Synthesis and Structural Characterization of the New Clathrates K8Cd4Ge42, Rb8Cd4Ge42, and Cs8Cd4Ge42
Abstract
:1. Introduction
2. Results
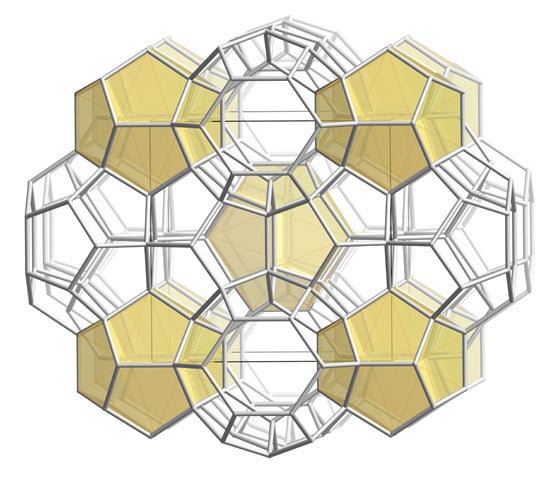
2.1. Crystallographic Analysis
2.2. Structural Metrics
3. Discussion
4. Materials and Methods
4.1. Synthesis
4.2. Powder X-ray Diffraction
4.3. Single-Crystal X-ray Diffraction
4.4. Energy-Dispersive Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PGEC | Phonon-Glass Electron-Crystal |
| Tt | Tetrel, group 14 elements Si, Ge and Sn |
| A | group 1, 2 and 3 elements, which partially or fully occupy the cages within the clathrate framework |
| M | group 13 and 12 elements, as well as late transition metals, substituting framework atoms |
| ☐ | vacancy |
References
- Kasper, J.S.; Hagenmuller, P.; Pouchard, M.; Cros, C. Clathrate structure of silicon Na8Si46 and NaxSi136 (x < 11). Science 1965, 150, 1713–1714. [Google Scholar] [PubMed]
- Slack, G.A. New materials and performance limits for thermoelectric cooling. In CRC Handbook of Thermoelectrics; Rowe, D.M., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 407–440. [Google Scholar]
- Christensen, M.; Johnson, S.; Iversen, B.B. Thermoelectric clathrates of type-I. Dalton Trans. 2010, 39, 978–992. [Google Scholar] [CrossRef] [PubMed]
- Nolas, G.S.; Cohn, J.L.; Slack, G.A.; Schjuman, S.B. Semiconducting Ge clathrates: Promising candidates for thermoelectric applications. Appl. Phys. Lett. 1998, 73, 178–180. [Google Scholar] [CrossRef]
- Sales, B.C.; Chakoumakos, B.C.; Mandrus, D.; Sharp, J.W. Atomic displacement parameters and the lattice thermal conductivity of clathrate-like thermoelectric compounds. J. Solid State Chem. 1999, 146, 528–532. [Google Scholar] [CrossRef]
- Bobev, S.; Sevov, S.C. Clathrates of group 14 with alkali metals: An exploration. J. Solid State Chem. 2000, 153, 92–105. [Google Scholar] [CrossRef]
- Beekman, M.; Nolas, G.S. Inorganic clathrate-II materials of group 14: Synthetic routes and physical properties. J. Mater. Chem. 2008, 18, 842–851. [Google Scholar] [CrossRef]
- Shevelkov, A.V.; Kovnir, K. Zintl clathrates. In Structure and Bonding; Springer: Berlin, Germany, 2011; Volume 990, pp. 97–142. [Google Scholar]
- Paschen, S.; Carrillo Cabrera, W.; Bentien, A.; Tran, V.H.; Baenitz, M.; Grin, Y.; Steglich, F. Structural, transport, magnetic and thermal properties of Eu8Ga16Ge30. Phys. Rev. B 2001, 64. [Google Scholar] [CrossRef]
- Prokofiev, A.; Sidorenko, A.; Hradil, K.; Ikeda, M.; Svagera, R.; Waas, M.; Winkler, H.; Neumaier, K.; Paschen, S. Thermopower enhancement by encapsulating cerium in clathrate cages. Nat. Mater. 2013, 12, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Paschen, S.; Budnyk, S.; Köhler, U.; Prots, Y.; Hiebl, K.; Steglich, F.; Grin, Y. New type-I clathrates with ordered Eu distribution. Physica. B 2006, 383, 89–92. [Google Scholar] [CrossRef]
