1. Introduction
Scots pine (
Pinus sylvestris L.) is the most widely spread species in genus Pinus and economically it is a very important tree species in the Northern hemisphere. Its wood is used as building and construction wood, for furniture, with further processing as veneer, fiberboards, or chipboards; and to some extent also for pulp and paper production [
1]. As organic material, wood in construction is prone to deterioration due to attacks by microbes and fungi. Wood decay resistance has been increased by using super effective wood preservation chemicals, but in recent years, their application has been legally restricted due to the increasing concern about their environmental impacts [
2]. This has raised interest in the natural durability of construction wood, in which chemical properties of Scots pine heartwood are considered important.
Heartwood is transformed from the inner layers of the ageing sapwood and is a normally occurring part of the xylem in mature trees [
3,
4]. In Scots pine, the formation of heartwood is related to the constitutive synthesis of phenolic compounds, stilbenes, and accumulation of resin acids [
5]. Scots pine heartwood timber is generally quite durable against brown rot decay caused by cellar fungus
Coniophora puteana (Schum. ex Fr.), but the resistance varies widely [
6,
7], which is connected to the variation in the content of phenolic compounds, mainly stilbenes [
8,
9,
10,
11,
12,
13]. The variation in heartwood stilbene content between individual trees has been found to be highly inherited [
14,
15,
16], and depending on the application it offers a possibility to direct breeding for special Scots pine lines producing higher or lower content of stilbenes in their heartwood. An obstacle in breeding the chemical quality of heartwood is the late age of the beginning of heartwood formation, which delays the selection for several decades, making it practically unprofitable.
The solution for developing tools for early testing might arise from the fact that stilbenes are stress-inducible defense compounds as well. Various types of tissues in Scots pine seedlings have been found to activate stilbene synthesis and produce stilbenes in response to, e.g., ultraviolet irradiation [
17,
18], ozone fumigation [
18,
19], fungal infection [
20,
21], or mechanical wounding [
22,
23]. In the study [
22] of Harju et al., there was indication that the mechanically induced production of phenolic stilbenes in the seedlings (
h2 = 0.62) was related to the developmentally programmed content of stilbenes in the heartwood of their mature maternal parents. Thus, methods for early selection by combining the phenotypic measurements of parent trees to the measurements of their young progenies could possibly be developed.
To get selected material, e.g., high-phenolic Scots pine genotypes, available for forest regeneration, vegetative propagation could be utilized instead of traditional seed production in seed orchards. In conifers, somatic embryogenesis (SE) is expected to be a highly potential propagation method tool due to its high multiplication rate and the maintenance of material’s juvenility via cryopreservation allowing, e.g., early testing of the materials [
24]. In Scots pine, however, there is wide variation among donor trees in their SE initiation frequency, and the embryo production capacity varies among the genotypes, as reviewed by [
25]. SE is a complicated process controlled by complex regulatory networks that remains partly unrevealed but is known to be affected by genetic and physiological factors [
26]. Production of phenolic compounds involved in defense reactions has been suggested to interfere with SE process [
27,
28]. Due to the connection between constitutive and mechanically induced content of stilbenes in Scots pine wood tissue [
22], effects of mechanical excision of explants during SE initiation may differ among parent trees and be further reflected in the success in SE.
The prospect of using SE for producing Scots pine forest regeneration material having a high tendency to synthetize stilbenes in their heartwood exists only if there is no trade-off between SE propagation and capacity for high stilbene synthesis. We hypothesize that in the case, where there is no trade-off, there is no difference in SE initiation rate, culture proliferation, somatic embryo production capacity, or embling performance between parent genotypes having high or low stilbene content in their heartwood.
The aim of this pilot study was to examine whether the inherent capacity for high stilbene synthesis of parent trees interferes with SE-plant production in Scots pine. Furthermore, we studied the content of stilbenes during the SE propagation to evaluate whether detectable differences in SE progenies can be already found at this stage. As far as we know, there are no studies where SE has been initiated and emblings regenerated from Scots pine parent trees according to their stilbene content.
