Thickness Influence on In Vitro Biocompatibility of Titanium Nitride Thin Films Synthesized by Pulsed Laser Deposition
Abstract
:1. Introduction
2. Experimental Section
2.1. PLD Experiment
2.2. Physical-Chemical Characterization of Deposited Structures
2.2.1. Biological Assays
2.2.1.1. Cell Cultures
2.2.1.2. Cell Morphology
2.2.1.3. Proliferation Assay
2.2.1.4. Cell Toxicity Assay
2.2.2. Profilometry
2.2.3. Scanning Electron Microscopy
2.2.4. Atomic Force Microscopy
2.2.5. X-ray Photoelectron Spectroscopy
2.2.6. X-ray Diffraction
2.2.7. Wetting
2.2.8. Pull-Out
2.2.9. Statistical Analysis
3. Results
3.1. Thickness and Bonding Strength of TiN Films Deposited on 410SS Substrates

| Sample Type | Film Thickness (nm) |
|---|---|
| 5A film | 60.6 ± 11.7 |
| 10B film | 86.6 ± 13.8 |
| 20C film | 133.1 ± 18.2 |
| Sample Type | Bonding Strength (MPa) |
|---|---|
| Control (bare 410SS) | ~65 |
| 5A film | 55.7 ± 3.9 |
| 10B film | 57.5 ± 7.5 |
| 20C film | 55.0 ± 7.1 |
3.2. Biological Assessment of TiN Films
3.2.1. Cell Morphology

3.2.2. Cell Proliferation

3.2.3. Cell Death

3.3. Physical-Chemical Investigations of TiN Films
3.3.1. Morphological Examination of TiN Films



| Sample Type | RRMS (nm) | Ra (nm) |
|---|---|---|
| Reference (bare glass substrate) | 1.76 ± 0.02 | 1.33 ± 0.03 |
| 5A film | 0.30 ± 0.01 | 0.23 ± 0.01 |
| 10B film | 0.60 ± 0.03 | 0.44 ± 0.02 |
| 20C film | 0.37 ± 0.02 | 0.29 ± 0.02 |
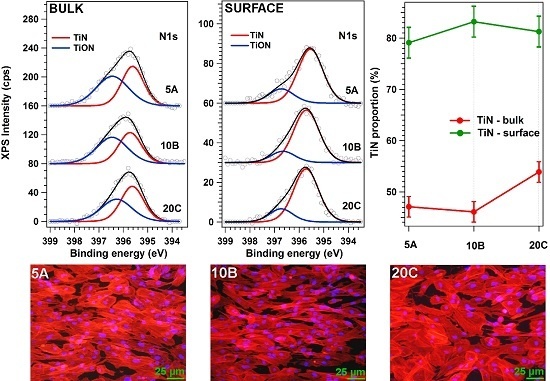
3.4. Compositional Analyses of TiN Films


| Sample Type | Stoichiometry | ||
|---|---|---|---|
| 5A | 10B | 20C | |
| Volume | TiN1.19 | TiN1.27 | TiN1.37 |
| Surface | TiN1.10 | TiN1.39 | TiN1.36 |
3.5. Structural Investigation of TiN Films

3.6. Wetting Behavior of TiN Films

| Sample Type | Contact Angle (°) | Surface Free Energy | |||
|---|---|---|---|---|---|
| Water | Ethylene Glycol | γsd | γsp | γtotal | |
| Bare glass | 23.2 ± 0.4 | 31.4 ± 0.3 | 3.8 | 72.3 | 76.1 |
| Bare 410SS | 51.0 ± 1.9 | 56.0 ± 1.1 | 2.4 | 53.2 | 55.6 |
| 5A film | 88.8 ± 2.12 | 66.4 ± 0.3 | 15.2 | 8.7 | 24.0 |
| 10B film | 93.2 ± 0.07 | 66.3 ± 0.2 | 23.2 | 2.7 | 25.9 |
| 20C film | 88.5 ± 1.27 | 66.7 ± 0.5 | 16.9 | 6.9 | 23.8 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- ISO 10993-5: 2009. Biological evaluation of medical devices—Part 5: Tests for in vitro cytotoxicity; ISO: Geneva, Switzerland, 2009.
