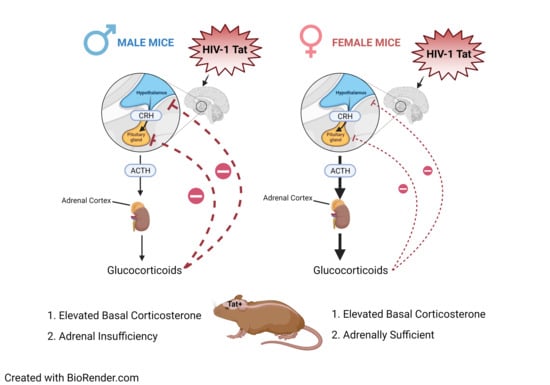
HIV-1 Tat Protein Promotes Neuroendocrine Dysfunction Concurrent with the Potentiation of Oxycodone’s Psychomotor Effects in Female Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects & Housing
2.2. Surgical Manipulation
2.3. Chemicals
2.4. Determination of Estrous Cycle Phase
2.5. Behavioral Assays
2.5.1. Forced Swim Stress
2.5.2. Open Field Test
2.5.3. Light-Dark Transition Test
2.6. Biochemical Assays
2.6.1. Tissue Collection
2.6.2. Enzyme-Linked Immunosorbant Assay (ELISA)
2.6.3. Ultra-Performance Liquid Chromatography (UPLC)-Mass Spectrometry (MS)
2.7. Procedure
2.8. Statistical Analyses
3. Results
3.1. HIV-1 Tat and Oxycodone-Mediated Psychostimulation Is Moderated by Stress and Estrous Cycle
3.2. Gonadal Steroids Are Necessary for Tat to Potentiate Oxycodone-Mediated Psychostimulation
3.3. Tat, Oxycodone, and Gonadal Steroids Interact to Influence Circulating Steroids
3.4. Acute Oxycodone Interacted with Tat Exposure to Influence Hypothalamic Allopregnanolone
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wallet, C.; De Rovere, M.; Van Assche, J.; Daouad, F.; De Wit, S.; Gautier, V.; Mallon, P.W.G.; Marcello, A.; Van Lint, C.; Rohr, O.; et al. Microglial Cells: The Main HIV-1 Reservoir in the Brain. Front. Cell. Infect. Microbiol. 2019, 9, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nath, A.; Anderson, C.; Jones, M.; Maragos, W.; Booze, R.; Mactutus, C.; Bell, J.; Hauser, K.F.; Mattson, M. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J. Psychopharmacol. 2000, 14, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.; Hauser, K.F.; Wojna, V.; Booze, R.M.; Maragos, W.; Prendergast, M.; Cass, W.; Turchan, J.T. Molecular basis for interactions of HIV and drugs of abuse. J. Acquir. Immune Defic. Syndr. 2002, 31, S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.B.; Kaul, M. Neuronal Stress and Injury Caused by HIV-1, cART and Drug Abuse: Converging Contributions to HAND. Brain Sci. 2017, 7, 25. [Google Scholar] [CrossRef]
- Saylor, D.; Dickens, A.M.; Sacktor, N.; Haughey, N.; Slusher, B.; Pletnikov, M.; Mankowski, J.L.; Brown, A.; Volsky, D.J.; McArthur, J.C. HIV-associated neurocognitive disorder-pathogenesis and prospects for treatment. Nat. Rev. Neurol. 2016, 12, 309. [Google Scholar] [CrossRef] [Green Version]
- McArthur, J.C.; Steiner, J.; Sacktor, N.; Nath, A. Human immunodeficiency virus-associated neurocognitive disorders: Mind the gap. Ann. Neurol. 2010, 67, 699–714. [Google Scholar] [CrossRef]
- Jeevanjee, S.; Penko, J.; Guzman, D.; Miaskowski, C.; Bangsberg, D.R.; Kushel, M.B. Opioid analgesic misuse is associated with incomplete antiretroviral adherence in a cohort of HIV-infected indigent adults in San Francisco. AIDS Behav. 2014, 18, 1352–1358. [Google Scholar] [CrossRef] [Green Version]
- Merlin, J.S.; Tamhane, A.; Starrels, J.L.; Kertesz, S.; Saag, M.; Cropsey, K. Factors Associated with Prescription of Opioids and Co-prescription of Sedating Medications in Individuals with HIV. AIDS Behav. 2016, 20, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Silverberg, M.J.; Ray, G.T.; Saunders, K.; Rutter, C.M.; Campbell, C.I.; Merrill, J.O.; Sullivan, M.D.; Banta-Green, C.J.; Von Korff, M.; Weisner, C. Prescription long-term opioid use in HIV-infected patients. Clin. J. Pain 2012, 28, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Canan, C.E.; Chander, G.; Monroe, A.K.; Gebo, K.A.; Moore, R.D.; Agwu, A.L.; Alexander, G.C.; Lau, B.; HIV Research Network. High-Risk Prescription Opioid Use among People Living with HIV. J. Acquir. Immune Defic. Syndr. 2018, 78, 283–290. [Google Scholar] [CrossRef]
- Brunet, L.; Napravnik, S.; Heine, A.D.; Leone, P.A.; Eron, J.J. Brief Report: Longitudinal Opioid Use among HIV-Infected Patients, 2000 to 2014. J. Acquir. Immune Defic. Syndr. 2017, 75, 77–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, P.J.; Pontones, P.; Hoover, K.W.; Patel, M.R.; Galang, R.R.; Shields, J.; Blosser, S.J.; Spiller, M.W.; Combs, B.; Switzer, W.M.; et al. Indiana HIV Outbreak Investigation Team. HIV Infection Linked to Injection Use of Oxymorphone in Indiana, 2014–2015. N. Engl. J. Med. 2016, 375, 229–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henderson, L.J.; Johnson, T.P.; Smith, B.R.; Reoma, L.B.; Santamaria, U.A.; Bachani, M.; Demarino, C.; Barclay, R.A.; Snow, J.; Sacktor, N.; et al. Presence of Tat and transactivation response element in spinal fluid despite antiretroviral therapy. AIDS 2019, 33, S145–S157. [Google Scholar] [CrossRef] [PubMed]
- Hudson, L.; Liu, J.; Nath, A.; Jones, M.; Raghavan, R.; Narayan, O.; Male, D.; Everall, I. Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J. Neurovirol. 2000, 6, 145–155. [Google Scholar] [CrossRef]
- Mousseau, G.; Valente, S.T. Role of Host Factors on the Regulation of Tat-Mediated HIV-1 Transcription. Curr. Pharm. Des. 2017, 23, 4079–4090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitting, S.; Zou, S.; Chen, W.; Vo, P.; Hauser, K.F.; Knapp, P.E. Regional heterogeneity and diversity in cytokine and chemokine production by astroglia: Differential responses to HIV-1 Tat, gp120, and morphine revealed by multiplex analysis. J. Proteome Res. 2010, 9, 1795–1804. [Google Scholar] [CrossRef] [Green Version]