- Kishimoto, K.; Sasaki, Y.; Koyanagi, T.; Ohoyama, K.; Akai, K. Crystal structure and thermoelectric properties of KxBa8–xZnyGe46–y clathrates. J. Appl. Phys. 2012, 111. [Google Scholar] [CrossRef]
- Kaltzoglou, A.; Ponou, S.; Faessler, T.F. A4Ge9 (A = K, Rb) as precursors for Hg-substituted clathrate-I synthesis: Crystal structure of A8Hg3Ge43. Eur. J. Inorg. Chem. 2008, 29, 4507–4510. [Google Scholar] [CrossRef]
- Baran, V.; Faessler, T.F. Si-based clathrates with partial substitution by Zn and Ga: K8Zn3.5Si42.5, Rb7.9Zn3.6Si42.4, and Cs8–xGa8–ySi38+y. Z. Anorg. Allg. Chem. 2015, 641, 1435–1443. [Google Scholar] [CrossRef]
- Schäfer, M.C.; Bobev, S. Copper and zinc substitutions in clathrates of tin: Synthesis, structural characterization, and physical properties of A8Cu2.67Sn43.33 and A8Zn4Sn42 (A = K, Rb, Cs) with the type-I structure. Chem. Mater. 2013, 25, 3737–3744. [Google Scholar] [CrossRef]
- Nasir, N.; Grytsiv, A.; Melnychenko-Koblyuk, N.; Rogl, P.; Bauer, E.; Lackner, R.; Royanian, E.; Giester, G.; Saccone, A. Clathrates Ba8{Zn,Cd}xSi46–x, x~7: Synthesis, crystal structure and thermoelectric properties. J. Phys. 2009, 21, 385404. [Google Scholar] [CrossRef] [PubMed]
- Kauzlarich, S.M. Chemistry, Structure, and Bonding of Zintl. Phases and Ions; VCH: New York, NY, USA, 1996. [Google Scholar]
- Melnychenko-Koblyuk, N.; Grytsiv, A.; Fornasari, L.; Kaldarar, H.; Michor, H.; Roehrbacher, F.; Koza, M.; Royanian, E.; Bauer, E.; Rogl, P.; et al. Ternary clathrates Ba–Zn–Ge: Phase equilibria, crystal chemistry and physical properties. J. Phys. Condens. Matter. 2007, 19, 216223. [Google Scholar] [CrossRef]
- Melnychenko-Koblyuk, N.; Grytsiv, A.; Berger, S.T.; Kaldarar, H.; Michor, H.; Roehrbacher, F.; Royanian, E.; Bauer, E.; Rogl, P.; Schmid, H.; et al. Ternary clathrates Ba-Cd-Ge: Phase equilibria, crystal chemistry and physical properties. J. Phys. Condens. Matter. 2007, 19, 046203. [Google Scholar] [CrossRef]
- Schäfer, M.C.; Bobev, S. K and Ba distribution in the structures of the clathrate compounds KxBa16−x(Ga,Sn)136 (x = 0.8, 4.4, and 12.9) and KxBa8−x(Ga,Sn)46 (x = 0.3). Acta. Cryst. 2013, C69, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.C.; Yamasaki, Y.; Fritsch, V.; Bobev, S. Ternary compounds in the Sn-rich section of the Ba-Ga-Sn system: Ba8Ga16–xSn30+x (1.1 ≤ x ≤ 2.8) clathrates of type-I and type-VIII, and BaGa2–xSn4+x (x ≈ 0.2) with a clathrate-like structure. Crystals 2011, 1, 145–162. [Google Scholar] [CrossRef]
- Von Schnering, H.-G.; Carillo-Cabrera, W.; Kröner, R.; Peters, E.-M.; Peters, K. Crystal structure of the clathrate β-Ba8Ga16Sn30. Z. Kristallogr. New Cryst. Struct. 1998, 213, 679. [Google Scholar]
- Chakoumakos, B.C.; Sales, B.C.; Mandrus, D.; Nolas, G.S. Structural disorder and thermal conductivity of the semiconducting clathrate Sr8Ga16Ge30. J. Alloys Compd. 2000, 296, 80–86. [Google Scholar] [CrossRef]
