Fischer, R.A; Wöll, C. Step-by-step route for the synthesis of metal-organic frameworks. J. Am. Chem. Soc. 2007, 129, 15118–15119. [Google Scholar] [CrossRef]
- Allara, D.; Nuzzo, R. Spontaneously organized molecular assemblies. 1. Formation, dynamics, and physical-properties of normal-alkanoic acids adsorbed from solution on an oxidized aluminum surface. Langmuir 1985, 1, 45–52. [Google Scholar] [CrossRef]
- Love, J.; Estroff, L.; Kriebel, J.; Nuzzo, R.; Whitesides, G. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005, 105, 1103–1169. [Google Scholar] [CrossRef] [PubMed]
- Chui, S.; Lo, S.; Charmant, J.; Orpen, A.; Williams, I. A chemically functionalizable nanoporous material [Cu-3(TMA)(2)(H2O)(3)](n). Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Shekhah, O.; Wang, H.; Zacher, D.; Fischer, R.A.; Wöll, C. Growth mechanism of metal-organic frameworks: Insights into the nucleation by employing a step-by-step route. Angew Chem. Int. Ed. 2009, 48, 5038–5041. [Google Scholar] [CrossRef]
- Hermes, S.; Zacher, D.; Baunemann, A.; Woll, C.; Fischer, R.A. Selective growth and MOCVD loading of small single crystals of MOF-5 at alumina and silica surfaces modified with organic self-assembled monolayers. Chem. Mater. 2007, 19, 2168–2173. [Google Scholar] [CrossRef]
- Evans, S.; Ulman, A.; Goppertberarducci, K.; Gerenser, L. Self-assembled multilayers of omega-mercaptoalkanoic acids - selective ionic interactions. J. Am. Chem. Soc. 1991, 113, 5866–5868. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Shekhah, O.; Wang, H.; Strunskus, T.; Cyganik, P.; Zacher, D.; Fischer, R; Wöll, C. Layer-by-layer growth of oriented metal organic polymers on a functionalized organic surface. Langmuir 2007, 23, 7440–7442. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G. Soft lithography. Annu. Rev. Mater. Sci. 1998, 28, 84–153. [Google Scholar] [CrossRef]
- Zacher, D.; Baunemann, A.; Hermes, S.; Fischer, R.A. Deposition of microcrystalline [Cu-3(btc)(2)] and [Zn-2(bdc)(2)(dabco)] at alumina and silica surfaces modified with patterned self assembled organic monolayers: Evidence of surface selective and oriented growth. J. Mater. Chem. 2007, 17, 2785–2792. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Moler, D.; Li, H.; Chen, B.; Reinke, T.; O'Keeffe, M.; Yaghi, O. Modular chemistry: Secondary building units as a basis for the design of highly porous and roboust metal-organic carboxylate frameworks. Acc. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Seki, K.; Takamizawa, S; Mori, W. Design and gas adsorption property of a three-dimensional coordination polymer with a stable and highly porous framwork. Chem. Lett. 2001, 332–333. [Google Scholar] [CrossRef]
- Seki, K.; Takamizawa, S; Mori, W. Characterization of microporous copper(II) dicarboxylates (fumarate, terephthalate, and trans-1,4-cyclohexanedicarboxylate) by gas adsorption. Chem. Lett. 2001, 122–123. [Google Scholar] [CrossRef]
- Dybtsev, D.; Chun, H.; Kim, K. Rigid and flexible: A highly porous metal-organic framework with unusual guest-dependent dynamic behavior. Angew Chem Int. Ed. 2004, 43, 5033–5036. [Google Scholar] [CrossRef]
- Zacher, D.; Baunemann, A.; Hermes, S.; Fischer, R.A. Deposition of microcrystalline [Cu-3(btc)(2)] and [Zn-2(bdc)(2)(dabco)] at alumina and silica surfaces modified with patterned self assembled organic monolayers: Evidence of surface selective and oriented growth. J. Mater. Chem 2007, 17, 2785–2792. [Google Scholar] [CrossRef]
- Chen, B.; Liang, C.; Yang, J.; Contreras, D.; Clancy, Y.; Lobkovsky, E.; Yaghi, O; Dai, S. A microporous metal-organic framework for gas-chromatographic separation of alkanes. Angew Chem. Int. Ed. 2006, 45, 1390–1393. [Google Scholar] [CrossRef]
- Shekhah, O.; Wang, H.; Paradinas, M.; Ocal, C.; Schupbach, B.; Terfort, A.; Zacher, D.; Fischer, R.A; Wöll, C. Controlling interpenetration in metal-organic frameworks by liquid-phase epitaxy. Nat. Mater. 2009, 8, 481–484. [Google Scholar] [CrossRef]
- Kind, M.; Wöll, C. Organic surfaces exposed by self-assembled organothiol monolayers: Preparation, characterization, and application. Prog. Surf. Sci. 2009, 84, 230–278. [Google Scholar] [CrossRef]
- Shekhah, O.; Roques, N.; Mugnaini, V.; Munuera, C.; Ocal, C.; Veciana, J; Wöll, C. Grafting of monocarboxylic substituted polychlorotriphenylmethyl radicals onto a COOH-functionalized self-assembled monolayer through copper (II) metal ions. Langmuir 2008, 24, 6640–6648. [Google Scholar] [CrossRef]
- Munuera, C.; Shekhah, O.; Wang, H.; Wöll, C.; Ocal, C. The controlled growth of oriented metal organic frameworks on functionalized surfaces as followed by scanning force microscopy. Phys. Chem. Chem. Phys. 2008, 10, 7257–7261. [Google Scholar] [CrossRef] [PubMed]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shekhah, O. Layer-by-Layer Method for the Synthesis and Growth of Surface Mounted Metal-Organic Frameworks (SURMOFs). Materials 2010, 3, 1302-1315. https://doi.org/10.3390/ma3021302
Shekhah O. Layer-by-Layer Method for the Synthesis and Growth of Surface Mounted Metal-Organic Frameworks (SURMOFs). Materials. 2010; 3(2):1302-1315. https://doi.org/10.3390/ma3021302
Chicago/Turabian StyleShekhah, Osama. 2010. "Layer-by-Layer Method for the Synthesis and Growth of Surface Mounted Metal-Organic Frameworks (SURMOFs)" Materials 3, no. 2: 1302-1315. https://doi.org/10.3390/ma3021302
APA StyleShekhah, O. (2010). Layer-by-Layer Method for the Synthesis and Growth of Surface Mounted Metal-Organic Frameworks (SURMOFs). Materials, 3(2), 1302-1315. https://doi.org/10.3390/ma3021302