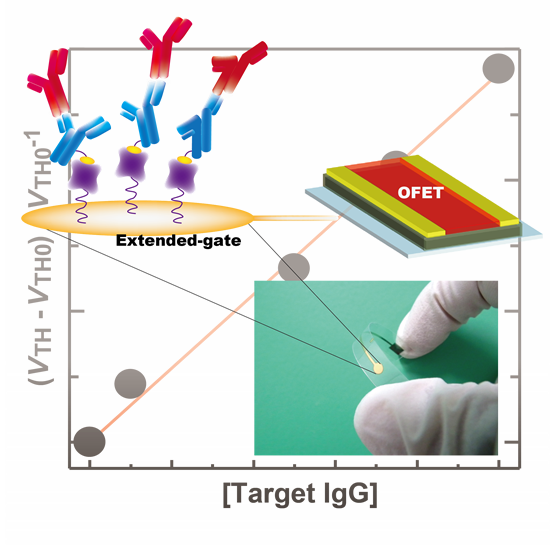
A Label-Free Immunosensor for IgG Based on an Extended-Gate Type Organic Field Effect Transistor
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design of the OFET Immunosensor Device and Functionalization of the Extended Gate


2.2. Immobilization of Anti-IgG Antibody

2.3. Label-Free Immunoassay for IgG

3. Experimental Section
3.1. Materials and Equipment
3.2. Fabrication of the Device
3.3. Estimation of LOD and LOQ
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Patterson, C.; Chambers, L.W. Preventive health care. Lancet 1995, 345, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.F. Immunoassay. Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Hage, D.S. Immunoassays. Anal. Chem. 1999, 71, 294R–304R. [Google Scholar] [CrossRef] [PubMed]
- Crosson, C.; Rossi, C. Quartz crystal microbalance immunosensor for the quantification of immunoglobulin G in bovinemilk. Biosens. Bioelectron. 2013, 42, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Shen, F.; Gurgel, P.V.; Rojas, O.J.; Carbonell, R.G. Dynamic and equilibrium performance of sensors based on short peptide ligands for affinity adsorption of human IgG using surface plasmon resonance. Biosens. Bioelectron. 2014, 58, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Hotani, K.; Hideshima, S.; Kuroiwa, S.; Nakanishi, T.; Hashimoto, M.; Mori, Y.; Osaka, T. Field effect transistor biosensor using antigen binding fragment for detecting tumor marker in human serum. Materials 2014, 7, 2490–2500. [Google Scholar] [CrossRef]
- Torsi, L.; Magliulo, M.; Manoli, K.; Palazzo, G. Organic field-effect transistor sensors: A tutorial review. Chem. Soc. Rev. 2013, 42, 8612–8628. [Google Scholar] [CrossRef] [PubMed]
- Minamiki, T.; Minami, T.; Kurita, R.; Niwa, O.; Wakida, S.; Fukuda, K.; Kumaki, D.; Tokito, S. Accurate and reproducible detection of proteins in water using an extended-gate type organic transistor biosensor. Appl. Phys. Lett. 2014, 104. [Google Scholar] [CrossRef]
- Bergveld, P. Thirty years of ISFETOLOGY what happened in the past 30 years and what may happen in the next 30 years. Sens. Actuators B 2003, 88, 1–20. [Google Scholar] [CrossRef]
- Kim, D.-S.; Park, J.-E.; Shin, J.-K.; Kim, P.K.; Lim, G.; Shoji, S. An extended gate FET-based biosensor integrated with a Si microfluidic channel for detection of protein complexes. Sens. Actuators B 2006, 117, 488–494. [Google Scholar] [CrossRef]
- Bian, C.; Tong, J.; Sun, J.; Zhang, H.; Xue, Q.; Xia, S. A field effect transistor (FET)-based immunosensor for detection of HbA1c and Hb. Biomed. Microdevices 2011, 13, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Kay, R.A.; Wood, K.J.; Bernstein, R.M.; Holt, P.J.L.; Pumphrey, R.S.H. An IgG subclass imbalance in connective tissue disease. Ann. Rheum. Dis. 1988, 47, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Kumaki, D.; Umeda, T.; Tokito, S. Influence of H2O and O2 on threshold voltage shift in organic thin-film transistors: deprotonation of SiOH on SiO2 gate-insulator surface. Appl. Phys. Lett. 2008, 92. [Google Scholar] [CrossRef]
- Chapman-Smith, A.; Cronan, J.E., Jr. Molecular biology of biotin attachment to proteins. J. Nutr. 1999, 129, 477S–484S. [Google Scholar]
- De Boer, B.; Hadipour, A.; Mandoc, M.M.; van Woudenbergh, T.; Blom, P.W.M. Tuning of metal work functions with self-assembled monolayers. Adv. Mater. 2005, 17, 621–625. [Google Scholar] [CrossRef]
