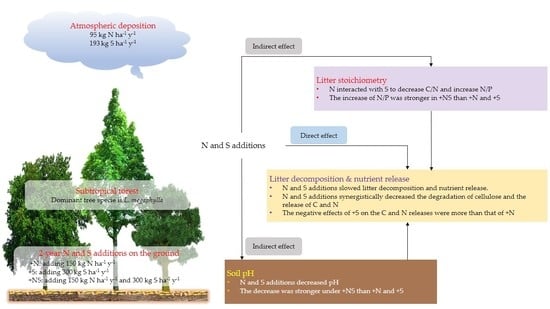
The Additions of Nitrogen and Sulfur Synergistically Decrease the Release of Carbon and Nitrogen from Litter in a Subtropical Forest
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Experimental Design
2.2.1. Leaf Collecting and Bagging
2.2.2. Plot Design and Fertilization
2.3. Litter and Soil Sampling and Analysis
2.3.1. Litter and Soil Sampling
2.3.2. Sample Analysis
2.4. Calculation of the Litter’s Stoichiometry, Remaining Mass, and Remaining Nutrients
2.5. Statistical Analyses
3. Results
3.1. Remaining Litter Mass and Decomposition Coefficient
3.2. Remaining Litter Lignin and Cellulose
3.3. Remaining Nutrients in Litter
3.4. Litter Stoichiometry Ratios and Soil pH
3.5. Release of Nutrient Elements and Degradation of Lignin and Cellulose
4. Discussion
4.1. Rate of Litter Decomposition
4.2. Degradation of Cellulose and Lignin
4.3. Release of C, N, and P from Litter
4.4. Interactive Effects of the Addition of N and S
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tan, J.; Fu, J.S.; Dentener, F.; Jian, S.; Keating, T. Multi-model study of HTAP II on sulfur and nitrogen deposition. Atmos. Chem. Phys. 2018, 18, 6847–6866. [Google Scholar] [CrossRef] [Green Version]
- Duan, L.; Yu, Q.; Zhang, Q.; Wang, Z.; Pan, Y.; Larssen, T.; Tang, J.; Mulder, J. Acid deposition in Asia: Emissions, deposition, and ecosystem effects. Atmos. Environ. 2016, 146, 55–69. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Ma, M.; Yang, T.; Chen, W.; Yang, T. Global atmospheric sulfur deposition and associated impaction on nitrogen cycling in ecosystems. J. Clean. Prod. 2018, 195, 1–9. [Google Scholar] [CrossRef]
- Jung, K.; Kwak, J.-H.; Gilliam, F.S.; Chang, S.X. Simulated N and S deposition affected soil chemistry and understory plant communities in a boreal forest in western Canada. J. Plant Ecol. 2017, 11, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Adrian, W.J. A comparison of a wet pressure digestion method with other commonly used wet and dry-ashing methods. Analyst 1973, 98, 213–216. [Google Scholar] [CrossRef]
- Du, E.; de Vries, W.; Liu, X.; Fang, J.; Galloway, J.N.; Jiang, Y. Spatial boundary of urban ‘acid islands’ in southern China. Sci. Rep. 2015, 5, 12625. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Zhang, Q.; Streets, D.G. Sulfur dioxide and primary carbonaceous aerosol emissions in China and India, 1996–2010. Atmos. Chem. Phys. 2011, 11, 9839–9864. [Google Scholar] [CrossRef] [Green Version]
- Frouz, J. Effects of soil macro- and mesofauna on litter decomposition and soil organic matter stabilization. Geoderma 2018, 332, 161–172. [Google Scholar] [CrossRef]
- Yarwood, S.A. The role of wetland microorganisms in plant-litter decomposition and soil organic matter formation: A critical review. FEMS Microbiol. Ecol. 2018, 94. [Google Scholar] [CrossRef] [Green Version]
- Sardans, J.; Peñuelas, J. The role of plants in the effects of global change on nutrient availability and stoichiometry in the plant-soil system. Plant Physiol. 2012, 160, 1741–1761. [Google Scholar] [CrossRef] [Green Version]