- King, J.E.; Eugenin, E.A.; Buckner, C.M.; Berman, J.W. HIV tat and neurotoxicity. Microbes Infect. 2006, 8, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Fitting, S.; Knapp, P.E.; Zou, S.; Marks, W.D.; Bowers, M.S.; Akbarali, H.I.; Hauser, K.F. Interactive HIV-1 Tat and morphine-induced synaptodendritic injury is triggered through focal disruptions in Na+ influx, mitochondrial instability, and Ca2+ overload. J. Neurosci. 2014, 34, 12850–12864. [Google Scholar] [CrossRef] [Green Version]
- Haughey, N.J.; Nath, A.; Mattson, M.P.; Slevin, J.T.; Geiger, J.D. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J. Neurochem. 2001, 78, 457–467. [Google Scholar] [CrossRef]
- Hu, X.T. HIV-1 Tat-Mediated Calcium Dysregulation and Neuronal Dysfunction in Vulnerable Brain Regions. Curr. Drug. Targets 2016, 17, 4–14. [Google Scholar] [CrossRef] [Green Version]
- Norman, J.P.; Perry, S.W.; Kasischke, K.A.; Volsky, D.J.; Gelbard, H.A. HIV-1 trans activator of transcription protein elicits mitochondrial hyperpolarization and respiratory deficit, with dysregulation of complex IV and nicotinamide adenine dinucleotide homeostasis in cortical neurons. J. Immunol. 2007, 178, 869–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thangaraj, A.; Periyasamy, P.; Liao, K.; Bendi, V.S.; Callen, S.; Pendyala, G.; Buch, S. HIV-1 TAT-mediated microglial activation: Role of mitochondrial dysfunction and defective mitophagy. Autophagy 2018, 14, 1596–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paris, J.J.; Liere, P.; Kim, S.; Mahdi, F.; Buchanan, M.E.; Nass, S.R.; Qrareya, A.N.; Salahuddin, M.F.; Pianos, A.; Fernandez, N.; et al. Pregnane steroidogenesis is altered by HIV-1 Tat and morphine: Physiological allopregnanolone is protective against neurotoxic and psychomotor effects. Neurobiol. Stress. 2020, 12, 100211. [Google Scholar] [CrossRef] [PubMed]
- González-González, J.G.; de la Garza-Hernández, N.E.; Garza-Morán, R.A.; Rivera-Morales, I.M.; Montes-Villarreal, J.; Valenzuela-Rendón, J.; Villarreal-Pérez, J.Z. Prevalence of abnormal adrenocortical function in human immunodeficiency virus infection by low-dose cosyntropin test. Int. J. STD AIDS 2001, 12, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Prasanthai, V.; Sunthornyothin, S.; Phowthongkum, P.; Suankratay, C. Prevalence of adrenal insufficiency in critically ill patients with AIDS. J. Med. Assoc. Thai. 2007, 90, 1768–1774. [Google Scholar]
- Marik, P.E.; Kiminyo, K.; Zaloga, G.P. Adrenal insufficiency in critically ill patients with human immunodeficiency virus. Crit. Care Med. 2002, 30, 1267–1273. [Google Scholar] [CrossRef]
- Afreen, B.; Khan, K.A.; Riaz, A. Adrenal Insufficiency in Pakistani HIV Infected Patients. J. Ayub. Med. Coll. Abbottabad 2017, 29, 428–431. [Google Scholar]
- Sharma, N.; Sharma, L.K.; Anand, A.; Gadpayle, A.K.; Gaurav, K.; Mukherjee, S.; Kulshreshtha, B.; Dutta, D. Presence, patterns & predictors of hypocortisolism in patients with HIV infection in India. Indian J. Med. Res. 2018, 147, 142–150. [Google Scholar] [CrossRef]
- Dobs, A.S.; Dempsey, M.A.; Ladenson, P.W.; Polk, B.F. Endocrine disorders in men infected with human immunodeficiency virus. Am. J. Med. 1988, 84, 611–616. [Google Scholar] [CrossRef]
- Dutta, D.; Sharma, L.K.; Sharma, N.; Gadpayle, A.K.; Anand, A.; Gaurav, K.; Gupta, A.; Poondla, Y.; Kulshreshtha, B. Occurrence, patterns & predictors of hypogonadism in patients with HIV infection in India. Indian J. Med. Res. 2017, 145, 804–814. [Google Scholar] [CrossRef]
- Grinspoon, S.; Corcoran, C.; Miller, K.; Biller, B.M.; Askari, H.; Wang, E.; Hubbard, J.; Anderson, E.J.; Basgoz, N.; Heller, H.M.; et al. Body composition and endocrine function in women with acquired immunodeficiency syndrome wasting. J. Clin. Endocrinol. Metab. 1997, 82, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.S.; Lo, Y.; Santoro, N.; Dobs, A.S. Androgen levels in older men who have or who are at risk of acquiring HIV infection. Clin. Infect. Dis. 2005, 41, 1794–1803. [Google Scholar] [CrossRef]
- Dube, M.P.; Parker, R.A.; Mulligan, K.; Tebas, P.; Robbins, G.K.; Roubenoff, R.; Grinspoon, S.K. Effects of potent antiretroviral therapy on free testosterone levels and fat-free mass in men in a prospective, randomized trial: A5005s, a substudy of AIDS Clinical Trials Group Study 384. Clin. Infect. Dis. 2007, 45, 120–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wunder, D.M.; Bersinger, N.A.; Fux, C.A.; Mueller, N.J.; Hirschel, B.; Cavassini, M.; Elzi, L.; Schmid, P.; Bernasconi, E.; Mueller, B.; et al. Swiss HIV Cohort Study. Hypogonadism in HIV-1-infected men is common and does not resolve during antiretroviral therapy. Antivir. Ther. 2007, 12, 261–265. [Google Scholar]
- Rietschel, P.; Corcoran, C.; Stanley, T.; Basgoz, N.; Klibanski, A.; Grinspoon, S. Prevalence of hypogonadism among men with weight loss related to human immunodeficiency virus infection who were receiving highly active antiretroviral therapy. Clin. Infect. Dis. 2000, 31, 1240–1244. [Google Scholar] [CrossRef]
