Binary Alkali-Metal Silicon Clathrates by Spark Plasma Sintering: Preparation and Characterization
Abstract
:1. Introduction
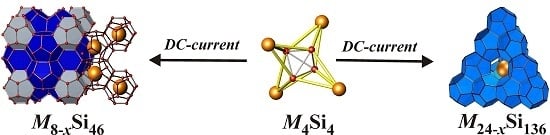
2. Results and Discussion
112 M+ + 112 e− → 112 M0 (cathode)
76 M+ + 76 e− → 76 M0 (cathode)
3. Materials and Methods
3.1. Synthesis of Precursors
3.2. Spark Plasma Synthesis
3.3. Sample Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hohmann, E. Silicide und Germanide der Alkalimetalle. Z. Anorg. Chem. 1948, 257, 113–126. [Google Scholar] [CrossRef]
- Cros, C.; Pouchard, M.; Hagenmuller, P. Two new phases of the silicon-sodium system. C. R. Hebd. Seances Acad. Sci. 1965, 260, 4764–4767. [Google Scholar]
- Cros, S.; Pouchard, M. Sur les phases de type clathrate du silicium et des éléments apparentés (C, Ge, Sn): Une approche historique. C. R. Chim. 2009, 12, 1014–1056. [Google Scholar] [CrossRef]
- Nolas, G.S. (Ed.) The Physics and Chemistry of Inorganic Clathrates; Springer: Berlin, Germany, 2014.
- Kasper, J.S.; Hagenmuller, P.; Pouchard, M.; Cros, C. Clathrate Structure of Silicon Na8Si46 and NaxSi136 (x < 11). Science 1965, 150, 1713–1714. [Google Scholar] [PubMed]
- Cros, C.; Pouchard, M.; Hagenmuller, P. Sur une nouvelle famille de clathrates minéraux isotypes des hydrates de gaz et de liquides. Interprétation des résultats obtenus. J. Solid State Chem. 1970, 2, 570–581. [Google Scholar] [CrossRef]
- Böhme, B.; Guloy, A.; Tang, Z.; Schnelle, W.; Burkhardt, U.; Baitinger, M.; Grin, Y. Oxidation of M4Si4 (M = Na, K) to Clathrates by HCl or H2O. J. Am. Chem. Soc. 2007, 129, 5348–5349. [Google Scholar] [CrossRef] [PubMed]
- Böhme, B.; Hoffmann, S.; Baitinger, M.; Grin, Y. Application of n-Dodecyltrimethylammonium Chloride for the Oxidation of Intermetallic Phases. Z. Naturforsch. 2011, 66, 230–238. [Google Scholar] [CrossRef]
- Liang, Y.; Böhme, B.; Reibold, M.; Schnelle, W.; Schwarz, U.; Baitinger, M.; Lichte, H.; Grin, Y. Synthesis of the Clathrate-I Phase Ba8-xSi46 via Redox Reactions. Inorg. Chem. 2011, 50, 4523–4528. [Google Scholar] [CrossRef] [PubMed]
- Krishna, L.; Chai, P.; Koh, C.A.; Toberer, E.S.; Nolas, G.S. Synthesis and structural properties of type I potassium SiGe alloy clathrates. Mater. Lett. 2015, 149, 123–126. [Google Scholar] [CrossRef]
- Ammar, A.; Cros, C.; Pouchard, M.; Jaussaud, N.; Bassat, J.-M.; Villeneuve, G.; Duttine, M.; Ménétrier, M.; Reny, E. On the clathrate form of elemental silicon, Si136: Preparation and characterisation of NaxSi136 (x → 0). Solid State Sci. 2004, 6, 393–400. [Google Scholar] [CrossRef]
- Guloy, A.M.; Ramlau, R.; Tang, Z.; Schnelle, W.; Baitinger, M.; Grin, Y. A guest-free germanium clathrate. Nature 2006, 443, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Gallmeier, J.; Schäfer, H.; Weiss, A. Cage structure as a common building principle of compounds K8E46 (E = silicon, germanium, tin). Z. Naturforsch. B 1969, 24, 665–667. [Google Scholar]