- Sovak, G.; Weiss, A.; Gotman, I. Osseointegration of Ti6Al4V alloy implants coated with titanium nitride by a new method. J. Bone Joint Surg. Br. 2000, 82, 290–296. [Google Scholar] [CrossRef]
- Patsalas, P.; Kalfagiannis, N.; Kassavetis, S. Optical properties and plasmonic performance of titanium nitride. Materials 2015, 8, 3128–3154. [Google Scholar] [CrossRef]
- Valvoda, V. Structure of TiN coatings. Surf. Coat. Technol. 1996, 80, 61–65. [Google Scholar] [CrossRef]
- Holmberg, K.; Matthews, A. Coatings-Tribology—Properties, Techniques and Applications in Surface Engineering; Elsevier: Amsterdam, The Netherlands, 1994; pp. 1–442. [Google Scholar]
- Lackner, J.M.; Waldhauser, W.; Berghauser, R.; Ebner, R.; Major, B.; Schoberl, T. Structural, mechanical and tribological investigations of pulsed laser deposited titanium nitride coatings. Thin Solid Films 2004, 453–454, 195–202. [Google Scholar] [CrossRef]
- Ferro, D.; Rau, J.V.; Albertini, V.R.; Generosi, A.; Teghil, R.; Barinov, S.M. Pulsed laser deposited hard TiC, ZrC, HfC and TaC films on titanium: Hardness and an energy-dispersive X-ray diffraction study. Surf. Coat. Technol. 2008, 202, 1455–1461. [Google Scholar] [CrossRef]
- Hassani, S.; Klemberg-Sapieha, J.E.; Bielawski, M.; Beres, W.; Martinu, L.; Balazinski, M. Design of hard coating architecture for the optimization of erosion resistance. Wear 2008, 265, 879–887. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; Lee, J.; Tsotsos, C.; Demas, N.G.; Meschewski, R.L.; Rebholz, C.; Polycarpou, A.A. Deposition and nanotribological characterization of sub-100-nm thick protective Ti-based coatings for miniature applications. Tribol. Lett. 2011, 44, 213–221. [Google Scholar] [CrossRef]
- Kao, C.T.; Ding, S.J.; Chen, Y.C.; Huang, T.H. The anticorrosion ability of titanium nitride (TiN) plating on an orthodontic metal bracket and its biocompatibility. J. Biomed. Mater. Res. 2002, 63, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.H.; Liu, G.T.; Duh, J.G.; Wang, J.H. Biocompatibility of a titanium-aluminum nitride film coating on a dental alloy. Surf. Coat. Technol. 2004, 188–189, 745–749. [Google Scholar] [CrossRef]
- Akbari, A.; Templier, C.; Beaufort, M.F.; Egidi, D.; Riviere, J.P. Ion beam assisted deposition of TiN-Ni nanocomposite coatings. Surf. Coat. Technol. 2011, 206, 972–975. [Google Scholar] [CrossRef]
- Subramanian, B.; Ananthakumar, R.; Jayachandran, M. Structural and tribological properties of DC reactive magnetron sputtered Titanium/Titanium Nitride (Ti/TiN) multilayered coatings. Surf. Coat. Technol. 2011, 205, 3485–3492. [Google Scholar] [CrossRef]
- Lu, F.H.; Chen, H.Y. Characterization of titanium nitride films deposited by cathodic arc plasma technique on copper substrates. Surf. Coat. Technol. 2000, 130, 290–296. [Google Scholar] [CrossRef]
- Mihailescu, I.N.; Chitica, N.; Gyorgy, E.; Teodorescu, V.S.; Marin, G.; Luches, A.; Perrone, A.; Martino, M.; Neamtu, J. A parametric study of the deposition of the TiN thin films by laser reactive ablation of Ti targets in N2: The roles of the total gas pressure and the contaminations with oxides. J. Mater. Sci. 1996, 31, 2909–2915. [Google Scholar] [CrossRef]
- Craciun, V.; Craciun, D.; Boyd, I.W. Reactive pulsed laser deposition of thin TiN films. Mater. Sci. Eng. B 1993, 18, 178–180. [Google Scholar] [CrossRef]
- Bailey, E.; Ray, N.M.T.; Hector, A.L.; Crozier, P.; Petuskey, W.T.; McMillan, P.F. Mechanical properties of pitanium nitride nanocomposites produced by chemical precursor synthesis followed by high-P,T treatment. Materials 2011, 4, 1747–1762. [Google Scholar] [CrossRef]
- Lee, D.K.; Lee, J.J.; Joo, J. Low temperature TiN deposition by ICP assisted chemical vapor deposition. Surf. Coat. Technol. 2003, 174–175, 1234–1237. [Google Scholar] [CrossRef]
- Gammer, K.M.S.; Wagner, J.; Hutter, H.; Kullmer, R.; Mitterer, C. Investigations on the effect of plasma-assisted pre-treatment for plasma-assisted chemical vapour deposition TiN coatings on tool steels. Thin Solid Films 2004, 461, 277–281. [Google Scholar] [CrossRef]
- Park, J.; Kim, D.J.; Kim, Y.K.; Lee, K.H.; Lee, K.H.; Lee, H.; Ahn, S. Improvement of the biocompatibility and mechanical properties of surgical tools with TiN coating by PACVD. Thin Solid Films 2003, 435, 102–107. [Google Scholar] [CrossRef]
- Bry Coat Homepage. Available online: http://www.brycoat.com/ (accessed on 28 October 2015).