- Crum-Cianflone, N.F.; Bavaro, M.; Hale, B.; Amling, C.; Truett, A.; Brandt, C.; Pope, B.; Furtek, K.; Medina, S.; Wallace, M.R. Erectile dysfunction and hypogonadism among men with HIV. AIDS Patient Care STDS 2007, 21, 9–19. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef] [Green Version]
- Frye, C.A.; Paris, J.J.; Osborne, D.M.; Campbell, J.C.; Kippin, T.E. Prenatal Stress Alters Progestogens to Mediate Susceptibility to Sex-Typical, Stress-Sensitive Disorders, such as Drug Abuse: A Review. Front. Psychiatry 2011, 2, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar] [CrossRef]
- Tomaselli, G.; Vallée, M. Stress and drug abuse-related disorders: The promising therapeutic value of neurosteroids focus on pregnenolone-progesterone-allopregnanolone pathway. Front. Neuroendocrinol. 2019, 55, 100789. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Salahuddin, M.F.; Mahdi, F.; Paris, J.J. HIV-1 Tat Dysregulates the Hypothalamic-Pituitary-Adrenal Stress Axis and Potentiates Oxycodone-Mediated Psychomotor and Anxiety-Like Behavior of Male Mice. Int. J. Mol. Sci. 2020, 21, 8212. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, M.F.; Qrareya, A.N.; Mahdi, F.; Jackson, D.; Foster, M.; Vujanovic, T.; Box, J.G.; Paris, J.J. Combined HIV-1 Tat and oxycodone activate the hypothalamic-pituitary-adrenal and -gonadal axes and promote psychomotor, affective, and cognitive dysfunction in female mice. Horm. Behav. 2020, 119, 104649. [Google Scholar] [CrossRef]
- Paris, J.J.; Chen, X.; Anderson, J.; Qrareya, A.N.; Mahdi, F.; Du, F.; McLaughlin, J.P.; Kaufman, M.J. In vivo proton magnetic resonance spectroscopy detection of metabolite abnormalities in aged Tat-transgenic mouse brain. Geroscience 2021. [Google Scholar] [CrossRef]
- Fitting, S.; Scoggins, K.L.; Xu, R.; Dever, S.M.; Knapp, P.E.; Dewey, W.L.; Hauser, K.F. Morphine efficacy is altered in conditional HIV-1 Tat transgenic mice. Eur. J. Pharmacol. 2012, 689, 96–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wodarski, R.; Bagdas, D.; Paris, J.J.; Pheby, T.; Toma, W.; Xu, R.; Damaj, M.I.; Knapp, P.E.; Rice, A.S.C.; Hauser, K.F. Reduced intraepidermal nerve fibre density, glial activation, and sensory changes in HIV type-1 Tat-expressing female mice: Involvement of Tat during early stages of HIV-associated painful sensory neuropathy. Pain Rep. 2018, 3, e654. [Google Scholar] [CrossRef] [PubMed]
- Bruce-Keller, A.J.; Turchan-Cholewo, J.; Smart, E.J.; Geurin, T.; Chauhan, A.; Reid, R.; Xu, R.; Nath, A.; Knapp, P.E.; Hauser, K.F. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia 2008, 56, 1414–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickens, A.M.; Yoo, S.W.; Chin, A.C.; Xu, J.; Johnson, T.P.; Trout, A.L.; Hauser, K.F.; Haughey, N.J. Chronic low-level expression of HIV-1 Tat promotes a neurodegenerative phenotype with aging. Sci. Rep. 2017, 7, 7748. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.J.; Fenwick, J.; McLaughlin, J.P. Progesterone protects normative anxiety-like responding among ovariectomized female mice that conditionally express the HIV-1 regulatory protein, Tat, in the CNS. Horm. Behav. 2014, 65, 445–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawley, J.N.; Paylor, R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm. Behav. 1997, 31, 197–211. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Wang, S.; Zeng, Z.; Li, F.; Montalvo-Ortiz, J.; Tucker, C.; Akhtar, S.; Shi, J.; Meltzer, H.Y.; Rice, K.C.; et al. Effects of corticotrophin-releasing factor receptor 1 antagonists on amyloid-β and behavior in Tg2576 mice. Psychopharmacology 2014, 231, 4711–4722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Wang, S.; Rice, K.C.; Munro, C.A.; Wand, G.S. Restraint stress and ethanol consumption in two mouse strains. Alcohol. Clin. Exp. Res. 2008, 32, 840–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofford, R.S.; Prendergast, M.A.; Bardo, M.T. Pharmacological manipulation of glucocorticoid receptors differentially affects cocaine self-administration in environmentally enriched and isolated rats. Behav. Brain Res. 2015, 283, 196–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefevre, E.M.; Medley, G.A.; Reeks, T.; Alexander, S.; Burne, T.H.J.; Eyles, D.W. Effect of the glucocorticoid receptor antagonist RU486 on MK-801 induced behavioural sensitisation. PLoS ONE 2017, 12, e0176156. [Google Scholar] [CrossRef] [PubMed]
- Paris, J.J.; Fenwick, J.; McLaughlin, J.P. Estrous cycle and HIV-1 Tat protein influence cocaine-conditioned place preference and induced locomotion of female mice. Curr. HIV Res. 2014, 12, 388–396. [Google Scholar] [CrossRef]
- Ferraz-de-Paula, V.; Stankevicius, D.; Ribeiro, A.; Pinheiro, M.L.; Rodrigues-Costa, E.C.; Florio, J.C.; Lapachinske, S.F.; Moreau, R.L.; Palermo-Neto, J. Differential behavioral outcomes of 3,4-methylenedioxymethamphetamine (MDMA-ecstasy) in anxiety-like responses in mice. Braz. J. Med. Biol. Res. 2011, 44, 428–437. [Google Scholar] [CrossRef]
