Targeting the Human Influenza a Virus: The Methods, Limitations, and Pitfalls of Virtual Screening for Drug-like Candidates Including Scaffold Hopping and Compound Profiling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Computer Programs
- 1.
- To determine the pharmacophore patterns, we studied binding mode specificities and structure–activity relationships (SAR) of hitherto known 3D structure complexes between influenza virus neuraminidase targets and sialic acid substrates or four reference antiviral drugs (oseltamivir, zanamivir, laninamivir, and peramivir) to generate 1D, 2D, and 3D fingerprints used as filters during virtual screening (VS).
- 2.
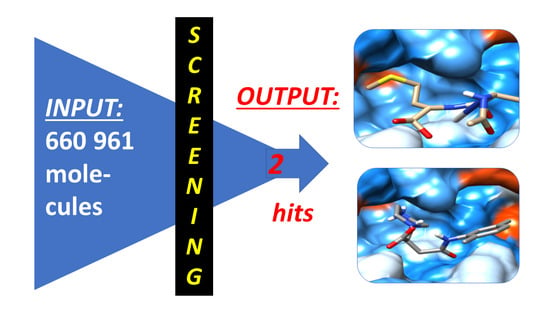
- We also aimed to carry out VS on drug-like compounds by 1D, 2D, and 3D fingerprints. The method facilitates a fully automated (unsupervised) selection of drug-like candidates. To this end, 1-, 2-, or 3D filters have to be predefined or are built-in search tools [27,28,29,30]. The input data collection comprises a total of 660,961 small organic molecules (SOMs) [18]. It is composed of basic commercial structures for next-step experimental lead optimization and scaffold diversification upon identifying selected VS hits.
- 3.
- The ligand affinities were then calculated to target for the selected VS hits by molecular docking. The self- or back-docking of reference inhibitors compares their predicted poses and affinities with observed (crystallographic) data and validates the computational study, besides docking new molecules to target. The molecular affinities were compared to the viral neuraminidase target by molecular docking each of the following ligands: natural substrates, sialic acid, reference drugs, and VS hits. Interaction energies and affinities were quantitated by means of the inhibition constant (Ki), and the results of the computed affinities were compared with experimental Ki values of reference drugs from the literature.
- 4.
- For ADMET profiling, smile codes were created for the VS hits and ADMET data were assessed using ADMET Predictor software.
2.2. Virtual Screening
- i.
- Based on molecular overall features, thousands of chemical substances were eliminated by of their size (molecular weight), lipophilicity (log P), and toxicity (toxic groups, SMILES patterns). Such screening methods are termed one-dimensional (1D) filters.
- ii.
- All molecules which passed the 1D filter were filtered through topological searches for 2D binding patterns.
- iii.
- Utilizing active conformations of known ligands at the binding site, a pharmacophore 3D filter was designed and a conformational database of the remaining substances was searched for spatial matches (hits) of atoms, groups, or properties (acidic, basic, polar, nonpolar, ionic, H-bond etc.).
- iv.
- Finally, the few 3D filter hits were screened by docking simulations, also sometimes called 4D filtering.
2.3. Molecular Docking
2.4. ADMET Profiling
3. Results
3.1. Binding Pattern and Pharmacophore Modeling
3.2. D Filtering (2D Fingerprint Design)
3.3. D Filtering (3D Fingerprint Design)
3.4. Prospective 2D VS
3.5. Prospective 3D VS
3.6. Virtual Library Performance under Fingerprint Model Number 24
3.7. Ligand–Target Docking
3.8. ADMET Profiling
4. Discussion
4.1. Implications and Limitations for Drug Screening
4.2. Implications and Limitations for Ligand–Target Docking
4.3. Implications and Limitations for ADMET Modeling
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, Y.; Hoang, P.; Kim, D.; Ayun, R.; Luong, Q.; Na, K.; Kim, T.; Oh, Y.; Kim, W.; Lee, S. A Therapeutically Active Minibody Exhibits an Antiviral Activity in Oseltamivir-Resistant Influenza-Infected Mice via Direct Hydrolysis of Viral RNAs. Viruses 2022, 14, 1105. [Google Scholar] [CrossRef]
- Dufrasne, F. Baloxavir Marboxil: An Original New Drug against Influenza. Pharmaceuticals 2022, 15, 28. [Google Scholar] [CrossRef]
