Anatomy of the Windmill Palm (Trachycarpus fortunei) and Its Application Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Morphological Observations and Measurement of Vascular Bundles
2.3. Measurement of Fiber Dimensions and Cell Walls
2.4. Nanoindentation of the Two Kinds of Fiber
2.5. Statistically Significant Differences Analysis
3. Results and Discussion
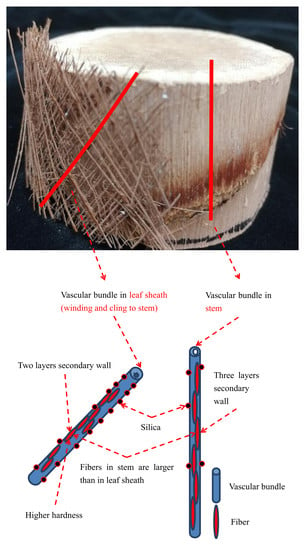
3.1. Anatomical Structure of Vascular Bundles
3.2. Shape of Vascular Bundles
3.3. Tissue Proportion of Vascular Bundles
3.4. Lateral Surface of the Vascular Bundles
3.5. Dimensions of Fibers
3.6. Fiber Outer Surface Microstructure
3.7. Fiber Cell Wall Ultrastructure
3.8. Mechanical Properties of Fiber Cell Wall
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ghaffar, S.H.; Fan, M. An aggregated understanding of physicochemical properties and surface functionalities of wheat straw node and internode. Ind. Crops Prod. 2017, 95, 207–215. [Google Scholar] [CrossRef]
- Tomlinson, P.B. The structural biology of palms. In Quarterly Review of Biology; Oxford University Press: Oxford, UK, 1991. [Google Scholar] [CrossRef]
- Eames, A.J.; Tomlinson, R.B. Anatomy of the monocotyledons, II Palmae. Bull. Torrey Bot. Club 1961, 89, 122. [Google Scholar] [CrossRef]
- Zhai, S.; Pan, B.; Horikawa, Y.; Li, D.; Itoh, T.; Sugiyama, J. Structure and development of fibrovascular bundles from leaf sheath of windmill palm. J. Nanjing For. Univ. 2014, 38, 90–96. [Google Scholar] [CrossRef]
- Zhai, S.; Pan, B.; Sugiyama, J.; Li, D.; Itoh, T. Tensile strength of windmill palm (Trachycarpus fortunei) fiber bundles and its structural implications. J. Mater. Sci. 2012, 47, 949–959. [Google Scholar] [CrossRef]
- Zhai, S.; Sugiyama, J.; Itoh, T.; Pan, B.; Li, D. Cell wall characterization of windmill palm (Trachycarpus fortunei) fibers and its functional implications. Iawa J. 2013, 34, 20–33. [Google Scholar] [CrossRef]
- Zhai, S. Structural, Chemical and Physical Properties of Palm Fiber; Nanjing Forest University: Nanjing, China, 2010. [Google Scholar]
- Zhai, S. Anatomical and mechanical features of palm fibrovascular bundles. In Sustainable Humanosphere Bulletin of Research Institute for Sustainable Humanosphere Kyoto University; Kyoto University: Kyoto, Japan, 2014; Volume 10, pp. 22–23. [Google Scholar]
- Chen, C.; Chen, G.; Li, X.; Guo, H.; Wang, G. The influence of chemical treatment on the mechanical properties of windmill palm fiber. Cellulose 2017, 24, 1611–1620. [Google Scholar] [CrossRef]
- Schmitt, U.; Weiner, G.; Liese, W. The fine structure of the stegmata in calamus axillaris becc during maturation. Iawa J. 1995, 16, 61–88. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, H.; Yue, L.; Pizzi, A.; Lu, X. Effects of steam explosion on the characteristics of windmill palm fiber and its application to fiberboard. Eur. J. Wood Wood Prod. 2018, 76, 601–609. [Google Scholar] [CrossRef]
- Al-Khanbashi, A.; Al-Kaabi, K.; Hammami, A. Date palm fibers as polymeric matrix reinforcement: Fiber characterization. Polym. Compos. 2010, 26, 486–497. [Google Scholar] [CrossRef]
- Rashid, B.; Leman, Z.; Jawaid, M.; Ghazali, M.J.; Ishak, M.R. Physicochemical and thermal properties of lignocellulosic fiber from sugar palm fibers: Effect of treatment. Cellulose 2016, 23, 2905–2916. [Google Scholar] [CrossRef]
- Essig, F.B.; Dong, Y.F. The many uses of Trachycarpus fortunei (Arecaceae) in China. Econ. Bot. 1987, 41, 411–417. [Google Scholar] [CrossRef]
- Anders, B.; Manju, B.; John, D.; Henrik, B. SE Asian palms for agroforestry and home gardens. Forests 2015, 6, 4607–4616. [Google Scholar] [CrossRef] [Green Version]