- Leite, L.M.; Carvalho, A.G.; Ferreira, P.L.; Pessoa, I.X.; Gonçalves, D.O.; Lopes Ade, A.; Góes, J.G.; Alves, V.C.; Leal, L.K.; Brito, G.A.; et al. Anti-inflammatory properties of doxycycline and minocycline in experimental models: An in vivo and in vitro comparative study. Inflammopharmacology 2011, 19, 99–110. [Google Scholar] [CrossRef]
- Paris, J.J.; Singh, H.D.; Ganno, M.L.; Jackson, P.; McLaughlin, J.P. Anxiety-like behavior of mice produced by conditional central expression of the HIV-1 regulatory protein, Tat. Psychopharmacology 2014, 231, 2349–2360. [Google Scholar] [CrossRef] [Green Version]
- Christeff, N.; Gherbi, N.; Mammes, O.; Dalle, M.T.; Gharakhanian, S.; Lortholary, O.; Melchior, J.C.; Nunez, E.A. Serum cortisol and DHEA concentrations during HIV infection. Psychoneuroendocrinology 1997, 22, S11–S18. [Google Scholar] [CrossRef]
- Christeff, N.; Nunez, E.A.; Gougeon, M.L. Changes in cortisol/DHEA ratio in HIV-infected men are related to immunological and metabolic perturbations leading to malnutrition and lipodystrophy. Ann. N. Y. Acad. Sci. 2000, 917, 962–970. [Google Scholar] [CrossRef]
- Chrousos, G.P.; Zapanti, E.D. Hypothalamic-pituitary-adrenal axis in HIV infection and disease. Endocrinol. Metab. Clin. N. Am. 2014, 43, 791–806. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.K.; Girdler, S.S. Neurosteroid, GABAergic and hypothalamic pituitary adrenal (HPA) axis regulation: What is the current state of knowledge in humans? Psychopharmacology 2014, 231, 3619–3634. [Google Scholar] [CrossRef] [Green Version]
- Girdler, S.S.; Klatzkin, R. Neurosteroids in the context of stress: Implications for depressive disorders. Pharmacol. Ther. 2007, 116, 125–139. [Google Scholar] [CrossRef] [Green Version]
- Gunn, B.G.; Cunningham, L.; Mitchell, S.G.; Swinny, J.D.; Lambert, J.J.; Belelli, D. GABAA receptor-acting neurosteroids: A role in the development and regulation of the stress response. Front. Neuroendocrinol. 2015, 36, 28–48. [Google Scholar] [CrossRef] [PubMed]
- Finkel, D.G.; John, G.; Holland, B.; Slim, J.; Smith, S.M. Women have a greater immunological response to effective virological HIV-1 therapy. AIDS 2003, 17, 2009–2011. [Google Scholar] [CrossRef] [PubMed]
- Grinsztejn, B.; Smeaton, L.; Barnett, R.; Klingman, K.; Hakim, J.; Flanigan, T.; Kumarasamy, N.; Campbell, T.; Currier, J.; PEARLS study team of the ACTG. Sex-associated differences in pre-antiretroviral therapy plasma HIV-1 RNA in diverse areas of the world vary by CD4(+) T-cell count. Antivir. Ther. 2011, 16, 1057–1062. [Google Scholar] [CrossRef] [Green Version]
- Jarrin, I.; Geskus, R.; Bhaskaran, K.; Prins, M.; Perez-Hoyos, S.; Muga, R.; Hernández-Aguado, I.; Meyer, L.; Porter, K.; del Amo, J.; et al. Gender differences in HIV progression to AIDS and death in industrialized countries: Slower disease progression following HIV seroconversion in women. Am. J. Epidemiol. 2008, 168, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Maki, P.M.; Rubin, L.H.; Springer, G.; Seaberg, E.C.; Sacktor, N.; Miller, E.N.; Valcour, V.; Young, M.A.; Becker, J.T.; Martin, E.M.; et al. Differences in Cognitive Function Between Women and Men with HIV. J. Acquir. Immune Defic. Syndr. 2018, 79, 101–107. [Google Scholar] [CrossRef]
- Qiao, X.; Lin, H.; Chen, X.; Ning, C.; Wang, K.; Shen, W.; Xu, X.; Xu, X.; Liu, X.; He, N.; et al. Sex differences in neurocognitive screening among adults living with HIV in China. J. Neurovirol. 2019, 25, 363–371. [Google Scholar] [CrossRef]
- Rubin, L.H.; Neigh, G.N.; Sundermann, E.E.; Xu, Y.; Scully, E.P.; Maki, P.M. Sex Differences in Neurocognitive Function in Adults with HIV: Patterns, Predictors, and Mechanisms. Curr. Psychiatry Rep. 2019, 21, 94. [Google Scholar] [CrossRef]
- Bing, E.G.; Burnam, M.A.; Longshore, D.; Fleishman, J.A.; Sherbourne, C.D.; London, A.S.; Turner, B.J.; Eggan, F.; Beckman, R.; Vitiello, B.; et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch. Gen. Psychiatry 2001, 58, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Olfson, M.; Rabkin, J.; Hasin, D.S.; Alegría, A.A.; Lin, K.H.; Grant, B.F.; Blanco, C. Gender, HIV status, and psychiatric disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psychiatry 2012, 73, 384–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsao, J.C.; Dobalian, A.; Moreau, C.; Dobalian, K. Stability of anxiety and depression in a national sample of adults with human immunodeficiency virus. J. Nerv. Ment. Dis. 2004, 192, 111–118. [Google Scholar] [CrossRef]
