Increased Levels of Txa2 Induced by Dengue Virus Infection in IgM Positive Individuals Is Related to the Mild Symptoms of Dengue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Aspects
2.2. Study Population
2.3. Eicosanoids Quantification
2.4. Immunofluorescence Staining
2.5. mRNA Quantification
2.6. Dengue Virus Load Quantification
2.7. Analyses of Vacuolated Monocytes
2.8. Statistical Analysis
3. Results
3.1. Dengue Diagnosis
3.2. Plasma from IgM-Positive Individuals Has Increased Levels of TXA2
3.3. Monocytes from IgM-Negative Individuals Displayed Increased Levels of LBs
3.4. Expression Levels of COX-2 and TXA2S Genes Were Lower in Leukocytes from IgM-Positive Individuals
3.5. The Dengue Virus Load Was Higher in Plasma from IgM-Negative Patients
3.6. An Increased Percentage of Vacuolated Monocytes Was Observed in IgM-Negative Individuals
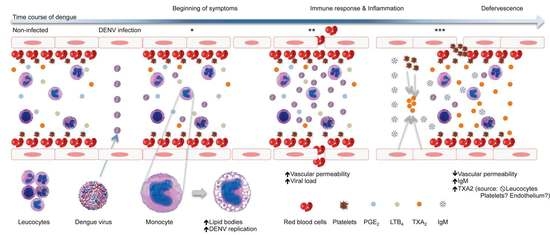
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| COX | Cyclooxygenase |
| LOX | Lipoxygenase |
| TXA2 | thromboxane A2 |
| PGE2 | prostaglandin E2 |
| LTB4 | leukotriene B4 |
| TXA2S | thromboxane A2 synthase |
| PGE2S | prostaglandin E2 synthase |
| LTA4H | leukotriene A4 hydrolase |
| COX-2 | cyclooxygenase-2 |
| 5-LOX | 5-lipoxygenase |
| DENV | Dengue virus |
| LB | lipid bodies |
| AA | arachidonic acid |
| PGH2 | prostaglandin H2 |
| MFI | median fluorescence intensity |
| N | Non-infected |
References
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue infection. Nat. Rev. Dis. Prim. 2016, 2, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.H.; Lee, L.K.; Lye, D.C.; Leo, Y.S. Current management of severe dengue infection. Expert Rev. Anti-Infect. Ther. 2017, 15, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Martina, B.E.E.; Koraka, P.; Osterhaus, A.D.M.E. Dengue Virus Pathogenesis: An Integrated View. Clin. Microbiol. Rev. 2009, 22, 564–581. [Google Scholar] [CrossRef] [PubMed]
- Malavige, G.N.; Ogg, G.S. Pathogenesis of vascular leak in dengue virus infection. Immunology 2017, 151, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Lannes, N.; Summerfield, A.; Filgueira, L. Regulation of inflammation in Japanese encephalitis. J. Neuroinflamm. 2017, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Meeran, H.; Messent, M. The systemic inflammatory response syndrome. Trauma 2001, 3, 89–100. [Google Scholar] [CrossRef]
- Harizi, H.; Corcuff, J.B.; Gualde, N. Arachidonic-acid-derived eicosanoids: Roles in biology and immunopathology. Trends Mol. Med. 2008, 14, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Preeyasombat, C.; Treepongkaruna, S.; Sriphrapradang, A.; Choubtum, L. The role of prostacyclin (PGI2) and thromboxane A2 (TXA2) in pathogenesis of dengue hemorrhagic fever (DHF). J. Med. Assoc. Thail. 1999, 82, 16–21. [Google Scholar]
- Loke, W.M.; Chow, A.Y.; Lam, K.; Sing, M.; Lee, C.J.; Halliwell, B.; Lim, E.C.H.; Quek, A.M.L.; Ooi, E.E.; Seet, R.C.S. Augmentation of 5-lipoxygenase activity and expression during dengue serotype-2 infection. Virol. J. 2013, 10, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.K.; Tseng, C.K.; Wu, Y.H.; Liaw, C.C.; Lin, C.Y.; Huang, C.H.; Chen, Y.H.; Lee, J.C. Cyclooxygenase-2 facilitates dengue virus replication and serves as a potential target for developing antiviral agentes. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Bozza, P.T.; Bakker-abreu, I.; Navarro-xavier, R.A.; Bandeira-melo, C. Lipid body function in eicosanoid synthesis: An update. Prostaglandins Leukot. Essent. Fat. Acids 2011, 85, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Bozza, P.T.; Melo, R.C.N.; Bandeira-Melo, C. Leukocyte lipid bodies regulation and function: Contribution to allergy and host defense. Pharmacol. Ther. 2007, 113, 30–49. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.C.N.; D’Avila, H.; Wan, H.C.; Bozza, P.T.; Dvorak, A.M.; Weller, P.F. Lipid Bodies in Inflammatory Cells: Structure, Function, and Current Imaging Techniques. J. Histochem. Cytochem. 