- Craine, J.M.; Morrow, C.; Fierer, N. Microbial nitrogen limitation increases decomposition. Ecology 2007, 88, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Corrales, A.; Turner, B.L.; Tedersoo, L.; Anslan, S.; Dalling, J.W. Nitrogen addition alters ectomycorrhizal fungal communities and soil enzyme activities in a tropical montane forest. Fungal Ecol. 2017, 27, 14–23. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, G.G.; Tang, C.; Fang, H.; Duan, J.; Yu, X. Effects of one-year simulated nitrogen and acid deposition on soil respiration in a subtropical plantation in China. Forests 2020, 11, 235. [Google Scholar] [CrossRef] [Green Version]
- Janssens, I.A.; Dieleman, W.; Luyssaert, S.; Subke, J.A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G.; et al. Reduction of forest soil respiration in response to nitrogen deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Peñuelas, J.; Sardans, J.; Rivas-Ubach, A.; Janssens, I.A. The human-induced imbalance between C, N and P in earth’s life system. Global Change Biol. 2012, 18, 3–6. [Google Scholar] [CrossRef]
- Sardans, J.; Rivas-Ubach, A.; Peñuelas, J. The C:N:P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Manning, P.; Saunders, M.; Bardgett, R.D.; Bonkowski, M.; Bradford, M.A.; Ellis, R.J.; Kandeler, E.; Marhan, S.; Tscherko, D. Direct and indirect effects of nitrogen deposition on litter decomposition. Soil Biol. Biochem. 2008, 40, 688–698. [Google Scholar] [CrossRef]
- Zhuang, L.; Liu, Q.; Liang, Z.; You, C.; Tan, B.; Zhang, L.; Yin, R.; Yang, K.; Bol, R.; Xu, Z. Nitrogen additions retard nutrient release from two contrasting foliar litters in a subtropical forest, southwest China. Forests 2020, 11, 377. [Google Scholar] [CrossRef] [Green Version]
- Mo, J.; Brown, S.; Xue, J.; Fang, Y.; Li, Z. Response of litter decomposition to simulated N deposition in disturbed, rehabilitated and mature forests in subtropical China. Plant Soil 2006, 282, 135–151. [Google Scholar] [CrossRef]
- Bejarano, M.; Crosby, M.M.; Parra, V.; Etchevers, J.D.; Campo, J. Precipitation regime and nitrogen addition effects on leaf litter decomposition in tropical dry forests. Biotropica 2014, 46, 415–424. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, C.Y.; Jia, Y.Y.; Wang, W.W.; Ma, X.; Du, J.J.; Pu, G.Z.; Tian, X.J. Effects of sulfuric, nitric, and mixed acid rain on litter decomposition, soil microbial biomass, and enzyme activities in subtropical forests of China. Appl. Soil Ecol. 2014, 79, 1–9. [Google Scholar] [CrossRef]
- Wang, Q.; Kwak, J.-H.; Choi, W.-J.; Chang, S.X. Decomposition of trembling aspen leaf litter under long-term nitrogen and sulfur deposition: Effects of litter chemistry and forest floor microbial properties. For. Ecol. Manag. 2018, 412, 53–61. [Google Scholar] [CrossRef]
- Singh, R.K.; Dutta, R.K.; Agrawal, M. Litter decomposition and nutrient release in relation to atmospheric deposition of S and N in a dry tropical region. Pedobiologia 2004, 48, 305–311. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, B.; Zhao, W.R.; Wang, L.; Xie, D.J.; Huo, W.T.; Wu, Y.W.; Zhang, J.C. Comparative effects of sulfuric and nitric acid rain on litter decomposition and soil microbial community in subtropical plantation of Yangtze River Delta region. Sci. Total Environ. 2017, 601, 669–678. [Google Scholar] [CrossRef]
- Tu, L.H.; Hu, T.X.; Zhang, J.; Li, X.W.; Hu, H.L.; Liu, L.; Xiao, Y.L. Nitrogen addition stimulates different components of soil respiration in a subtropical bamboo ecosystem. Soil Biol. Biochem. 2013, 58, 255–264. [Google Scholar] [CrossRef]
- Lin, H.J.; Hu, T.X. Preliminary study on the Chemistry Features of Atmospheric Precipitation of three Typical Regions in Sichuan (in Chinese with English Abstract); Sichuan Agricultural University: Ya’an, China, 2011. [Google Scholar]