- Bobev, S.; Sevov, S.C. Synthesis and Characterization of Stable Stoichiometric Clathrates of Silicon and Germanium: Cs8Na16Si136 and Cs8Na16Ge136. J. Am. Chem. Soc. 1999, 121, 3795–3796. [Google Scholar] [CrossRef]
- Nolas, G.S.; Vanderveer, D.G.; Wilkinson, A.P.; Cohn, J.L. Temperature dependent structural and transport properties of the type II clathrates A8Na16E136 (A = Cs or Rb and E = Ge or Si). J. Appl. Phys. 2002, 91, 8970–8973. [Google Scholar] [CrossRef]
- Beekman, M.; Baitinger, M.; Borrmann, H.; Schnelle, W.; Meier, K.; Nolas, G.S.; Grin, Y. Preparation and Crystal Growth of Na24Si136. J. Am. Chem. Soc. 2009, 131, 9642–9643. [Google Scholar] [CrossRef] [PubMed]
- Beekman, M.; Schnelle, W.; Borrmann, H.; Baitinger, M.; Grin, Y.; Nolas, G.S. Intrinsic Electrical and Thermal Properties from Single Crystals of Na24Si136. Phys. Rev. Lett. 2010, 104. [Google Scholar] [CrossRef] [PubMed]
- Stefanoski, S.; Beekman, M.; Wong-Ng, W.; Zavalij, P.; Nolas, G.S. Simple Approach for Selective Crystal Growth of Intermetallic Clathrates. Chem. Mater. 2011, 23, 1491–1495. [Google Scholar] [CrossRef]
- Stefanoski, S.; Nolas, G.S. Synthesis and Structural Characterization of Single-Crystal K7.5Si46 and K17.8Si136 Clathrates. Cryst. Growth Des. 2011, 11, 4533–4537. [Google Scholar] [CrossRef]
- Baranowski, L.L.; Krishna, L.; Martinez, A.D.; Raharjo, T.; Stevanovic, V.; Tamboli, A.C.; Toberer, E.S. Synthesis and optical band gaps of alloyed Si–Ge type II clathrates. J. Mater. Chem. B 2014, 2, 3231–3237. [Google Scholar] [CrossRef]
- Dong, Y.; Nolas, G.S. Rapid crystal growth of type-II clathrates A8Na16Si136 (A = K, Rb, Cs) by spark plasma sintering. Cryst. Eng. Commun. 2015, 17, 2242–2244. [Google Scholar] [CrossRef]
- Dong, Y.; Chai, P.; Beekman, M.; Zeng, X.; Tritt, T.M.; Nolas, G.S. Precursor Routes to Complex Ternary Intermetallics: Single-Crystal and Microcrystalline Preparation of Clathrate-I Na8Al8Si38 from NaSi + NaAlSi. Inorg. Chem. 2015, 54, 5316–5321. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Dong, Y.; Nolas, G.S. Precursor routes to quaternary intermetallics: Synthesis, crystal structure, and physical properties of clathrate-II Cs8Na16Al24Si112. J. Solid State Chem. 2016, 237, 81–85. [Google Scholar] [CrossRef]
- Reinfried, N.; Höhn, P.; Grin, Y. Spark-Plasma Synthesis an Inert Gas Atmosphere; Max-Planck-Institute for Chemical Physics of Solids: Dresden, Germany, 2006; p. 29. [Google Scholar]
- Akselrud, L.; Grin, Y. WinCSD: Software package for crystallographic calculations (Version 4). J. Appl. Crystallogr. 2014, 47, 803–805. [Google Scholar] [CrossRef]