- Sewell, M.C.; Goggin, K.J.; Rabkin, J.G.; Ferrando, S.J.; McElhiney, M.C.; Evans, S. Anxiety syndromes and symptoms among men with AIDS: A longitudinal controlled study. Psychosomatics 2000, 41, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Orlando, M.; Burnam, M.A.; Beckman, R.; Morton, S.C.; London, A.S.; Bing, E.G.; Fleishman, J.A. Re-estimating the prevalence of psychiatric disorders in a nationally representative sample of persons receiving care for HIV: Results from the HIV Cost and Services Utilization Study. Int. J. Methods Psychiatr. Res. 2002, 11, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Goggin, K.; Engelson, E.S.; Rabkin, J.G.; Kotler, D.P. The relationship of mood, endocrine, and sexual disorders in human immunodeficiency virus positive (HIV+) women: An exploratory study. Psychosom. Med. 1998, 60, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Andreano, J.M.; Touroutoglou, A.; Dickerson, B.; Barrett, L.F. Hormonal Cycles, Brain Network Connectivity, and Windows of Vulnerability to Affective Disorder. Trends Neurosci. 2018, 41, 660–676. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.M.; Jerram, M.; Abbs, B.; Whitfield-Gabrieli, S.; Makris, N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J. Neurosci. 2010, 30, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Viau, V.; Bingham, B.; Davis, J.; Lee, P.; Wong, M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology 2005, 146, 137–146. [Google Scholar] [CrossRef]
- MacLusky, N.J.; Yuan, H.; Elliott, J.; Brown, T.J. Sex differences in corticosteroid binding in the rat brain: An in vitro autoradiographic study. Brain Res. 1996, 708, 71–81. [Google Scholar] [CrossRef]
- Handa, R.J.; Burgess, L.H.; Kerr, J.E.; O′Keefe, J.A. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm. Behav. 1994, 28, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Babb, J.A.; Masini, C.V.; Day, H.E.; Campeau, S. Sex differences in activated corticotropin-releasing factor neurons within stress-related neurocircuitry and hypothalamic-pituitary-adrenocortical axis hormones following restraint in rats. Neuroscience 2013, 234, 40–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasaki-Sekino, A.; Mano-Otagiri, A.; Ohata, H.; Yamauchi, N.; Shibasaki, T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology 2009, 34, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Duncko, R.; Kiss, A.; Skultétyová, I.; Rusnák, M.; Jezová, D. Corticotropin-releasing hormone mRNA levels in response to chronic mild stress rise in male but not in female rats while tyrosine hydroxylase mRNA levels decrease in both sexes. Psychoneuroendocrinology 2001, 26, 77–89. [Google Scholar] [CrossRef]
- Oyola, M.G.; Handa, R.J. Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: Sex differences in regulation of stress responsivity. Stress 2017, 20, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Young, E.A. Sex differences and the HPA axis: Implications for psychiatric disease. J. Gend. Specif. Med. 1998, 1, 21–27. [Google Scholar]
- Hu, M.; Becker, J.B. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J. Neurosci. 2003, 23, 693–699. [Google Scholar] [CrossRef]
- Calipari, E.S.; Juarez, B.; Morel, C.; Walker, D.M.; Cahill, M.E.; Ribeiro, E.; Roman-Ortiz, C.; Ramakrishnan, C.; Deisseroth, K.; Han, M.H.; et al. Dopaminergic dynamics underlying sex-specific cocaine reward. Nat. Commun. 2017, 8, 13877. [Google Scholar] [CrossRef] [Green Version]
- Vandegrift, B.J.; You, C.; Satta, R.; Brodie, M.S.; Lasek, A.W. Estradiol increases the sensitivity of ventral tegmental area dopamine neurons to dopamine and ethanol. PLoS ONE 2017, 12, e0187698. [Google Scholar] [CrossRef] [Green Version]
- Ramôa, C.P.; Doyle, S.E.; Naim, D.W.; Lynch, W.J. Estradiol as a mechanism for sex differences in the development of an addicted phenotype following extended access cocaine self-administration. Neuropsychopharmacology 2013, 38, 1698–1705. [Google Scholar] [CrossRef]
- Zhang, J.J.; Kong, Q. Locomotor activity: A distinctive index in morphine self-administration in rats. PLoS ONE 2017, 12, e0174272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniell, H.W. Hypogonadism in men consuming sustained-action oral opioids. J. Pain 2002, 3, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.W. Opioid endocrinopathy in women consuming prescribed sustained-action opioids for control of nonmalignant pain. J. Pain 2008, 9, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.; Kreek, M.J. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am. J. Psychiatry 2007, 164, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Porter, R.J.; Gallagher, P. Abnormalities of the HPA axis in affective disorders: Clinical subtypes and potential treatments. Acta Neuropsychiatr. 2006, 18, 193–209. [Google Scholar] [CrossRef]
- Brown, T.T.; Wisniewski, A.B.; Dobs, A.S. Gonadal and Adrenal Abnormalities in Drug Users: Cause or Consequence of Drug Use Behavior and Poor Health Outcomes. Am. J. Infect. Dis. 2006, 2, 130–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaine, S.K.; Milivojevic, V.; Fox, H.; Sinha, R. Alcohol Effects on Stress Pathways: Impact on Craving and Relapse Risk. Can. J. Psychiatry 2016, 61, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Gonek, M.; McLane, V.D.; Stevens, D.L.; Lippold, K.; Akbarali, H.I.; Knapp, P.E.; Dewey, W.L.; Hauser, K.F.; Paris, J.J. CCR5 mediates HIV-1 Tat-induced neuroinflammation and influences morphine tolerance, dependence, and reward. Brain Behav. Immun. 2018, 69, 124–138. [Google Scholar] [CrossRef]