- Wei, S.Z.; Tie, L.H.; Liao, J.; Liu, X.; Du, M.L.; Lan, S.X.; Li, X.R.; Li, C.S.; Zhan, H.C.; Huang, C.D. Nitrogen and phosphorus co-addition stimulates soil respiration in a subtropical evergreen broad-leaved forest. Plant Soil 2020, 450, 171–182. [Google Scholar] [CrossRef]
- Zhou, S.X.; Xiang, Y.B.; Tie, L.H.; Han, B.H.; Huang, C.D. Simulated nitrogen deposition significantly reduces soil respiration in an evergreen broadleaf forest in western China. PLoS ONE 2018, 13, e0204661. [Google Scholar] [CrossRef]
- Peng, Y.; Song, S.Y.; Li, Z.Y.; Li, S.; Chen, G.T.; Hu, H.L.; Xie, J.L.; Chen, G.; Xiao, Y.L.; Liu, L.; et al. Influences of of nitrogen addition and aboveground litter-input manipulations on soil respiration and biochemical properties in a subtropical forest. Soil Biol. Biochem. 2020, 142, 107694. [Google Scholar] [CrossRef]
- Tie, L.; Zhang, S.; Peñuelas, J.; Sardans, J.; Zhou, S.; Hu, J.; Huang, C. Responses of soil C, N, and P stoichiometric ratios to N and S additions in a subtropical evergreen broad-leaved forest. Geoderma 2020, 379. [Google Scholar] [CrossRef]
- Berg, B.; Davey, M.P.; De Marco, A.; Emmett, B.; Faituri, M.; Hobbie, S.E.; Johansson, M.B.; Liu, C.; McClaugherty, C.; Norell, L.; et al. Factors influencing limit values for pine needle litter decomposition: A synthesis for boreal and temperate pine forest systems. Biogeochemistry 2010, 100, 57–73. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Reed, S.C.; Keller, A.B.; Nemergut, D.R.; O’Neill, S.P.; Ostertag, R.; Vitousek, P.M. Litter quality versus soil microbial community controls over decomposition: A quantitative analysis. Oecologia 2014, 174, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Fan, J.L.; Ding, W.X.; Gunina, A.; Chen, Z.M.; Bol, R.; Luo, J.F.; Bolan, N. Characterization of organic carbon in decomposing litter exposed to nitrogen and sulfur additions: Links to microbial community composition and activity. Geoderma 2017, 286, 116–124. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, X.M. The concept of the Rainy Zone of West China and its significance to the biodiversity conservation in China (Abstract in English). Biodiv. Sci. 2002, 10, 339–344. [Google Scholar] [CrossRef]
- Berg, B.; Matzner, E. Effect of N deposition on decomposition of plant litter and soil organic matter in forest systems. Environ. Rev. 1997, 5, 1–25. [Google Scholar] [CrossRef]
- Schinner, F.; Ohlinger, R.; Kandeler, E.; Margesin, R. Methods in Soil Biology; Springer: Berlin, Germany, 1996. [Google Scholar]
- Allen, S.E.; Grimshaw, H.M.; Parkinson, J.A.; Quarmby, C. Chemical Analysis of Ecological Materials; Blackwell Scientific Publications: Oxford/London, UK, 1974. [Google Scholar]
- Bragazza, L.; Siffi, C.; Iacumin, P.; Gerdol, R. Mass loss and nutrient release during litter decay in peatland: The role of microbial adaptability to litter chemistry. Soil Biol. Biochem. 2007, 39, 257–267. [Google Scholar] [CrossRef]
- Olson, J.S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef] [Green Version]
- Bockheim, J.G.; Jepsen, E.A.; Heisey, D.M. Nutrient dynamics in decomposing leaf litter of four tree species on a sandy soil in northwestern Wisconsin. Can. J. For. Res. 1991, 21, 803–812. [Google Scholar] [CrossRef]