- Vadiraj, A.; Kamaraj, M. Fretting fatigue studies of titanium nitride-coated biomedical titanium alloys. J. Mater. Eng. Perform. 2006, 15, 553–557. [Google Scholar] [CrossRef]
- Starosvetsky, D.; Gotman, I. Corrosion behavior of titanium nitride coated Ni-Ti shape memory surgical alloy. Biomaterials 2001, 22, 1853–1859. [Google Scholar] [CrossRef]
- Blackwood, D.J. Corrosion in body fluids. In Shreir’s Corrosion, 4th ed.; Richardson, T.J.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 2, pp. 1308–1322. [Google Scholar]
- Bártolo, P.J.; Bidanda, B. Bio-Materials and Prototyping Applications in Medicine, 1st ed.; Springer Science & Business Media, Technology & Engineering: New York, NY, USA, 2007; pp. 1–216. [Google Scholar]
- Pini, S.; Groppetti, R.; Mucchino, C.; Geretto, V. Evaluation of DLC, WC/C, and TiN coatings on martensitic stainless steel and yttria-stabilized tetragonal zirconia polycrystal substrates for reusable surgical scalpels. ISRN Ceram. 2013, 2013. [Google Scholar] [CrossRef]
- Chrisey, D.B.; Hubler, G.K. Pulsed Laser Deposition of Thin Films; John Wiley & Sons: Hoboken, NJ, USA, 1994; pp. 1–649. [Google Scholar]
- Eason, R. Pulsed Laser Deposition of Thin Films—Applications-Led Growth of Functional Materials; Wiley-Interscience: Hoboken, NJ, USA, 2006; pp. 1–648. [Google Scholar] [CrossRef]
- Perrone, A. State-of-the-art reactive pulsed laser deposition of nitrides. Jpn. J. Appl. Phys. 2002, 41, 2163–2170. [Google Scholar] [CrossRef]
- Martor Homepage. Available online: http://www.martor.de/tin-blades.html (accessed on 28 October 2015).