- Chakoumakos, B.C.; Sales, B.C.; Mandrus, D. Structural disorder and magnetism of the semiconducting clathrate Eu8Ga16Ge30. J. Alloys Compd. 2001, 322, 127–134. [Google Scholar] [CrossRef]
- Nolas, G.S.; Weakley, T.J.R.; Cohn, J.L.; Sharma, R. Structural properties and thermal conductivity of crystalline Ge clathrates. Phys. Rev. B 2000, 61, 3845–3850. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bonding, 3rd ed.; Cornell University Press: Ithaca, NY, USA, 1960; p. 403. [Google Scholar]
- Von Schnering, H.-G.; Llanos, J.; Peters, K.; Baitinger, M.; Grin, Y.; Nesper, R. Refinement of the crystal structure of K8Ge44☐2, an intermetallic clathrate I. Z. Kristallogr. New Cryst. Struct. 2011, 226, 9–10. [Google Scholar]
- Veremchuk, I.; Wosylus, A.; Boehme, B.; Baitinger, M.; Borrmann, H.; Prots, Yu.; Burkhardt, U.; Schwarz, U.; Grin, Y. Preparation and crystal structure of the clathrate-I Cs8–xGe44+y☐2–y. Z. Anorg. Allg. Chem. 2011, 637, 1281–1286. [Google Scholar] [CrossRef]
- Von Schnering, H.-G.; Menke, H.; Kroener, R.; Peters, E.M.; Peters, K.; Nesper, R. Crystal structure of the clathrates Rb8In8Ge38 and K8In8Ge38. Z. Kristallogr. New Cryst. Struct. 1998, 213, 673–674. [Google Scholar]
- Menke, H.; Carrillo Cabrera, W.; Peters, K.; Peters, E.M.; von Schnering, H.-G. Crystal structure of the clathrate Cs8In8Ge38. Z. Kristallogr. New Cryst. Struct. 1999, 214, 14. [Google Scholar] [CrossRef]
- Schäfer, M.C.; Bobev, S. New type-I and type-II clathrates in the systems Cs–Na–Ga–Si, Rb–Na–Ga–Si, and Rb–Na–Zn–Si. Inorganics 2014, 2, 79–95. [Google Scholar] [CrossRef]
- Bobev, S.; Sevov, S.C. Synthesis and characterization of stable stoichiometric clathrates of silicon and germanium: Cs8Na16Si136 and Cs8Na16Ge136. J. Am. Chem. Soc. 1999, 121, 3795–3796. [Google Scholar] [CrossRef]
- Beekman, M.; Wong-Ng, W.; Kaduk, J.A.; Shapiro, A.; Nolas, G.S. Synthesis and single-crystal X-ray diffraction studies of new framework substituted type II clathrates, Cs8Na16AgxGe136−x (x < 7). J. Solid State Chem. 2007, 180, 1076–1082. [Google Scholar]
- Beekman, M.; Kaduk, J.A.; Gryko, J.; Wong-Ng, W.; Shapiro, A.; Nolas, G.S. Synthesis and characterization of framework-substituted Cs8Na16Cu5Ge131. J. Alloys Compd. 2009, 470, 365–368. [Google Scholar] [CrossRef]
- Schäfer, M.C.; Bobev, S. Novel tin clathrates with the type-II structure. J. Am. Chem. Soc. 1999, 121, 3795–3796. [Google Scholar]
- SMART NT, version 5.63. Bruker Analytical X-ray Systems Inc.: Madison, WI, USA, 2003.
- SAINT NT, version 6.45. Bruker Analytical X-ray Systems Inc.: Madison, WI, USA, 2003.
- SADABS NT, version 2.10. Bruker Analytical X-ray Systems Inc.: Madison, WI, USA, 2001.
- SHELXTL, version 6.12. Bruker Analytical X-ray Systems Inc.: Madison, WI, USA, 2001.