2. Materials and Methods
2.1. Selection of Parent Genotypes and Their Controlled Crossings for Explant Production
Parent genotypes were selected in 2011 among 37-year-old grafts growing in a clonal collection of Scots pine in Punkaharju (61°48′ N and 29°19′ E, 90 m a.s.l.), Finland. The selection was based on the previously determined content of total phenolics in the heartwood of 23 genotypes using Folin Ciocalteu (FC) assay (unpublished data). FC assay had been found to predict well the Scots pine heartwood decay resistance against cellar fungus [
10]. For phenolic analysis, in March 2004, increment cores 5 mm in diameter had been drilled from stochastic directions to the pith of the stem, from two grafts in each genotype. Increment cores from the height of 130 cm included both sapwood and heartwood. The boundaries between them were marked with pencil immediately after drilling based on their moisture difference. The mean number of heartwood annual rings was 11.3 (SD = 2.6). From each increment core, on average 31-mm-long (SD = 4.6 mm) heartwood specimens were sampled and dried in +60 °C oven for 24 h, after which they were ground with an analysis mill (Kinematica AG, Malters, Switzerland) for FC assay to determine the content of total phenolic compounds. The heartwood specimen contained 4.5 annual rings on an average (SD = 0.7) and they were the annual rings from 5 to 8 counted as precisely as possible from the pith.
The protocol of FC assay was described in [
10,
29]. A 100 mg sample of milled heartwood was extracted with 5 mL of 80% (
v/
v) aqueous acetone for 30 min and was washed two times for 5 min (see [
30]). The absorption was measured at 735 nm using tannic acid as a standard, and thus, the results were expressed as tannic acid equivalents (TAE) per gram dry mass of wood.
The content of total phenolics in the heartwood samples of the selected six genotypes in 2004 are presented in
Table 1. These results were used later to plan reciprocal controlled crosses that were performed in 2011 within low-phenolic-content (genotypes K917, K699, and K927) and high-phenolic-content (genotypes K836, K912, and K803) grafted parents (
Table 1) using forced pollen. Due to poor pollen production in 2011, the two controlled crosses with clone K699 pollen were performed in 2012.
2.2. SE Initiation
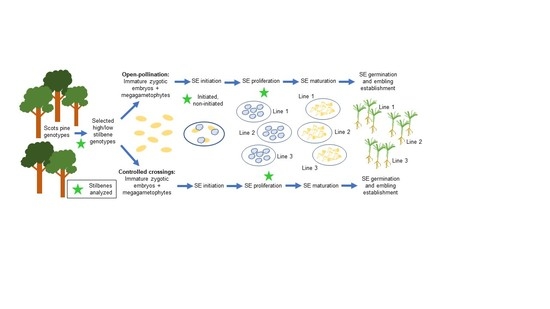
One-year-old immature seed cones were collected from the six selected Scots pine parent genotypes, in 2011 following open-pollination, and in 2012–2013 from controlled crossings, as described in
Table 1. Every year, the cones were collected during several sequential stages, when the temperature sum was between 480 and 700 d.d. (degree days, temperature sum with a threshold of +5 °C). The cones were stored in the cold room (+2 °C) until used for tissue culture initiation within the following month.
SE initiations were performed according to [
31]. In brief, immature seeds removed from the cones were surface-sterilized for 5 min in 70% ethanol and rinsed three times with sterile water, after which the immature zygotic embryos (ZE) surrounded by megagametophyte were put on initiation medium. Initiations were then kept without subculturing in the dark at room temperature for 10 weeks, followed by examination and picking up induced embryogenic tissues onto proliferation medium.
In 2011, the cone collections were performed at the following d.d.s: 480 (28.6), 526 (1.7), 576 (5.7), 623 (8.7), and 693 (12.7). Both DCR- [
32,
33] medium containing 13.5 µM 2,4-D and 2.2 µM BA and LM- [
34] medium containing 2.2 µM 2,4-D and 2.3 µM BA were used for SE initiation. The DCR medium was solidified with 2.5 g/L Phytagel, and the LM medium with 4 g/L Phytagel, and both media contained 30 g/L sucrose. Thirty explants per maternal genotype and collection time was put on each medium, i.e., altogether 1800 initiations were made.
In 2012, the cones from the 2011 controlled crossings were collected when the d.d. was 554 (9.7), 593 (12.7)., 638 (16.7), and 665 (19.7). Only LM-medium was used, and the number of explants per genotype and collection time varied from 50 to 120, the total number of initiations being 2645. A part of explants, i.e., 465, 30–245 per a collection time, was treated slightly differently, i.e., the megagametophyte surrounding the ZE was cut into halves, trying not to harm the ZE that was picked up after a few days and transferred directly onto SE initiation medium.