- Kesby, J.P.; Najera, J.A.; Romoli, B.; Fang, Y.; Basova, L.; Birmingham, A.; Marcondes, M.C.G.; Dulcis, D.; Semenova, S. HIV-1 TAT protein enhances sensitization to methamphetamine by affecting dopaminergic function. Brain Behav. Immun. 2017, 65, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Lovallo, W.R. The hypothalamic-pituitary-adrenocortical axis in addiction. Int. J. Psychophysiol. 2006, 59, 193–194. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, Y.; Yamashita, N.; Nakamura, T.; Iwamoto, A. Prospective examination of adrenocortical function in advanced AIDS patients. Endocr. J. 2002, 49, 641–647. [Google Scholar] [CrossRef] [Green Version]
- Membreno, L.; Irony, I.; Dere, W.; Klein, R.; Biglieri, E.G.; Cobb, E. Adrenocortical function in acquired immunodeficiency syndrome. J. Clin. Endocrinol. Metab. 1987, 65, 482–487. [Google Scholar] [CrossRef]
- Kino, T.; Chrousos, G.P. Human immunodeficiency virus type-1 accessory protein Vpr: A causative agent of the AIDS-related insulin resistance/lipodystrophy syndrome? Ann. N. Y. Acad. Sci. 2004, 1024, 153–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norbiato, G.; Bevilacqua, M.; Vago, T.; Baldi, G.; Chebat, E.; Bertora, P.; Moroni, M.; Galli, M.; Oldenburg, N. Cortisol resistance in acquired immunodeficiency syndrome. J. Clin. Endocrinol. Metab. 1992, 74, 608–613. [Google Scholar] [CrossRef] [Green Version]
- Leung, D.Y.; Hamid, Q.; Vottero, A.; Szefler, S.J.; Surs, W.; Minshall, E.; Chrousos, G.P.; Klemm, D.J. Association of glucocorticoid insensitivity with increased expression of glucocorticoid receptor beta. J. Exp. Med. 1997, 186, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Bamberger, C.M.; Bamberger, A.M.; de Castro, M.; Chrousos, G.P. Glucocorticoid receptor beta, a potential endogenous inhibitor of glucocorticoid action in humans. J. Clin. Investig. 1995, 95, 2435–2441. [Google Scholar] [CrossRef] [Green Version]
- Charmandari, E.; Chrousos, G.P.; Ichijo, T.; Bhattacharyya, N.; Vottero, A.; Souvatzoglou, E.; Kino, T. The human glucocorticoid receptor (hGR) beta isoform suppresses the transcriptional activity of hGRalpha by interfering with formation of active coactivator complexes. Mol. Endocrinol. 2005, 19, 52–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekhbat, M.; Mehta, C.C.; Kelly, S.D.; Vester, A.; Ofotokun, I.; Felger, J.; Wingood, G.; Anastos, K.; Gustafson, D.R.; Kassaye, S.; et al. HIV and symptoms of depression are independently associated with impaired glucocorticoid signaling. Psychoneuroendocrinology 2018, 96, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Eledrisi, M.S.; Verghese, A.C. Adrenal insufficiency in HIV infection: A review and recommendations. Am. J. Med. Sci. 2001, 321, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Freda, P.U.; Bilezikian, J.P. The hypothalamus-pituitary-adrenal axis in HIV disease. AIDS Read. 1999, 9, 43–50. [Google Scholar]
- Mayo, J.; Collazos, J.; Martínez, E.; Ibarra, S. Adrenal Function in the Human Immunodeficiency Virus–Infected Patient. Arch. Intern. Med. 2002, 162, 1095–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bangasser, D.A.; Curtis, A.; Reyes, B.A.; Bethea, T.T.; Parastatidis, I.; Ischiropoulos, H.; Van Bockstaele, E.J.; Valentino, R.J. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: Potential role in female vulnerability to stress-related psychopathology. Mol. Psychiatry 2010, 877, 896–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, B.B.; Weaver, D.A. Sexual dimorphism of glucocorticoid binding in rat brain. Brain Res. 1985, 343, 16–23. [Google Scholar] [CrossRef]
- Solomon, M.B.; Loftspring, M.; de Kloet, A.D.; Ghosal, S.; Jankord, R.; Flak, J.N.; Wulsin, A.C.; Krause, E.G.; Zhang, R.; Rice, T.; et al. Neuroendocrine Function After Hypothalamic Depletion of Glucocorticoid Receptors in Male and Female Mice. Endocrinology 2015, 156, 2843–2853. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, B.; Rowe, W.; Sharma, S.; Diorio, J.; Steverman, A.; Walker, M.; Meaney, M.J. Dynamic variations in plasma corticosteroid-binding globulin and basal HPA activity following acute stress in adult rats. J. Neuroendocrinol. 1997, 9, 163–168. [Google Scholar] [CrossRef]
- Majewska, M.D.; Harrison, N.L.; Schwartz, R.D.; Barker, J.L.; Paul, S.M. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 1986, 232, 1004–1007. [Google Scholar] [CrossRef] [Green Version]
- Morrow, A.L.; Suzdak, P.D.; Paul, S.M. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur. J. Pharmacol. 1987, 142, 483–485. [Google Scholar] [CrossRef]
- Lambert, J.J.; Cooper, M.A.; Simmons, R.D.; Weir, C.J.; Belelli, D. Neurosteroids: Endogenous allosteric modulators of GABA(A) receptors. Psychoneuroendocrinology 2009, 34, S48–S58. [Google Scholar] [CrossRef]
- Reddy, D.S.; Rogawski, M.A. Stress-induced deoxycorticosterone-derived neurosteroids modulate GABA(A) receptor function and seizure susceptibility. J. Neurosci. 2002, 22, 3795–3805. [Google Scholar] [CrossRef] [Green Version]
- Miklós, I.H.; Kovács, K.J. GABAergic innervation of corticotropin-releasing hormone (CRH)-secreting parvocellular neurons and its plasticity as demonstrated by quantitative immunoelectron microscopy. Neuroscience 2002, 113, 581–592. [Google Scholar] [CrossRef]