| Compound | 1 | 2 | 3 |
|---|---|---|---|
| Fw/g·mol−1 | 3803.2 | 4170.2 | 4519.1 |
| Crystal system | Cubic | ||
| Space group | Pm (No. 223), Z = 1 | ||
| a/Å | 10.8710(4) | 10.9099(5) | 10.9643(7) |
| V/Å3 | 1284.72(8) | 1298.56(10) | 1318.08(15) |
| T/K | 200(2) | ||
| Radiation | Mo Kα, λ = 0.71073 Å | ||
| ρ/g·cm−3 | 4.92 | 5.33 | 5.69 |
| μ/cm−1 | 264.3 | 329.7 | 304.7 |
| data/restraints/parameters | 291/0/17 | 318/0/17 | 327/0/18 |
| R1 (I >2σ(I)) a | 0.0182 | 0.0178 | 0.0138 |
| wR2 (I >2σ(I)) a | 0.0359 | 0.0365 | 0.0298 |
| R1 (all data) a | 0.0241 | 0.0232 | 0.0180 |
| wR2 (all data) a | 0.0378 | 0.0386 | 0.0314 |
| GOF | 1.156 | 1.064 | 1.116 |
| largest peak & hole/e−·Å−3 | 0.54 & −0.57 | 0.62 & −0.85 | 0.63 & −0.56 |
| Atom | Site | x/a | y/b | z/c | Occupancy | Ueq a |
|---|---|---|---|---|---|---|
| K8Cd3.77(7)Ge42.23 | ||||||
| K1 | 6d | 0 | 1/4 | 1/2 | 100% | 0.0358(7) |
| K2 | 2a | 0 | 0 | 0 | 100% | 0.0175(9) |
| Ge1 | 24k | 0 | 0.30355(5) | 0.11589(5) | 100% | 0.0129(2) |
| Ge2 | 16i | 0.18327(4) | x | x | 100% | 0.0119(2) |
| Ge/Cd3 | 6c | 1/4 | 0 | 1/2 | 37(1)/63(1)% | 0.0137(3) |
| Rb8Cd3.65(7)Ge42.35 | ||||||
| Rb1 | 6d | 0 | 1/4 | 1/2 | 100% | 0.0254(3) |
| Rb2 | 2a | 0 | 0 | 0 | 100% | 0.0124(3) |
| Ge1 | 24k | 0 | 0.30365(5) | 0.11637(5) | 100% | 0.0123(2) |
| Ge2 | 16i | 0.18356(3) | x | x | 100% | 0.0114(2) |
| Ge/Cd3 | 6c | 1/4 | 0 | 1/2 | 39(1)/61(1)% | 0.0127(3) |
| Cs7.80(1)Cd3.65(6)Ge42.35 | ||||||
| Cs1 | 6d | 0 | 1/4 | 1/2 | 100% | 0.0204(2) |
| Cs2 | 2a | 0 | 0 | 0 | 89.8(4)% | 0.0117(3) |
| Ge1 | 24k | 0 | 0.30334(4) | 0.11719(4) | 100% | 0.0123(1) |
| Ge2 | 16i | 0.18370 (3) | x | x | 100% | 0.0116(2) |
| Ge/Cd3 | 6c | 1/4 | 0 | 1/2 | 39(1)/61(1)% | 0.0136(3) |
| Atom | U11 | U22 | U33 | U23 | U13 | U12 |
|---|---|---|---|---|---|---|
| K8Cd3.77(7)Ge42.23 | ||||||
| K1 | 0.038(1) | 0.032(2) | =U11 | 0 | 0 | 0 |
| K2 | 0.0175(9) | =U11 | =U11 | 0 | 0 | 0 |
| Ge1 | 0.0121(3) | 0.0137(3) | 0.0130(3) | 0.0000(2) | 0 | 0 |
| Ge2 | 0.0119(2) | =U11 | =U11 | −0.0006(1) | =U23 | =U23 |
| Ge/Cd3 | 0.0157(5) | 0.0127(4) | =U22 | 0 | 0 | 0 |
| Rb8Cd3.65(7)Ge42.35 | ||||||
| Rb1 | 0.0278(4) | 0.0205(6) | =U11 | 0 | 0 | 0 |
| Rb2 | 0.0124(3) | =U11 | =U11 | 0 | 0 | 0 |
| Ge1 | 0.0119(3) | 0.0127(3) | 0.0124(3) | 0.0003(2) | 0 | 0 |