In 2013, the cones from the 2012 controlled crossings were collected at the d.d. 550 (27.6), 600 (30.6), and 650 (3.7). As in 2012, only LM medium was used, and 100 explants per genotype and collection time were subjected for SE initiation, the total number of initiations being 600.
2.3. SE Proliferation, Maturation, Embryo Germination, and Conversion
Embryogenic tissues that continued to grow on proliferation media following excision from the explants were considered as established embryogenic cultures (ECs). Proliferation of ECs was performed according to [
31]: the ECs initiated on DCR medium were cultured as small tissue clumps on DCR medium containing 9.1 µM 2,4-D and 2.2 µM BA and 30 g/L sucrose, solidified with 2.5 g/L of Phytagel, and, respectively, the ECs initiated on LM medium were cultured on the same LM medium as used for initiation. Subculturing onto fresh medium was done in 2-week intervals by dividing the tissue clumps into smaller pieces and discarding the brownish inner parts (if any). The proliferation rate of ECs was measured with 32 SE lines originating in the controlled crossings and growing on LM medium. The tissue was weighed, transferred onto a fresh medium, and reweighted after 2 weeks of proliferation. The achieved weight was divided by starting weight to get the growth factor. For each line, three replicates were weighted, and the 2-week proliferation period repeated twice (first subculture and second subculture).
Maturation of somatic embryos was performed according to [
34] on LM maturation medium containing 80 µM abscisic acid (ABA) and 0.2 M sucrose, solidified with 10 g/L of Phytagel. Briefly, after one week from the last subculture, approximately 150–200 mg of EC was weighed, suspended in liquid LM medium, and spread onto filter paper using Büchner funnel and suction, as described by [
25], preparing 3–5 replicates per genotype. Following eight weeks on maturation medium, the number of mature cotyledonary somatic embryos was counted, and the embryos picked up for germination.
Germination took place according to [
25], i.e., the embryos were placed horizontally on DCR-based medium, MB5, containing 0.09 M sucrose and 2 g/L Phytagel without growth regulators, in the dark at +22 °C. Germinating embryos with a developing root were transferred into a vertical position, with the root in the medium, onto modified half-strength DCR medium, MB6, containing 0.06 M sucrose, 250 mg/L L-glutamine, and 10 g/L agar in glass jars, and grown under a 16/8 h light/dark photoperiod, under the cool white fluorescent lamps, with 45–75 µmol m
−2 s
−1, at 22 °C. Following the cultivation in glass jars, the germinants were potted in peat:perlite (1:1) and grown in the greenhouse as described by Aronen et al. (2009), 0.2% Taimi Superex (NPK: 19-4-20, manufactured by Kekkilä LtD., Vantaa, Finland) being used as fertilizer, and Entonem (Koppert, Berkel en Rodenrijs, The Netherlands) for controlling dark-winged fungus gnats (Sciaridae) and Mogeton WP (Agro-Kanesho Co., Ltd., Tokyo, Japan) for controlling moss and liverwort, both according to the manufacturer’s instructions. The survival of the germinants at the greenhouse was observed, and the height of the living ones measured at the end of the first growing season.
2.4. Chemical Analysis of SE Lines
Samples for stilbene analysis were collected (1) from parent heartwood (increment cores sampled in 2012), (2) from initiated and non-initiated explants originating from open pollination and their proliferating ECs, and (3) from proliferating ECs from the controlled crossings, and freeze-dried. Within each maternal genotype, the explants originating from open pollination were pooled into a few separate batches according to their initiation success. The pooling was partly done according to the cone collection d.d., but some of the batches included explants from all the collection dates. Only part of each batch was included in the stilbene analysis.