- Chen, C.P.; Ganguly, A.; Lu, C.Y.; Chen, T.Y.; Kuo, C.C.; Chen, R.S.; Tu, W.H.; Fischer, W.B.; Chen, K.H.; Chen, L.C. Ultrasensitive in situ label-free DNA detection using a GaN nanowire-based extended-gate field-effect-transistor sensor. Anal. Chem. 2011, 83, 1938–1943. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.G.R.; Vieira, N.C.S.; Queiroz, A.A.A.D.; Guimarães, F.E.G.; Zucolotto, V. Immobilization of poly(propylene imine) dendrimer/nickel phtalocyanine as nanostructured multilayer films to be used as gate membranes for SEGFET pH sensors. J. Phys. Chem. C 2010, 114, 6478–6483. [Google Scholar]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson: Harlow, UK, 2010. [Google Scholar]
- Khan, H.U.; Jang, J.; Kim, J.-J.; Knoll, W. Effect of passivation on the sensitivity and stability of pentacene transistor sensors in aqueous media. Biosens. Bioelectron. 2011, 26, 4217–4221. [Google Scholar] [CrossRef] [PubMed]
- Sergeyeva, T.A.; Lavrik, N.V.; Piletsky, S.A.; Rachkov, A.E.; El’skaya, A.V. Polyaniline label-based conductometric sensor for IgG detection. Sens. Actuators B 1996, 34, 283–288. [Google Scholar] [CrossRef]
- Darain, F.; Park, S.-U.; Shim, Y.-B. Disposable amperometric immunosensor system for rabbit IgG using a conducting polymer modified screen-printed electrode. Biosens. Bioelectron. 2003, 18, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Mcculloch, I.; Heeney, M.; Bailey, C.; Genevicius, K.; Macdonald, I.; Shkunov, M.; Sparrowe, D.; Tierney, S.; Wagner, R.; Zhang, W.; et al. Liquid-crystalline semiconducting polymers with high charge-carrier mobility. Nat. Mater. 2006, 5, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Umeda, T.; Kumaki, D.; Tokito, S. Surface-energy-dependent field-effect mobilities up to 1 cm2/Vs for polymer thin-film transistor. J. Appl. Phys. 2009, 105. [Google Scholar] [CrossRef]
- Klauk, H.; Zschieschang, U.; Pflaum, J.; Halik, M. Ultralow-power organic complementary circuits. Nature 2007, 445, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Hamamoto, T.; Yokota, T.; Sekitani, T.; Zschieschang, U.; Klauk, H.; Someya, T. Effects of the alkyl chain length in phosphonic acid self-assembled monolayer gate dielectrics on the performance and stability of low-voltage organic thin-film transistors. Appl. Phys. Lett. 2009, 95. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Minamiki, T.; Minami, T.; Kurita, R.; Niwa, O.; Wakida, S.-i.; Fukuda, K.; Kumaki, D.; Tokito, S. A Label-Free Immunosensor for IgG Based on an Extended-Gate Type Organic Field Effect Transistor. Materials 2014, 7, 6843-6852. https://doi.org/10.3390/ma7096843
Minamiki T, Minami T, Kurita R, Niwa O, Wakida S-i, Fukuda K, Kumaki D, Tokito S. A Label-Free Immunosensor for IgG Based on an Extended-Gate Type Organic Field Effect Transistor. Materials. 2014; 7(9):6843-6852. https://doi.org/10.3390/ma7096843
Chicago/Turabian StyleMinamiki, Tsukuru, Tsuyoshi Minami, Ryoji Kurita, Osamu Niwa, Shin-ichi Wakida, Kenjiro Fukuda, Daisuke Kumaki, and Shizuo Tokito. 2014. "A Label-Free Immunosensor for IgG Based on an Extended-Gate Type Organic Field Effect Transistor" Materials 7, no. 9: 6843-6852. https://doi.org/10.3390/ma7096843
APA StyleMinamiki, T., Minami, T., Kurita, R., Niwa, O., Wakida, S. -i., Fukuda, K., Kumaki, D., & Tokito, S. (2014). A Label-Free Immunosensor for IgG Based on an Extended-Gate Type Organic Field Effect Transistor. Materials, 7(9), 6843-6852. https://doi.org/10.3390/ma7096843