- Bourmaud, A.; Dhakal, H.; Habrant, A.; Padovanic, J.; Siniscalcoa, D.; Ramaged, M.H.; Beaugrandce, J.; Darshil, U.S. Exploring the potential of waste leaf sheath date palm fibres for composite reinforcement through a structural and mechanical analysis. Compos. Part A Appl. Sci. Manuf. 2017, 103, 292–303. [Google Scholar] [CrossRef] [Green Version]
- Clara, F.G.; Antonio, F.G.; Manuel, F.V.; Juan, F.; Teresa, G.O.; María, F.G. Physical and mechanical properties of particleboard made from palm tree prunings. Forests 2018, 9, 755. [Google Scholar] [CrossRef] [Green Version]
- Tran, L.Q.; Minh, T.N.; Fuentes, C.A.; Chi, T.T.; Vuure, A.W.; Verpoest, I. Investigation of microstructure and tensile properties of porous natural coir fibre for use in composite materials. Ind. Crops Prod. 2015, 65, 437–445. [Google Scholar] [CrossRef] [Green Version]
- Shinoj, S.; Visvanathan, R.; Panigrahi, S.; Kochubabua, M. Oil palm fiber (OPF) and its composites: A review. Ind. Crops Prod. 2011, 33, 7–22. [Google Scholar] [CrossRef]
- Li, J.; Li, K.; Zhang, T.; Wang, S.; Jiang, Y.; Bao, Y.; Tie, M. Development of activated carbon from windmill palm sheath fiber by, K.O.H activation. Fibers Polym. 2016, 17, 880–887. [Google Scholar] [CrossRef]
- Oushabi, A.; Sair, S.; Abboud, Y.; Tanane, O. Natural thermal-insulation materials composed of renewable resources: Characterization of local date palm fibers (LDPF). J. Mater. Environ. Sci. 2015, 6, 3395–3402. [Google Scholar]
- Zhang, T.; Guo, M.; Cheng, L.; Li, X. Investigations on the structure and properties of palm leaf sheath fiber. Cellulose 2015, 22, 1039–1051. [Google Scholar] [CrossRef]
- Chen, C.; Chen, G.; Sun, G. Windmill Palm Fiber/Polyvinyl Alcohol Nonwoven Fibrous Polymeric Materials. J. Eng. Fibers Fabr. 2016, 11, 4. [Google Scholar] [CrossRef] [Green Version]
- Chieng, B.; Lee, S.; Ibrahim, N.; Yoon, T.; Yuet, L. Isolation and characterization of cellulose nanocrystals from oil palm mesocarp fiber. Polymers 2017, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Caramyshev, A.V.; Firsova, Y.N.; Slastya, E.A.; Tagaev, A.; Potapenko, N.; Lobakova, E.; Pletjushkina, O.; Sakharov, I. Purification and characterization of windmill palm tree (Trachycarpus fortunei) peroxidase. J. Agric. Food Chem. 2006, 54, 9888–9894. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, H.; Fang, L.; Tian, G.; Lin, J. Bamboo fibers for composite applications: A mechanical and morphological investigation. J. Mater. Sci. 2014, 49, 2559–2566. [Google Scholar] [CrossRef]
- Khalil, H.P.; Alwani, M.S.; Ridzuan, R.; Kamarudin, H.; Khairul, A. Chemical composition, morphological characteristics, and cell wall structure of malaysian oil palm fibers. J. Macromol. Sci. Part D 2008, 47, 273–280. [Google Scholar] [CrossRef]
- Zhai, S.; Imai, T.; Horikawa, Y. Anatomical and mechanical characteristics of leaf-sheath fibrovascular bundles in palms. Iawa J. 2013, 34, 285–300. [Google Scholar] [CrossRef] [Green Version]
- Kanzawa, E.; Aoyagi, S.; Nakano, T. Vascular bundle shape in cross-section and relaxation properties of Moso bamboo (Phyllostachys pubescens). Mater. Sci. Eng. C 2011, 31, 1050–1054. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R. Palm stem anatomy and computer-aided identification: The Coryphoideae (Arecaceae). Am. J. Bot. 2013, 100, 289–313. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Yu, Z.; Yu, Y.; Wang, H. Compressive failure mechanism and buckling analysis of the graded hierarchical bamboo structure. J. Mater. Sci. 2017, 52, 6999–7007. [Google Scholar] [CrossRef]