- Stefanoski, S.; Blosser, M.C.; Nolas, G.S. Pressure Effects on the Size of Type-I and Type-II Si-Clathrates Synthesized by Spark Plasma Sintering. Cryst. Growth Des. 2013, 13, 195–197. [Google Scholar] [CrossRef]
- Blosser, M.; Nolas, G.S. Synthesis of Na8Si46 and Na24Si136 by oxidation of Na4Si4 from ionic liquid decomposition. Mater. Lett. 2013, 99, 161–163. [Google Scholar] [CrossRef]
- Ramachandran, G.K.; McMillan, P.F.; Dong, J.; Sankey, O.F. K7.62(1)Si46 and Rb6.15(2)Si46: Two Structure I Clathrates with Fully Occupied Framework Sites. J. Solid State Chem. 2000, 154, 626–634. [Google Scholar] [CrossRef]
- Bobev, S.; Sevov, S.C. Clathrates of Group 14 with Alkali Metals: An Exploration. J. Solid State Chem. 2000, 153, 92–105. [Google Scholar] [CrossRef]
- Beekman, M.; Nenghabi, E.N.; Biswas, K.; Myles, C.W.; Baitinger, M.; Grin, Y.; Nolas, G.S. Framework Contraction in Na-Stuffed Si(cF136). Inorg. Chem. 2010, 49, 5338–5340. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Reny, E.; Gravereau, P.; Cros, C.; Pouchard, M. Structural characterisations of the NaxSi136 and Na8Si46 silicon clathrates using the Rietveld method. J. Mater. Chem. 1998, 8, 2839–2844. [Google Scholar] [CrossRef]
- Stefanoski, S.; Martin, J.; Nolas, G.S. Low temperature transport properties and heat capacity of single-crystal Na8Si46. J. Phys. Condens. Matter 2010, 22. [Google Scholar] [CrossRef] [PubMed]
- Beekman, M.; Nolas, G.S. Transport Properties of the Binary Type I Clathrate K8Ge44□2. Int. J. Appl. Ceram. Technol. 2007, 4, 332–338. [Google Scholar] [CrossRef]
- Cros, C.; Benejat, J.-C. Preparation and properties of a clathrate having an extensive domain of existence. Sodium silicide NaxSi136 compounds. Bull. Soc. Chim. Fr. 1972, 5, 1739–1743. [Google Scholar]
- Hutchins, P.T.; Leynaud, O.; O’Dell, L.A.; Smith, M.E.; Barnes, P.; McMillan, P.F. Time-Resolved in Situ Synchrotron X-ray Diffraction Studies of Type 1 Silicon Clathrate Formation. Chem. Mater. 2011, 23, 5160–5167. [Google Scholar] [CrossRef] [Green Version]







| Precursor | TR (°C) a | tR (min) b | Die/Punches c | Products d |
|---|---|---|---|---|
| Na4Si4 | 550 | 180 | C/C | Na24Si136 (M) + Na8Si46 (m) |
| 600 | 180 | C/C | Na24Si136 (M) | |
| 700 | 180 | C/C | Na24Si136 (M) + Si (m) | |
| K4Si4 | 500 | 60 | BN/SS | K8-xSi46 (tr) |
| 550 | 60 | BN/SS | K8-xSi46 (M) | |
| 600 | 60 | BN/SS | K8-xSi46 (M) + Si (m) | |
| 650 | 60 | BN/SS | Si (M) + K8-xSi46 (m) | |
| Rb4Si4 | 450 | 60 | BN/SS | Rb8-xSi46 (M) + Rb24-xSi136 (m) + Si (tr) |
| 500 | 60 | BN/SS | Si (M) + Rb8-xSi46 (m) + Rb24-xSi136 (m) | |
| Cs4Si4 | 350 | 60 | BN/SS | Cs8-xSi136 (M) + Si (tr) |
| Phase | K7.6(1)Si46 | K6.8(1)Si46 | Rb6.2(1)Si46 |
|---|---|---|---|
| Lattice parameter a, Å | 10.2776 (1) | 10.2747 (1) | 10.2848 (1) |