- Zgura, I.; Beica, T.; Mitrofan, I.L.; Mateias, C.G.; Pirvu, D.; Patrascu, I. Assessment of the impression materials by investigation of the hydrophilicity. Dig. J. Nanomater. Biostruct. 2010, 5, 749–755. [Google Scholar]
- Zgura, I.; Moldovan, R.; Negrila, C.C.; Frunza, S.; Cotorobai, V.F.; Frunza, L. Surface free energy of smooth and dehydroxylated fused quartz from contact angle measurements using some particular organics as probe liquids. J. Optoelectron. Adv. Mater. 2013, 15, 627–634. [Google Scholar]
- Duta, L.; Popescu, A.C.; Dorcioman, G.; Mihailescu, I.N.; Stan, G.E.; Zgura, I.; Enculescu, I.; Dumitrescu, I. ZnO thin films deposited on textile material substrates for biomedical applications. In Technological Innovations in Sensing and Detection of Chemical, Biological, Radiological, Nuclear Threats and Ecological Terrorism; Vaseashta, A., Braman, E., Susmann, P., Eds.; Springer Science: New York, NY, USA, 2012; pp. 207–210. [Google Scholar] [CrossRef]
- Preda, N.; Enculescu, M.; Zgura, I.; Socol, M.; Matei, E.; Vasilache, V.; Enculescu, I. Superhydrophobic properties of cotton fabrics functionalized with ZnO by electroless deposition. Mater. Chem. Phys. 2013, 138, 253–261. [Google Scholar] [CrossRef]
- Popescu, A.C.; Duta, L.; Dorcioman, G.; Mihailescu, I.N.; Stan, G.E.; Pasuk, I.; Zgura, I.; Beica, T.; Enculescu, I.; Ianculescu, A.; et al. Radical modification of the wetting behavior of textiles coated with ZnO thin films and nanoparticles when changing the ambient pressure in the pulsed laser deposition process. J. Appl. Phys. 2011, 110. [Google Scholar] [CrossRef]
- Rosenman, T. Wettability modification of nanomaterials by low-energy electron flux. Nanoscale Res. Lett. 2009, 4, 1209–1217. [Google Scholar] [CrossRef]
- ASTM D4541-09e1: 2009—Standard test method for pull-off strength of coatings using portable adhesion testers; ASTM International: West Conshohocken, PA, USA, 2009.
- ISO 4624:2002—Paints and varnishes—Pull-off test for adhesion; ISO: Berlin, Germany, 2002.
- Bäuerle, D.W. Laser Processing and Chemistry; Springer-Verlag: Berlin, German; Heidelberg, German, 2011; pp. 1–851. [Google Scholar] [CrossRef]
- Mihailescu, I.N.; Teodorescu, V.S.; Gyorgy, E.; Ristoscu, C.; Cristescu, R. Particulates in pulsed laser deposition: Formation mechanisms and possible approaches to their elimination. In Proceedings of the SPIE of the International Conference of ALT’01, Constanta, Romania, 11–14 September 2001; Dumitras, D.C., Dinescu, M., Konov, V.I., Eds.; 2002; pp. 64–74. [Google Scholar] [CrossRef]
- Saha, N.C.; Tompkins, H.G. Titanium nitride oxidation chemistry: An X-ray photoelectron spectroscopy study. J. Appl. Phys. 1992, 72, 3072–3079. [Google Scholar] [CrossRef]
- Castillo, R.; Koch, B.; Ruiz, P.; Delmon, B. Influence of the amount of titania on the texture and structure of titania supported on silica. J. Catal. 1996, 161, 524–529. [Google Scholar] [CrossRef]
- Biwer, B.M.; Bernasek, S.L. Electron spectroscopic study of the iron surface and its interaction with oxygen and nitrogen. J. Electron Spectrosc. Relat. Phenom. 1986, 40, 339–351. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer formula for X-ray particle size determination. Phys. Rev. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Bico, J.; Marzolin, C.; Quere, D. Pearl drops. Europhys. Lett. 1999, 47, 220–226. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Beica, T.; Moldovan, R.; Zgura, I.; Frunza, S.; Poterasu, M. Mechanism of homeotropic alignment of a doped liquid crystal. J. Optoelectron. Adv. Mater. 2006, 8, 1512–1515. [Google Scholar]
- Kim, H.; Horwitz, J.S.; Kushto, G.P.; Pique, A.; Kafafi, Z.H.; Gilmore, C.M.; Chrisey, D. Effect of film thickness on the properties of indium tin oxide thin films. J. Appl. Phys. 2000, 88, 6021. [Google Scholar] [CrossRef]