| Ge2 | 0.0114(2) | =U11 | =U11 | −0.0005(1) | =U23 | =U23 |
| Ge/Cd3 | 0.0144(5) | 0.0119 (3) | =U22 | 0 | 0 | 0 |
| Cs7.80(1)Cd3.65(6)Ge42.35 | ||||||
| Cs1 | 0.0226(2) | 0.0159(3) | =U11 | 0 | 0 | 0 |
| Cs2 | 0.0117(3) | =U11 | =U11 | 0 | 0 | 0 |
| Ge1 | 0.0115(2) | 0.0122(3) | 0.0132(3) | 0.0003(2) | 0 | 0 |
| Ge2 | 0.0116(2) | =U11 | =U11 | −0.0002(1) | =U23 | =U23 |
| Ge/Cd3 | 0.0158(4) | 0.0125(3) | =U22 | 0 | 0 | 0 |
| Compound 1 | d/Å | Compound 2 | d/Å | Compound 3 | d/Å |
|---|---|---|---|---|---|
| Ge1-Ge2 (2×) | 2.4931(4) | Ge1-Ge2 (2×) | 2.5029(4) | Ge1-Ge2 (2×) | 2.5118(4) |
| Ge1-Ge1 | 2.520(1) | Ge1-Ge1 | 2.539(1) | Ge1-Ge1 | 2.570(1) |
| Ge1-Ge/Cd3 | 2.5858(6) | Ge1-Ge/Cd3 | 2.5912(5) | Ge1-Ge/Cd3 | 2.6019(5) |
| Ge2-Ge2 | 2.513(1) | Ge2-Ge2 | 2.511(1) | Ge2-Ge2 | 2.518(1) |
| Ge2-Ge1 (3×) | 2.4931(4) | Ge2-Ge1 (3×) | 2.5029(4) | Ge2-Ge1 (3×) | 2.5118(4) |
| Ge/Cd3-Ge1 (4×) | 2.5858(6) | Ge/Cd3-Ge1 (4×) | 2.5912(5) | Ge/Cd3-Ge1 (4×) | 2.6019(5) |
| K1-Ge1 (8×) | 3.6789(4) | Rb1-Ge1 (8×) | 3.6932(4) | Cs1-Ge1 (8×) | 3.7167(4) |
| K1-Ge2 (8×) | 4.0438(3) | Rb1-Ge2 (8×) | 4.0564(3) | Cs1-Ge2 (8×) | 4.0758(3) |
| K1-Ge/Cd3 (4×) | 3.8435(2) | Rb1-Ge/Cd3 (4×) | 3.8572(2) | Cs1-Ge/Cd3 (4×) | 3.8765(2) |
| K1-Ge1 (4×) | 4.2157(6) | Rb1-Ge1 (4×) | 4.2262(5) | Cs1-Ge1 (4×) | 4.2378(5) |
| K2-Ge2 (8×) | 3.4507(7) | Rb2-Ge2 (8×) | 3.4686(6) | Cs2-Ge2 (8×) | 3.4885(6) |
| K2-Ge1 (12×) | 3.5322(6) | Rb2-Ge1 (12×) | 3.5477(5) | Cs2-Ge1 (12×) | 3.5654(5) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schäfer, M.C.; Bobev, S. Synthesis and Structural Characterization of the New Clathrates K8Cd4Ge42, Rb8Cd4Ge42, and Cs8Cd4Ge42. Materials 2016, 9, 236. https://doi.org/10.3390/ma9040236
Schäfer MC, Bobev S. Synthesis and Structural Characterization of the New Clathrates K8Cd4Ge42, Rb8Cd4Ge42, and Cs8Cd4Ge42. Materials. 2016; 9(4):236. https://doi.org/10.3390/ma9040236
Chicago/Turabian StyleSchäfer, Marion C., and Svilen Bobev. 2016. "Synthesis and Structural Characterization of the New Clathrates K8Cd4Ge42, Rb8Cd4Ge42, and Cs8Cd4Ge42" Materials 9, no. 4: 236. https://doi.org/10.3390/ma9040236
APA StyleSchäfer, M. C., & Bobev, S. (2016). Synthesis and Structural Characterization of the New Clathrates K8Cd4Ge42, Rb8Cd4Ge42, and Cs8Cd4Ge42. Materials, 9(4), 236. https://doi.org/10.3390/ma9040236