| Radiation, wavelength λ, Å | Cu Kα1, 1.54056 | ||
| Maximal diffraction angle 2θ, ° | 100.30 | ||
| Residuals RI/RP | 0.04/0.10 | 0.04/0.09 | 0.02/0.10 |
| M1 2a (0 0 0) | Occ * = 0.783 (1) | Occ = 0.695 (4) | Occ = 0.244 (3) |
| M2 6d (¼ ½ 0) | Occ = 1.013 (3) ** | Occ = 0.918 (3) | Occ = 0.961 (2) |
| Si1 6c (¼ 0 ½) | – | – | – |
| Si2 16i (x x x) | x = 0.1845 (1) | x = 0.1846 (1) | x = 0.1840 (1) |
| Si3 24k (0 y z) | y = 0.3068 (1) | y = 0.3060 (1) | y = 0.3043 (1) |
| z = 0.1183 (1) | z = 0.1185 (1) | z = 0.1190 (1) | |
| Phase | Rb11.1(1)Si136 | Cs7.8(1)Si136 |
|---|---|---|
| Lattice parameter a, Å | 14.7142 (9) | 14.6733 (3) |
| Radiation, wavelength λ, Å | Cu Kα1, 1.54056 | |
| Maximal diffraction angle 2θ, ° | 100.30 | |
| Residuals RI/RP | 0.10/0.10 | 0.05/0.13 |
| M1 8b (⅜ ⅜ ⅜) | Occ * = 0.85 (2) | Occ = 0.968 (2) |
| M2 16c (0 0 0) | Occ = 0.272 (1) | Occ = 0.0 |
| Si1 8a (⅛ ⅛ ⅛) | – | – |
| Si2 32e (x x x) | x = 0.2198 (6) | x = 0.2168 (2) |
| Si3 96g (x x z) | x = 0.1839 (2) | x = 0.1822 (1) |
| z = 0.3698 (6) | z = 0.3699 (2) | |
| Precursor | Preparation Technique a | Clathrate Phases Obtained | References |
|---|---|---|---|
| Na4Si4 | CTC | Na8Si46 and Na24-xSi136 | [6,33] |
| KCTD | Na8Si46 and Na24-xSi136 | [18] | |
| CO | Na8Si46 and Na24-xSi136 | [7,25] | |
| SPS | Na8Si46 and Na24-xSi136 | [16,24], this work | |
| K4Si4 | CTC | K8-xSi46 | [6,26] |
| KCTD | K8-xSi46 and K24-xSi136 | [19] | |
| CO | K8-xSi46 | [7] | |
| SPS | K8-xSi46 | this work | |
| Rb4Si4 | CTC | Rb8-xSi46 | [6,26] |
| SPS | Rb8-xSi46 and Rb24-xSi136 | this work | |
| Cs4Si4 | CTC | Cs24-xSi136 | [6] |
| SPS | Cs24-xSi136 | this work |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veremchuk, I.; Beekman, M.; Antonyshyn, I.; Schnelle, W.; Baitinger, M.; Nolas, G.S.; Grin, Y. Binary Alkali-Metal Silicon Clathrates by Spark Plasma Sintering: Preparation and Characterization. Materials 2016, 9, 593. https://doi.org/10.3390/ma9070593
Veremchuk I, Beekman M, Antonyshyn I, Schnelle W, Baitinger M, Nolas GS, Grin Y. Binary Alkali-Metal Silicon Clathrates by Spark Plasma Sintering: Preparation and Characterization. Materials. 2016; 9(7):593. https://doi.org/10.3390/ma9070593
Chicago/Turabian StyleVeremchuk, Igor, Matt Beekman, Iryna Antonyshyn, Walter Schnelle, Michael Baitinger, George S. Nolas, and Yuri Grin. 2016. "Binary Alkali-Metal Silicon Clathrates by Spark Plasma Sintering: Preparation and Characterization" Materials 9, no. 7: 593. https://doi.org/10.3390/ma9070593
APA StyleVeremchuk, I., Beekman, M., Antonyshyn, I., Schnelle, W., Baitinger, M., Nolas, G. S., & Grin, Y. (2016). Binary Alkali-Metal Silicon Clathrates by Spark Plasma Sintering: Preparation and Characterization. Materials, 9(7), 593. https://doi.org/10.3390/ma9070593