- Entwistle, E.M.; Zak, D.R.; Edwards, I.P. Long-term experimental nitrogen deposition alters the composition of the active fungal community in the forest floor. Soil Sci. Soc. Am. J. 2013, 77, 1648–1658. [Google Scholar] [CrossRef]
- Zhou, S.X.; Huang, C.D.; Han, B.H.; Xiao, Y.X.; Tang, J.D.; Xiang, Y.B.; Luo, C. Simulated nitrogen deposition significantly suppresses the decomposition of forest litter in a natural evergreen broad-leaved forest in the Rainy Area of Western China. Plant Soil 2017, 420, 135–145. [Google Scholar] [CrossRef]
- Zhang, T.A.; Luo, Y.Q.; Chen, H.Y.H.; Ruan, H.H. Responses of litter decomposition and nutrient release to N addition: A meta-analysis of terrestrial ecosystems. Appl. Soil Ecol. 2018, 128, 35–42. [Google Scholar] [CrossRef]
- Tu, L.H.; Hu, H.L.; Chen, G.; Peng, Y.; Xiao, Y.L.; Hu, T.X.; Zhang, J.; Li, X.W.; Liu, L.; Tang, Y. Nitrogen addition significantly affects forest litter decomposition under high levels of ambient nitrogen deposition. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, T.R.; Trofymow, J.A.; Prescott, C.E.; Fyles, J.; Titus, B.D. Patterns of carbon, nitrogen and phosphorus dynamics in decomposing foliar litter in Canadian forests. Ecosystems 2006, 9, 46–62. [Google Scholar] [CrossRef]
- Wang, C.Y.; Guo, P.; Han, G.M.; Feng, X.G.; Zhang, P.; Tian, X.J. Effect of simulated acid rain on the litter decomposition of Quercus acutissima and Pinus massoniana in forest soil microcosms and the relationship with soil enzyme activities. Sci. Total Environ. 2010, 408, 2706–2713. [Google Scholar] [CrossRef] [PubMed]
- Jian, S.Y.; Li, J.W.; Chen, J.; Wang, G.S.; Mayes, M.A.; Dzantor, K.E.; Hui, D.F.; Luo, Y.Q. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: A meta-analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Keuskamp, J.A.; Feller, I.C.; Laanbroek, H.J.; Verhoeven, J.T.A.; Hefting, M.M. Short- and long-term effects of nutrient enrichment on microbial exoenzyme activity in mangrove peat. Soil Biol. Biochem. 2015, 81, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Aerts, R.; van Bodegom, P.M.; Cornelissen, J.H.C. Litter stoichiometric traits of plant species of high-latitude ecosystems show high responsiveness to global change without causing strong variation in litter decomposition. New Phytol. 2012, 196, 181–188. [Google Scholar] [CrossRef]
- Fioretto, A.; Di Nardo, C.; Papa, S.; Fuggi, A. Lignin and cellulose degradation and nitrogen dynamics during decomposition of three leaf litter species in a Mediterranean ecosystem. Soil Biol. Biochem. 2005, 37, 1083–1091. [Google Scholar] [CrossRef]
- Ahmad, M.; Taylor, C.R.; Pink, D.; Burton, K.; Eastwood, D.; Bending, G.D.; Bugg, T.D.H. Development of novel assays for lignin degradation: Comparative analysis of bacterial and fungal lignin degraders. Mol. Biosyst. 2010, 6, 815–821. [Google Scholar] [CrossRef]
- Brown, M.E.; Chang, M.C.Y. Exploring bacterial lignin degradation. Curr. Opin. Chem. Biol. 2014, 19, 1–7. [Google Scholar] [CrossRef]
- Du, E.; Vries, W.D.; Han, W.; Liu, X.; Yuan, J. Imbalanced phosphorus and nitrogen deposition in China’s forests. Atmos. Chem. Phys. 2016, 16, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Mieczan, T.; Adamczuk, M.; Tarkowska-Kukuryk, M.; Wojciech, P.; Pawlik-Skowronska, B. Effects of experimental addition of nitrogen and phosphorus on microbial and metazoan communities in a peatbog. Eur. J. Protistol. 