Samples (20–40 mg) were homogenized in 600 μL of ice-cold methanol for 30 s at 5500 g with a Precellys homogenizer (Bertin Instruments, Montigny-le-Bretonneux, France), incubated in an ice bath for 15 min before re-homogenization (according to [
35]). After that, the samples were centrifuged at +4 °C for 3 min at 13,000×
g using an Eppendorf 5415R Centrifuge (Marshall Scientific, Hampton, NH, USA). The extraction process was repeated three times. The combined extracts were evaporated to dryness in a vacuum centrifuge Eppendorf 270 Concentrator (Merk KGaA, Darmstad, Germany). The dried extracts were re-dissolved in 600 μL of methanol-water (50:50,
v/
v) and run with the high-performance liquid chromatography (HPLC). The injected volume was 10 μL for each sample. The HPLC system used (Series 1100, Agilent, City of Waldbronn, Germany) was equipped with a binary pump (G1312A), an ALS autosampler (G1329A), a vacuum degasser (G1322A), a column compartment (G1316A) with a reverse-phase column (Zorbax SB-C18, 4.6 × 75 mm, particle size 3.5 μm, Agilent), and a diode array detector (G1315B). Eluent A (1.5% tetrahydrofuran and 0.25% orthophosphoric acid in Milli-Q ultrapure water) and eluent B (100% methanol) constituted the mobile phase with a flow rate of 2 mL min
−1. The following gradient was used for eluent A: 0–5 min 100%; 5–40 min 100–50%; 40–60 min 50%; and 60–62 min 50–100%. The injector and column temperatures were set at +22 °C and +30 °C, respectively.
HPLC runs were monitored at 220, 270, 320, and 360 nm wavelengths. Retention times and UV-spectra were used for identifying the pinosylvin (PS) and pinosylvin monomethyl ether (PSM). The compounds were quantified (mg/g, on a dry weight basis) against commercial standards. The total concentration of stilbenes (STB) was calculated as a sum of the concentrations of PS and PSM.
Stilbene analysis of parent heartwood revealed that genotype K803 had to be reclassified as low stilbene genotype because of the low content of PS and PSM (
Table 1). Reclassification occurred after controlled crossings had been performed, which resulted in four classes of parent combinations (High × High, High × Low, Low × High, Low × Low) instead of planned two.
2.5. Statistical Analysis
Multiplication rate and embryo production capacity of the ECs, somatic embryo germination percentage, greenhouse survival of the germinants, and height of the emblings following the first growing season at the greenhouse was studied by non-parametric Kruskall–Wallis test. the connection between stilbene content of the ECs and the other measured traits was examined by Spearman’s rank-order correlation. All the statistical analyses were performed using IBM SPSS Statistics 27.0 (Armonk, NY, USA).
4. Discussion
This pilot study reported the results of somatic embryogenesis (SE) from Scots pine trees selected for a specific trait of the end-use interest of timber, i.e., stilbene content of their heartwood. As far as we know, this study was the first of its kind. The results suggest that SE propagation success and the trait of interest, stilbene content of the parent heartwood, are independent of each other, although many factors, including parent genotypes, were found to affect the success of SE, especially at the initiation phase.
The genotypes for this study were selected from a local Scots pine graft collection. The selection was based on the analysis of their heartwood total phenolic content, performed several years earlier, which was determined using a non-selective, low-cost, relatively fast, and easy spectrophotometric Folin Ciocalteu (FC) assay. The result of FC assay from Scots pine heartwood had been found to correlate well with the decay resistance against cellar fungus,
C. puteana [
9,
10]. The main phenolic compounds in Scots pine heartwood are stilbenes pinosylvin and pinosylvin monomethyl ether [
36]. The later-performed HPLC analysis of the heartwood stilbenes found that one of the studied genotypes (K803) was misclassified to be a high stilbene producing genotype. Thus, in future studies the selection of parents should be based on more detailed analysis of heartwood extractives instead of FC assay.
Generally, SE initiation success in Scots pine is relatively low compared with many other conifers, with published initiation rates varying from 0.2 to 30% for open-pollinated explant materials [
31,
37,
38,
39,
40,
41], and from 1 to 42% for controlled crossings, even with selection for their SE propagation ability [
34,
42]. Low initiation frequencies were also realized in this study. In open-pollinated material, the initiation rate was somewhat higher for maternal genotypes having a low compared to the high content of constitutive stilbenes in their heartwood; the average initiation rate was 6% compared to 2%, respectively. SE propagation was, however, possible also from genotypes having a high tendency to produce constitutive stilbenes in their heartwood, as demonstrated in one of the controlled crossings of high stilbene parents having SE initiation frequency of almost 8%.
When examining the factors affecting SE initiation, the present results support the earlier Scots pine studies for genotype, d.d. sum, and medium effects. Initiation frequencies varied among the present parent tree genotypes, the K803 being the best maternal genotype both in open-pollinated material and in controlled crossings, and the K836 was the best paternal genotype. Previously, both maternal and paternal effects had been found significant, the maternal effects considered greater than paternal [
31,
34,
42]. With the present material, the highest numbers of initiated ECs were seen when the cones containing the explants were collected either at 526 or 576 (2011) or 593 (2012) d.d., that is, in the middle of the period recommended in the earlier studies, i.e., 400–650 d.d. [
31,
40]. Basal medium had also been shown to influence SE initiation significantly: In [
43], 16% initiation was obtained on LM medium compared with 6% on DCR, whereas the corresponding figures in the present study were approximately 7% on LM and 2% on DCR.