2011, 59, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Herker, E.; Ott, M. Emerging Role of Lipid Droplets in Host/Pathogen Interactions. J. Biol. Chem. 2012, 287, 2280–2287. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.A.; Carneiro, F.A.; Martins, I.C.; Assunção-Miranda, I.; Faustino, A.F.; Pereira, R.M.; Bozza, P.T.; Castanho, M.A.R.B.; Mohana-Borges, R.; et al. Dengue Virus Capsid Protein Binding to Hepatic Lipid Droplets (LD) is Potassium Ion Dependent and is Mediated by LD Surface Proteins. J. Virol. 2012, 86, 2096–2108. [Google Scholar] [CrossRef] [PubMed]
- Jindal, A.; Bruzzì, S.; Sutti, S.; Locatelli, I.; Bozzola, C.; Paternostro, C.; Parola, M.; Albano, E. Fat-laden macrophages modulate lobular inflammation in nonalcoholic steatohepatitis (NASH). Exp. Mol. Pathol. 2015, 99, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, J.A.; Hernandez, J.P.; Valero, N.; Espina, L.M.; Añez, G.J. Ultrastructural studies on dengue virus type 2 infection of cultured human monocytes. Virol. J. 2005, 2, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; Fitzgerald, G.A. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. 2011, 31, 986–1001. [Google Scholar] [CrossRef] [PubMed]
- Hedi, H.; Norbert, G. 5-Lipoxygenase Pathway, Dendritic Cells, and Adaptive Immunity. J. Biomed. Biotechnol. 2004, 2004, 99–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Applied Biosystems. Relative Quantitation Using Comparative C. 2006. Available online: papers3://publication/uuid/BDBB14AF-36A9-4B7B-875F-4E9A02186C90.pdf (accessed on 11 May 2017).
- Pacidônio, E.C.; Caragata, E.P.; Alves, D.M.; Marques, J.T.; Moreira, L.M. The impact of Wolbachia infection on the rate of vertical transmission of dengue virus in Brazilian Aedes aegypti. Parasites Vectors 2017, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Molina-cruz, A.; Salazar, M.I.; Black, W. Quantitative Analysis of Dengue-2 Virus RNA during the Extrensic Incubation Period in Individual Aedes aegypti. Am. J. Trop. Med. Hyg. 2006, 74, 132–141. [Google Scholar] [PubMed]
- Oliveira, E.S. Imunologia Celular e Molecular, LB in Different Populations of Leukocytes of Dengue Patients; Instituto René Rachou: Belo Horizonte, Brazil, 2018. [Google Scholar]
- Simmons, C.P.; Mcpherson, K.; van Vinh, N.; Tam, D.T.H.; Young, P.; Mackenzie, J.; Wills, B. Recent advances in dengue pathogenesis and clinical management. Vaccine 2015, 33, 7061–7068. [Google Scholar] [CrossRef] [PubMed]
- Dorn, G.W.; Becker, M.W. Thromboxane A2 stimulated signal transduction in vascular smooth muscle. J. Pharmacol. Exp. Ther. 1993, 265, 447–456. [Google Scholar] [PubMed]
- Hoggatt, J.; Pelus, L.M. Eicosanoid regulation of hematopoiesis and hematopoietic stem and progenitor trafficking. Leukemia 2010, 24, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Remuzzi, G.; FitzGerald, G.; Patrono, C. Thromboxane synthesis and action within the kidney. Kidney Int. 1992, 41, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, P. Regulation of Immune Responses by Prostaglandin E2. J. Immunol. 2012, 27, 339–351. [Google Scholar] [CrossRef] [PubMed]
- White, R.K.; Montgomery, S. Leukotrienes: Inflammatory mediators—A review. Oral Surg. Oral Med. Oral Pathol. 1986, 61, 514–518. [Google Scholar] [CrossRef]
- Samsa, M.M.; Mondotte, J.; Iglesias, N.G.; Assunção-Miranda, I.; Barbosa-Lima, G.; Da Poian, A.T.; Bozza, P.T.; Gamarnik, A.V. Dengue Virus Capsid Protein Usurps Lipid Droplets for Viral Particle Formation. PLoS Pathog. 2009, 5. [Google Scholar] [CrossRef] [PubMed]