2017, 59, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Floudas, D.; Binder, M.; Riley, R.; Barry, K.; Blanchette, R.A.; Henrissat, B.; Martinez, A.T.; Otillar, R.; Spatafora, J.W.; Yadav, J.S.; et al. The paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 2012, 336, 1715–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talbot, J.M.; Treseder, K.K. Interactions among lignin, cellulose, and nitrogen drive litter chemistry-decay relationships. Ecology 2012, 93, 345–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, S.D.; Vitousek, P.M. Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Hu, Y.L.; Jung, K.; Zeng, D.H.; Chang, S.X. Nitrogen- and sulfur-deposition-altered soil microbial community functions and enzyme activities in a boreal mixedwood forest in western Canada. Can. J. For. Res. 2013, 43, 777–784. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant litter. In Decomposition, Humus Formation, Carbon Sequestration, 2nd ed.; Springer: Heidelberg, Germany, 2008. [Google Scholar]
- Tan, B.; Yin, R.; Yang, W.; Zhang, J.; Xu, Z.; Liu, Y.; He, S.; Zhou, W.; Zhang, L.; Li, H.; et al. Soil fauna show different degradation patterns of lignin and cellulose along an elevational gradient. Appl. Soil Ecol. 2020, 155. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z. Responses of litter decomposition and nutrient release of Bothriochloa ischaemum to soil petroleum contamination and nitrogen fertilization. Int. J. Environ. Sci. Technol. 2019, 16, 719–728. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.J.; Zhao, J.; Xiao, K.C.; Wang, K.L. Effects of nitrogen addition on activities of soil nitrogen acquisition enzymes: A meta-analysis. Agric. Ecosyst. Environ. 2018, 252, 126–131. [Google Scholar] [CrossRef]
- Pei, G.; Liu, J.; Peng, B.; Wang, C.; Jiang, P.; Bai, E. Non-linear coupling of carbon and nitrogen release during litter decomposition and its responses to nitrogen addition. J. Geophys. Res. Biogeosciences 2020, 125. [Google Scholar] [CrossRef]





| Treatments | Coefficient of Determination (R2) | k-Value (Year−1) | T50% (Year) |
|---|---|---|---|
| CK | 0.975 ** | 0.452 ± 0.005 a | 1.535 ± 0.015 d |
| +N | 0.947 ** | 0.384 ± 0.002 b | 1.804 ± 0.007 c |
| +S | 0.974 ** | 0.378 ± 0.002 c | 1.832 ± 0.010 b |
| +NS | 0.955 ** | 0.374 ± 0.001 d | 1.854 ± 0.006 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tie, L.; Fu, R.; Peñuelas, J.; Sardans, J.; Zhang, S.; Zhou, S.; Hu, J.; Huang, C. The Additions of Nitrogen and Sulfur Synergistically Decrease the Release of Carbon and Nitrogen from Litter in a Subtropical Forest. Forests 2020, 11, 1280. https://doi.org/10.3390/f11121280
Tie L, Fu R, Peñuelas J, Sardans J, Zhang S, Zhou S, Hu J, Huang C. The Additions of Nitrogen and Sulfur Synergistically Decrease the Release of Carbon and Nitrogen from Litter in a Subtropical Forest. Forests. 2020; 11(12):1280. https://doi.org/10.3390/f11121280
Chicago/Turabian StyleTie, Liehua, Rao Fu, Josep Peñuelas, Jordi Sardans, Shibin Zhang, Shixing Zhou, Junxi Hu, and Congde Huang. 2020. "The Additions of Nitrogen and Sulfur Synergistically Decrease the Release of Carbon and Nitrogen from Litter in a Subtropical Forest" Forests 11, no. 12: 1280. https://doi.org/10.3390/f11121280
APA StyleTie, L., Fu, R., Peñuelas, J., Sardans, J., Zhang, S., Zhou, S., Hu, J., & Huang, C. (2020). The Additions of Nitrogen and Sulfur Synergistically Decrease the Release of Carbon and Nitrogen from Litter in a Subtropical Forest. Forests, 11(12), 1280. https://doi.org/10.3390/f11121280