- Cullinan, W.E. GABA(A) receptor subunit expression within hypophysiotropic CRH neurons: A dual hybridization histochemical study. J. Comp. Neurol. 2000, 419, 344–351. [Google Scholar] [CrossRef]
- Marks, W.D.; Paris, J.J.; Schier, C.J.; Denton, M.D.; Fitting, S.; McQuiston, A.R.; Knapp, P.E.; Hauser, K.F. HIV-1 Tat causes cognitive deficits and selective loss of parvalbumin, somatostatin, and neuronal nitric oxide synthase expressing hippocampal CA1 interneuron subpopulations. J. Neurovirol. 2016, 22, 747–762. [Google Scholar] [CrossRef] [PubMed]
- Patchev, V.K.; Shoaib, M.; Holsboer, F.; Almeida, O.F. The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience 1994, 62, 265–271. [Google Scholar] [CrossRef]
- Patchev, V.K.; Hassan, A.H.; Holsboer, D.F.; Almeida, O.F. The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology 1996, 15, 533–540. [Google Scholar] [CrossRef] [Green Version]
- Gunn, B.G.; Cunningham, L.; Cooper, M.A.; Corteen, N.L.; Seifi, M.; Swinny, J.D.; Lambert, J.J.; Belelli, D. Dysfunctional astrocytic and synaptic regulation of hypothalamic glutamatergic transmission in a mouse model of early-life adversity: Relevance to neurosteroids and programming of the stress response. J. Neurosci. 2013, 33, 19534–19554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitran, D.; Dugan, M.; Renda, P.; Ellis, R.; Foley, M. Anxiolytic effects of the neuroactive steroid pregnanolone (3 alpha-OH-5 beta-pregnan-20-one) after microinjection in the dorsal hippocampus and lateral septum. Brain Res. 1999, 850, 217–224. [Google Scholar] [CrossRef]
- Carboni, E.; Wieland, S.; Lan, N.C.; Gee, K.W. Anxiolytic properties of endogenously occurring pregnanediols in two rodent models of anxiety. Psychopharmacology 1996, 126, 173–178. [Google Scholar] [CrossRef]
- Owens, M.J.; Ritchie, J.C.; Nemeroff, C.B. 5 alpha-pregnane-3 alpha, 21-diol-20-one (THDOC) attenuates mild stress-induced increases in plasma corticosterone via a non-glucocorticoid mechanism: Comparison with alprazolam. Brain Res. 1992, 573, 353–355. [Google Scholar] [CrossRef]
- Romeo, E.; Ströhle, A.; Spalletta, G.; di Michele, F.; Hermann, B.; Holsboer, F.; Pasini, A.; Rupprecht, R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am. J. Psychiatry 1998, 155, 910–913. [Google Scholar] [CrossRef]
- Uzunova, V.; Sheline, Y.; Davis, J.M.; Rasmusson, A.; Uzunov, D.P.; Costa, E.; Guidotti, A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc. Natl. Acad. Sci. USA 1998, 95, 3239–3244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serra, M.; Pisu, M.G.; Littera, M.; Papi, G.; Sanna, E.; Tuveri, F.; Usala, L.; Purdy, R.H.; Biggio, G. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABA(A) receptor function in rat brain. J. Neurochem. 2000, 75, 732–740. [Google Scholar] [CrossRef]
- Matsumoto, K.; Pinna, G.; Puia, G.; Guidotti, A.; Costa, E. Social isolation stress-induced aggression in mice: A model to study the pharmacology of neurosteroidogenesis. Stress 2005, 8, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Guennoun, R.; Labombarda, F.; Gonzalez Deniselle, M.C.; Liere, P.; De Nicola, A.F.; Schumacher, M. Progesterone and allopregnanolone in the central nervous system: Response to injury and implication for neuroprotection. J. Steroid. Biochem. Mol. Biol. 2015, 146, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Callachan, H.; Cottrell, G.A.; Hather, N.Y.; Lambert, J.J.; Nooney, J.M.; Peters, J.A. Modulation of the GABAA receptor by progesterone metabolites. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1987, 231, 359–369. [Google Scholar] [CrossRef]
- Earl, D.E.; Tietz, E.I. Inhibition of recombinant L-type voltage-gated calcium channels by positive allosteric modulators of GABAA receptors. J. Pharmacol. Exp. Ther. 2011, 337, 301–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, A.Q.; Wang, Z.M.; Lan, D.M.; Fu, Y.M.; Zhu, Y.H.; Dong, Y.; Zheng, P. Inhibition of evoked glutamate release by neurosteroid allopregnanolone via inhibition of L-type calcium channels in rat medial prefrontal cortex. Neuropsychopharmacology 2007, 32, 1477–1489. [Google Scholar] [CrossRef] [Green Version]
- Paris, J.J.; Zou, S.; Hahn, Y.K.; Knapp, P.E.; Hauser, K.F. 5α-reduced progestogens ameliorate mood-related behavioral pathology, neurotoxicity, and microgliosis associated with exposure to HIV-1 Tat. Brain Behav. Immun. 2016, 55, 202–214. [Google Scholar] [CrossRef] [Green Version]
- Raber, J.; Toggas, S.M.; Lee, S.; Bloom, F.E.; Epstein, C.J.; Mucke, L. Central nervous system expression of HIV-1 Gp120 activates the hypothalamic-pituitary-adrenal axis: Evidence for involvement of NMDA receptors and nitric oxide synthase. Virology 1996, 226, 362–373. [Google Scholar] [CrossRef] [Green Version]
- Costa, A.; Nappi, R.E.; Polatti, F.; Poma, A.; Grossman, A.B.; Nappi, G. Stimulating effect of HIV-1 coat protein gp120 on corticotropin-releasing hormone and arginine vasopressin in the rat hypothalamus: Involvement of nitric oxide. Exp. Neurol. 2000, 166, 376–384. [Google Scholar] [CrossRef]