The growth of the established ECs in the present study was in line with earlier studies: 2-week multiplication rates observed for the present ECs were around 4–4.5×, whereas for 6-week multiplication including two subcultures, rates from 5× to 13× depending on tissue clump size had been reported, and much higher rates, i.e., 24–52× in six weeks achieved with ECs spread on filter papers [
31]. Of the present embryogenic lines, 62% of the ones with the open-pollinated origin and 82% of the ones from controlled crossings were able to produce mature somatic embryos, close to previously reported proportions, 70–95% [
31,
34]. Likewise, the huge variation found in the number of somatic embryos produced per gFW among the genotypes is known from the earlier studies [
31,
34,
42].
In Scots pine SE, both normal and abnormal cotyledonary embryos are produced, with embryogenic lines varying in this aspect and showing differences in degeneration of embryos [
44]. Further, somatic embryo germination and greenhouse survival rates are known to vary depending on both maturation and germination conditions, as well as the quality of the picked-up embryos. Using the present methods, over 90% survival has been achieved with top-quality embryos, whereas applying the same methods for inferior, stub-type embryos may result in less than 10% survival [
31]. Thus, the present results with the average germination rate of 32% for the open-pollinated material and 53% for lines originating in the controlled crossings, and the overall greenhouse survival of 74% suggest that selection of the somatic embryos to be picked-up for germination was not completely successful in all cases. More emblings were lost during in vitro conversion than following acclimatization into the greenhouse, as also previously seen [
1].
There were great differences between parent trees in SE-initiation success, and thus the wide variation in the number of lines from each maternal parent or parent combinations (0–35, average 11.2, SD = 10.6). With the typically low and highly variable SE initiation rate, it was impossible to have equal sample sizes for stilbene analyses, which resulted in the chosen analysis scheme. It gave an idea of stilbene levels in different stages of SE procedure. When compared to the average stilbene content in mechanically wounded seedlings [
22] or to the constitutive contents in older trees [
16], the stilbene content in the explants was approximately 5–30%, depending on the maternal parent or crossing, and in the proliferating ECs it was approximately 2%. The low stilbene content in the ECs refers to low constitutive expression of defense genes in embryogenic tissue growing without any inducing agents.
Overall, no connection between the parent tree stilbene content and the proliferation of embryogenic cultures, embryo maturation, or embling performance could be shown in this study. Stilbenes measured from the explants showed, however, an interesting trend: The average stilbene content both in the initiated and non-initiated explants decreased with increasing d.d. sum, with the exception for non-initiated ones in the last collection time. This phenomenon can be related to the size of developing explants that are very tiny at the earliest collection dates and thus easily wounded, the preparation process becoming easier with time. In Scots pine, stilbene synthesis is known to be induced by mechanical wounding [
22,
23], explaining the higher stilbene content of smaller and probably more wounded explants. Experience in preparation work also plays an important role in its success: e.g., the higher stilbene content of non-initiated explants in the last collection time deviating from the overall trend could be partly due to increased wounding during preparation work.
Pine SE cultures have been studied previously for their chemical content, but these studies focused mostly on carbohydrates and storage proteins [
45,
46], as well as polyamines [
41,
47] related to oxidative cellular stress. Phenolic substances, if studied, have been found to be typical for non-embryogenic tissue in comparison with embryogenic one, as found in
Pinus koraiensis Sieb. et Zucc. [
48]. Likewise, when proteomics during prolonged culture of two embryogenic cell lines of
Pinus nigra Arn. were studied [
49], pinosylvin-forming stilbene synthase was observed after the lines had lost their embryogenic capacity. However, the pinosylvin content of these ECs was not analyzed. Non-embryogenic, ageing pine calli, however, have been indicated to produce stilbenes up to amounts high enough for stilbene extraction and suggested production of biocides against nematodes [
50]. Induction of stilbene synthesis and stilbene accumulation has also been shown in non-embryogenic
P. sylvestris cell suspension culture by applying an elicitor from a fungal pine needle pathogen [
51].