- Bollen, L.; Lambrecht, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Quirynen, M.; Bollen, C.; Papaioannou, W.; van Eldere, J.; van Steenberghe, D. The influence of titanium abutments surface roughness on plaque accumulation and gingivitis. Short term observations. Int. J. Oral Maxillofac. Implant. 1996, 11, 169–178. [Google Scholar]
- Größner-Schreiber, B.; Griepentrog, M.; Haustein, I.; Muller, W.D.; Lange, K.P.; Briedigkeit, H.; Gobel, U.B. Plaque formation on surface modified dental implants. Clin. Oral Implan. Res. 2001, 12, 543–551. [Google Scholar] [CrossRef]
- Katsikogianni, M.; Missirlis, Y.F. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cells Mater. 2004, 8, 37–57. [Google Scholar]
- Tsang, C.S.; Ng, H.; Mc Millan, A.S. Antifungal susceptibility of Candida Albicans biofilms on titanium discs with different surface roughness. Clin. Oral Investig. 2007, 11, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Pier-Francesco, A.; Adams, R.; Waters, M.; Williams, D. Titanium surface modification and its effect on the adherence of Porphyromonas gingivalis: An in vitro study. Clin. Oral Implan. Res. 2007, 17, 633–637. [Google Scholar] [CrossRef] [PubMed]
- Bull, S.J.; Bhat, D.G.; Staia, M.H. Properties and performance of commercial TiCN coatings. Part 1: Coating architecture and hardness modelling. Surf. Coat. Technol. 2003, 163–164, 499–506. [Google Scholar] [CrossRef]
- Tamada, Y.; Ikada, Y. Fibroblast growth on polymer surfaces and biosynthesis of collagen. J. Biomed. Mater. Res. 1994, 28, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.I.; Wang, Y. Cell responses to surface and architecture of tissue engineering scaffolds. In Regenerative Medicine and Tissue Engineering—Cells and Biomaterials, 1st ed.; Eberli, D., Ed.; Intech: Rijeka, Croatia, 2011; pp. 569–588. [Google Scholar]
- Sushant, K.R.; Chawla, A.K.; Jayaganthan, R.; Chandra, R. Structural, wettability and optical investigation of titanium oxynitride coatings: Effect of various sputtering parameters. J. Mater. Sci. Technol. 2012, 28, 512–523. [Google Scholar] [CrossRef]
- Banakh, O.; Moussa, M.; Matthey, J.; Pontearso, A.; Cattani-Lorente, M.; Sanjines, P.; Fontana, P.; Wiskot, A.; Durual, S. Sputtered titanium oxynitride coatings for endosseous applications: Physical and chemical evaluation and first bioactivity assays. Appl. Surf. Sci. 2014, 317, 986–993. [Google Scholar] [CrossRef]
- Mihailescu, I.N.; Ursu, I.; Nistor, L.C.; Teodorescu, V.S.; Prokhorov, A.M.; Konov, V.I.; Uglov, S.A. Multipulse laser nitridation of titanium, zirconium and hafnium in a nitrogen atmosphere containing oxygen. J. Phys. D Appl. Phys. 1987, 20, 1519–1524. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duta, L.; Stan, G.E.; Popa, A.C.; Husanu, M.A.; Moga, S.; Socol, M.; Zgura, I.; Miculescu, F.; Urzica, I.; Popescu, A.C.; et al. Thickness Influence on In Vitro Biocompatibility of Titanium Nitride Thin Films Synthesized by Pulsed Laser Deposition. Materials 2016, 9, 38. https://doi.org/10.3390/ma9010038
Duta L, Stan GE, Popa AC, Husanu MA, Moga S, Socol M, Zgura I, Miculescu F, Urzica I, Popescu AC, et al. Thickness Influence on In Vitro Biocompatibility of Titanium Nitride Thin Films Synthesized by Pulsed Laser Deposition. Materials. 2016; 9(1):38. https://doi.org/10.3390/ma9010038
Chicago/Turabian StyleDuta, Liviu, George E. Stan, Adrian C. Popa, Marius A. Husanu, Sorin Moga, Marcela Socol, Irina Zgura, Florin Miculescu, Iuliana Urzica, Andrei C. Popescu, and et al. 2016. "Thickness Influence on In Vitro Biocompatibility of Titanium Nitride Thin Films Synthesized by Pulsed Laser Deposition" Materials 9, no. 1: 38. https://doi.org/10.3390/ma9010038
APA StyleDuta, L., Stan, G. E., Popa, A. C., Husanu, M. A., Moga, S., Socol, M., Zgura, I., Miculescu, F., Urzica, I., Popescu, A. C., & Mihailescu, I. N. (2016). Thickness Influence on In Vitro Biocompatibility of Titanium Nitride Thin Films Synthesized by Pulsed Laser Deposition. Materials, 9(1), 38. https://doi.org/10.3390/ma9010038