- Assunção-Miranda, I.; Amaral, F.; Bozza, F.; Fagundes, C.T.; Sousa, L.P.; Souza, D.G.; Pacheco, P.; Barbosa-Lima, G.; Gomes, R.N.; Bozza, P.T.; et al. Contribution of macrophage migration inhibitory fator to the pathogenesis of dengue virus infection. FASEB J. 2010, 24, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.; Chen, W.; Balakrishnan, T.; Toh, Y.X.; Fink, K.; Wong, S.C. Susceptibility and Response of Human Blood Monocyte Subsets to Primary Dengue Virus Infection. PLoS ONE 2012, 7, e36435. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.; Nandi, D.; Batra, H.; Singhal, R.; Annarapu, G.K.; Bhattacharyya, S.; Seth, T.; Dar, L.; Medigeshi, G.R.; Vrati, S.; et al. Platelet activation determines the severity of thrombocytopenia in dengue infection. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, N.A.; Mackow, E.R. Roles for Endothelial Cells in Dengue Virus Infection. Adv. Virol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, F.M.; Upchurch, G.R.; Keagy, B.A.; Johnson, G. Endothelial Cell Thromboxane Production and Its Inhibition by a Calcium-Channel Blocker. Ann. Thorac. Surg. 1990, 49, 916–919. [Google Scholar] [CrossRef]
- Rocca, B.; Secchiero, P.; Ciabattoni, G.; Ranelletti, F.O.; Catani, L.; Guidotti, L.; Melloni, E.; Maggiano, N.; Zauli, G.; Patrono, C. Cyclooxygenase-2 expression is induced during human megakaryopoiesis and caracterizes newly formed platelets. Proc. Natl. Acad. Sci. USA 2002, 99, 7634–7639. [Google Scholar] [CrossRef] [PubMed]
- Rocca, B.; Secchiero, P.; Celeghini, C.; Ranelletti, F.O.; Ciabattoni, G.; Maggiano, N.; Habib, A.; Ricerca, B.M.; Barbarotto, E.; Patrono, C.; et al. Modulation of the expression and activity of cyclooxygenases in normal and accelerated erythropoiesis. Exp. Hematol. 2004, 32, 925–934. [Google Scholar] [CrossRef] [PubMed]






| Volunteer | RT-qPCR | ELISA NS1 | ELISA IgM |
|---|---|---|---|
| 1 | + | + | + |
| 2 | + | − | − |
| 3 | − | − | + |
| 4 | + | + | + |
| 5 | + | + | − |
| 6 | − | − | + |
| 7 | − | − | + |
| 8 | + | + | − |
| 9 | + | + | − |
| 10 | − | + | − |
| 11 | − | + | + |
| 12 | + | + | − |
| 13 | − | + | + |
| 14 | − | + | − |
| 15 | − | + | + |
| 16 | + | + | + |
| 17 | − | + | + |
| 18 | + | + | + |
| 19 | + | + | − |
| 20 | + | + | − |
| 21 | + | − | + |
| 22 | − | − | + |
| 23 | + | + | − |
| 24 | + | + | − |
| 25 | + | + | − |
| 26 | − | − | + |
| 27 | + | + | + |
| 28 | + | − | + |
| 29 | − | − | − |
| 30 | − | − | − |
| 31 | − | − | − |
| 32 | − | − | − |
| 33 | − | − | − |
| 34 | − | − | − |
| 35 | − | − | − |
| 36 | − | − | − |
| 37 | − | − | − |
| 38 | − | − | − |
| 39 | − | − | − |
| 40 | − | − | − |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, E.S.; Colombarolli, S.G.; Nascimento, C.S.; Batista, I.C.A.; Ferreira, J.G.G.; Alvarenga, D.L.R.; De Sousa, L.O.B.; Assis, R.R.; Rocha, M.N.; Alves, É.A.R.; et al. Increased Levels of Txa2 Induced by Dengue Virus Infection in IgM Positive Individuals Is Related to the Mild Symptoms of Dengue. Viruses 2018, 10, 104. https://doi.org/10.3390/v10030104
Oliveira ES, Colombarolli SG, Nascimento CS, Batista ICA, Ferreira JGG, Alvarenga DLR, De Sousa LOB, Assis RR, Rocha MN, Alves ÉAR, et al. Increased Levels of Txa2 Induced by Dengue Virus Infection in IgM Positive Individuals Is Related to the Mild Symptoms of Dengue. Viruses. 2018; 10(3):104. https://doi.org/10.3390/v10030104
Chicago/Turabian StyleOliveira, Eneida S., Stella G. Colombarolli, Camila S. Nascimento, Izabella C. A. Batista, Jorge G. G. Ferreira, Daniele L. R. Alvarenga, Laís O. B. De Sousa, Rafael R. Assis, Marcele N. Rocha, Érica A. R. Alves, and et al. 2018. "Increased Levels of Txa2 Induced by Dengue Virus Infection in IgM Positive Individuals Is Related to the Mild Symptoms of Dengue" Viruses 10, no. 3: 104. https://doi.org/10.3390/v10030104
APA StyleOliveira, E. S., Colombarolli, S. G., Nascimento, C. S., Batista, I. C. A., Ferreira, J. G. G., Alvarenga, D. L. R., De Sousa, L. O. B., Assis, R. R., Rocha, M. N., Alves, É. A. R., & Calzavara-Silva, C. E. (2018). Increased Levels of Txa2 Induced by Dengue Virus Infection in IgM Positive Individuals Is Related to the Mild Symptoms of Dengue. Viruses, 10(3), 104. https://doi.org/10.3390/v10030104