- Kino, T.; Gragerov, A.; Kopp, J.B.; Stauber, R.H.; Pavlakis, G.N.; Chrousos, G.P. The HIV-1 virion-associated protein Vpr is a coactivator of the human glucocorticoid receptor. J. Exp. Med. 1999, 189, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Kino, T.; Gragerov, A.; Slobodskaya, O.; Tsopanomichalou, M.; Chrousos, G.P.; Pavlakis, G.N. Human immunodeficiency virus type 1 (HIV-1) accessory protein Vpr induces transcription of the HIV-1 and glucocorticoid-responsive promoters by binding directly to p300/CBP coactivators. J. Virol. 2002, 76, 9724–9734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherman, M.P.; de Noronha, C.M.; Pearce, D.; Greene, W.C. Human immunodeficiency virus type 1 Vpr contains two leucine-rich helices that mediate glucocorticoid receptor coactivation independently of its effects on G(2) cell cycle arrest. J. Virol. 2000, 74, 8159–8165. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Non-Stressed | ||||||||
|---|---|---|---|---|---|---|---|---|
| Saline (0.9%, i.p.) | Oxycodone (3 mg/kg, i.p.) | |||||||
| Proestrous | Diestrous | Proestrous | Diestrous | |||||
| Tat(−) | Tat(+) | Tat(−) | Tat(+) | Tat(−) | Tat(+) | Tat(−) | Tat(+) | |
| Light Zone Time (s) | 78 ± 14 | 88 ± 34 | 24 ± 5 | 141 ± 42 ^ | 61 ± 19 | 49 ± 13 | 71 ± 13 | 20 ± 4 |
| Mean Velocity (m/s) | 0.036 ± 0.003 | 0.030 ± 0.004 | 0.024 ± 0.004 | 0.032 ± 0.003 | 0.072 ± 0.005 † | 0.103 ± 0.017 §,† | 0.062 ± 0.011 † | 0.104 ± 0.013 §,† |
| Number of transitions | 15 ± 2 | 8 ± 1 * | 6 ± 1 | 5 ± 1 * | 19 ± 4 † | 15 ± 3 †,* | 19 ± 5 † | 14 ± 3 †,* |
| Rearing Time (s) | 19 ± 4 | 20 ± 3 | 27 ± 6 # | 26 ± 7 # | 5 ± 1 | 15 ± 4 | 60 ± 23 # | 33 ± 20 # |
| Forced Swim-Stressed | ||||||||
|---|---|---|---|---|---|---|---|---|
| Saline (0.9%, i.p.) | Oxycodone (3 mg/kg, i.p.) | |||||||
| Proestrous | Diestrous | Proestrous | Diestrous | |||||
| Tat(−) | Tat(+) | Tat(−) | Tat(+) | Tat(−) | Tat(+) | Tat(−) | Tat(+) | |
| Light Zone Time (s) | 60 ± 16 | 43 ± 4 | 112 ± 30 | 81 ± 28 | 79 ± 36 | 38 ± 9 | 45 ± 6 | 44 ± 10 |
| Mean Velocity (m/sec) | 0.002 ± 0.001 | 0.007 ± 0.003 | 0.013 ± 0.003 # | 0.015 ± 0.002 # | 0.006 ± 0.002 | 0.021 ± 0.008 § | 0.018 ± 0.003 # | 0.047 ± 0.009 #,§ |
| Number of transitions | 10 ± 2 | 12 ± 2 ‡ | 14 ± 3 ‡ | 7 ± 1 | 5 ± 1 | 19 ± 5 ‡,§ | 14 ± 2 ‡ | 14 ± 3 § |
| Rearing Time (s) | 0.35 ± 0.15 | 1.97 ± 0.82 * | 1.56 ± 0.44 | 2.10 ± 0.66 * | 0.01 ± 0.01 | 1.65 ± 1.11 * | 0.21 ± 0.13 | 1.02 ± 0.50 * |
| HPA or HPG Blockade | ||||||||
|---|---|---|---|---|---|---|---|---|
| Vehicle | Antalarmin | |||||||
| Saline | Oxycodone | Saline | Oxycodone | |||||
| Tat(−) | Tat(+) | Tat(−) | Tat(+) | Tat(−) | Tat(+) | Tat(−) | Tat(+) | |
| Light Zone Time (s) | 112 ± 38 | 95 ± 43 * | 119 ± 35 † | 23 ± 7 † | 165 ± 34 | 100 ± 35 * | 85 ± 31 † | 31 ± 12 † |
| Mean Velocity (m/sec) | 0.022 ± 0.003 | 0.025 ± 0.004 | 0.046 ± 0.009 † | 0.103 ± 0.009 †,§ | 0.024 ± 0.004 | 0.023 ± 0.005 | 0.054 ± 0.013 † | 0.098 ± 0.015 †,§ |
| Number of transitions | 3.4 ± 0.3 | 7.5 ± 1.5 | 14.3 ± 2.9 | 7.7 ± 2.3 | 11.8 ± 2.3 @ | 5.1 ± 1.1 | 11.2 ± 3.3 | 12.5 ± 4.7 |
| Rearing Time (s) | 62 ± 33 | 15 ± 4 | 61 ± 37 | 34 ± 17 | 9 ± 3 # | 12 ± 2 # | 4 ± 2 # | 7 ± 3 # |
| HPA or HPG Blockade | ||||||||
| RU-486 | OVX | |||||||
| Saline | Oxycodone | Saline | Oxycodone | |||||
| Tat(−) | Tat(+) | Tat(−) | Tat(+) | Tat(−) | Tat(+) | Tat(−) | Tat(+) | |
| Light Zone Time (s) | 50 ± 12 #,^ | 29 ± 8 *,^ | 83 ± 28 †,^ | 29 ± 7 †,^ | 129 ± 39 | 88 ± 30 * | 137 ± 39 † | 27 ± 6 † |
| Mean Velocity (m/sec) | 0.022 ± 0.003 | 0.029 ± 0.004 | 0.061 ± 0.008 † | 0.093 ± 0.008 †,§ | 0.024 ± 0.003 | 0.024 ± 0.002 | 0.045 ± 0.013 †,‡ | 0.049 ± 0.011 †,§,‡ |
| Number of transitions | 4.9 ± 0.6 | 4.3 ± 0.6 | 9.3 ± 2.6 | 10.5 ± 1.5 | 9.4 ± 2.5 | 6.8 ± 1.0 | 9.1 ± 2.5 | 10.8 ± 2.3 |
| Rearing Time (s) | 9 ± 2 # | 17 ± 4 # | 3 ± 1 # | 11 ± 2 # | 11 ± 2 # | 15 ± 2 # | 16 ± 11 # | 2 ± 1 # |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salahuddin, M.F.; Mahdi, F.; Sulochana, S.P.; Paris, J.J. HIV-1 Tat Protein Promotes Neuroendocrine Dysfunction Concurrent with the Potentiation of Oxycodone’s Psychomotor Effects in Female Mice. Viruses 2021, 13, 813. https://doi.org/10.3390/v13050813
Salahuddin MF, Mahdi F, Sulochana SP, Paris JJ. HIV-1 Tat Protein Promotes Neuroendocrine Dysfunction Concurrent with the Potentiation of Oxycodone’s Psychomotor Effects in Female Mice. Viruses. 2021; 13(5):813. https://doi.org/10.3390/v13050813
Chicago/Turabian StyleSalahuddin, Mohammed F., Fakhri Mahdi, Suresh P. Sulochana, and Jason J. Paris. 2021. "HIV-1 Tat Protein Promotes Neuroendocrine Dysfunction Concurrent with the Potentiation of Oxycodone’s Psychomotor Effects in Female Mice" Viruses 13, no. 5: 813. https://doi.org/10.3390/v13050813
APA StyleSalahuddin, M. F., Mahdi, F., Sulochana, S. P., & Paris, J. J. (2021). HIV-1 Tat Protein Promotes Neuroendocrine Dysfunction Concurrent with the Potentiation of Oxycodone’s Psychomotor Effects in Female Mice. Viruses, 13(5), 813. https://doi.org/10.3390/v13050813