- Chen, C.J. Morphology Research of Windmill Palm (Trachycarpus fortunei) Material. Kemija U Industriji. 2015, 64, 467–472. [Google Scholar] [CrossRef] [Green Version]
- Lien, N.T.L.; Kolehmainen, H.; Hiltunen, E.; Hiltunen, E.; Nazhad, M. The impact of chemical composition of pulp fiber cell wall on paper recycling potential of fibers. In Improvement of Forest Resources for Recyclable Forest Products; Springer: Tokyo, Japan, 2004. [Google Scholar] [CrossRef]
- Zimmermann, T.; Thommen, V.; Reimann, P.; Hugc, H.J. Ultrastructural appearance of embedded and polished wood cell walls as revealed by atomic force microscopy. J. Struct. Biol. 2013, 156, 363–369. [Google Scholar] [CrossRef]
- Yu, H.; Liu, R.; Shen, D.; Wu, Z.; Huang, Y. Arrangement of cellulose microfibrils in the wheat straw cell wall. Carbohydr. Polym. 2008, 72, 122–127. [Google Scholar] [CrossRef]
- Gindl, W.; Gupta, H.S.; Grünwald, C. Lignification of spruce tracheid secondary cell walls related to longitudinal hardness and modulus of elasticity using nano-indentation. Can. J. Bot. 2002, 80, 1029–1033. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Zhou, D.; Xing, C.; Zhang, Y. Use of nanoindentation and silviscan to determine the mechanical properties of 10 hardwood species. Wood Fiber Sci. 2009, 41, 64–73. [Google Scholar] [CrossRef]
- Yu, Y.; Fei, B.; Zhang, B.; Yu, X. Cell-wall mechanical properties of bamboo investigated by in-situ imaging nanoindentation. Wood Fiber Sci. 2007, 39, 527–535. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Zhou, D.; Xing, C.; Zhang, Y.; Cai, Z. Evaluation of elastic modulus and hardness of crop stalks cell walls by nano-indentation. Bioresour. Technol. 2010, 101, 2867–2871. [Google Scholar] [CrossRef]








| Length (μm) | SD | Width (μm) | SD | Length/Width | SD | |
|---|---|---|---|---|---|---|
| Fiber in stem | 1260.63 | 279.59 | 42.00 | 10.11 | 32.08 | 12.85 |
| Fiber in leaf sheath | 710.89 | 125.61 | 12.77 | 2.67 | 58.08 | 16.89 |
| Independent-Sample t-Test | |
|---|---|
| Sample | Significance (sig.) |
| Cross sectional area of vascular bundle (Factor 1) | 0.0000 |
| Fiber length (Factor 2) | 0.0000 |
| Fiber diameter (Factor 3) | 0.0000 |
| Fibril diameter (Factor 4) | 0.0000 |
| Cell wall indentation modulus (Factor 5) | 0.0394 |
| Cell wall hardness (Factor 6) | 0.0002 |
| Stem and leaf sheath (Total analysis) | Significant difference |
| Latin Name | Indentation Modulus (GPa) | SD | Hardness (MPa) | SD | |
|---|---|---|---|---|---|
| Poplar | Populus | 16.90 | 1.88 | 490.00 | 46.70 |
| Spruce | Picea abies | 17.40 | 1.04 | 380.00 | 26.60 |
| Bamboo | Phyllostachys pubescens | 16.00 | 3.15 | 359.90 | 104.30 |
| Rice straw | Oryza sativa | 19.40 | 1.48 | 500.00 | 66.00 |
| Fiber in stem | Trachycarpus fortunei | 8.38 | 0.99 | 324.97 | 43.33 |
| Fiber in leaf sheath | Trachycarpus fortunei | 9.68 | 1.81 | 420.81 | 62.45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Li, J.; Wang, C.; Wang, H. Anatomy of the Windmill Palm (Trachycarpus fortunei) and Its Application Potential. Forests 2019, 10, 1130. https://doi.org/10.3390/f10121130
Zhu J, Li J, Wang C, Wang H. Anatomy of the Windmill Palm (Trachycarpus fortunei) and Its Application Potential. Forests. 2019; 10(12):1130. https://doi.org/10.3390/f10121130
Chicago/Turabian StyleZhu, Jiawei, Jing Li, Chuangui Wang, and Hankun Wang. 2019. "Anatomy of the Windmill Palm (Trachycarpus fortunei) and Its Application Potential" Forests 10, no. 12: 1130. https://doi.org/10.3390/f10121130
APA StyleZhu, J., Li, J., Wang, C., & Wang, H. (2019). Anatomy of the Windmill Palm (Trachycarpus fortunei) and Its Application Potential. Forests, 10(12), 1130. https://doi.org/10.3390/f10121130