- Su, B.; Wurtzer, S.; Rameix-Welti, M.; Dwyer, D.; van der Werf, S.; Naffakh, N.; Clavel, F.; Labrosse, B. Enhancement of the Influenza A Hemagglutinin (HA)-Mediated Cell-Cell Fusion and Virus Entry by the Viral Neuraminidase (NA). PLoS ONE 2009, 4, e8495. [Google Scholar] [CrossRef]
- Rogers, G.N.; D’Souza, B.L. Receptor binding properties of human and animal H1 influenza virus isolates. Virology 1989, 173, 317–322. [Google Scholar] [CrossRef]
- Rossignol, J.; La Frazia, S.; Chiappa, L.; Ciucci, A.; Santoro, M. Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at post-translational level. J. Biol. Chem. 2009, 284, 29798–29808. [Google Scholar] [CrossRef]
- Monto, A. Epidemiology and Virology of Influenza Illness. Am. J. Manag. Care 2000, 6 (Suppl. S5), S255–S264. [Google Scholar]
- Yamada, S.; Suzuki, Y.; Suzuki, T.; Le, M.Q.; Nidom, C.A.; Sakai-Tagawa, Y.; Mu-ramoto, Y.; Ito, M.; Kiso, M.; Horimoto, T.; et al. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature 2006, 444, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Kosik, I.; Yewdell, J.W. Influenza Hemagglutinin and Neuraminidase: Yin-Yang Proteins Coevolving to Thwart Immunity. Viruses 2019, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Guo, H.; Nijman, V.S.; Doedt, J.; van der Vries, E.; van der Lee, J.; Li, Z.; Boons, G.J.; van Kuppeveld, F.J.M.; de Vries, E.; et al. The 2nd sialic acid-binding site of influenza A virus neuraminidase is an important determinant of the hemagglutinin-neuraminidase-receptor balance. PLoS Pathog. 2019, 15, e1007860. [Google Scholar] [CrossRef]
- Kurt, M.; Ercan, S.; Pirinccioglu, N. Designing new drug candidates as inhibitors against wild and mutant type neuraminidases: Molecular docking, molecular dynamics and binding free energy calculations. J. Biomol. Struct. Dyn. 2022, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jeyaram, R.A.; Anu Radha, C. N1 neuraminidase of H5N1 avian influenza a virus complexed with sialic acid and zanamivir—A study by molecular docking and molecular dynamics simulation. J. Biomol. Struct. Dyn. 2022, 40, 11434–11447. [Google Scholar] [CrossRef]
- Colombo, C.; Podlipnik, C.; Lo Presti, L.; Niikura, M.; Bennet, A.J.; Bernardi, A. Design and synthesis of constrained bicyclic molecules as candidate inhibitors of influenza a neuraminidase. PLoS ONE 2018, 28, e0193623. [Google Scholar] [CrossRef] [PubMed]
- Pedretti, A.; Mazzolari, A.; Gervasoni, S.; Fumagalli, L.; Vistoli, G. The VEGA suite of programs: An versatile platform for cheminformatics and drug design projects. Bioinformatics 2021, 37, 1174–1175. [Google Scholar] [CrossRef] [PubMed]
- Bolton, E.E.; Wang, Y.; Thiessen, P.A.; Bryant, S.H. PubChem: Integrated platform of small molecules and biological activities. In Annual Reports in Computational Chemistry; Elsevier: Amsterdam, The Netherlands, 2008; Volume 4, pp. 217–241. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Seiler, K.P.; George, G.A.; Happ, M.P.; Bodycombe, N.E.; Carrinski, H.A.; Norton, S.; Brudz, S.; Sullivan, J.P.; Muhlich, J.; Serrano, M.; et al. ChemBank: A small-molecule screening and cheminformatics resource database. Nucleic Acids Res. 2007, 36, D351–D359. [Google Scholar] [CrossRef]
- Guha, R.; Howard, M.T.; Hutchison, G.R.; Murray-Rust, P.; Rzepa, H.; Steinbeck, C.; Wegner, J.; Willighagen, E.L. The Blue Obelisk-interoperability in chemical informatics. J. Chem. Inf. Model. 2006, 46, 991–998. [Google Scholar] [CrossRef]
- Molecular Operating Environment Software (MOE). Available online: http://www.chemcomp.com (accessed on 26 July 2018).
- ADMET Predictor™ Tool. Available online: https://www.simulations-plus.com (accessed on 29 August 2014).
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Pokorná, J.; Pachl, P.; Karlukova, E.; Hejdánek, J.; Rezácová, P.; Machara, A.; Hudlický, J.; Konvalinka, J.; Kožíšek, M. Kinetic, Thermodynamic, and Structural Analysis of Drug Resistance Mutations in Neuraminidase from the 2009 Pandemic Influenza Virus. Viruses 2018, 10, 339. [Google Scholar] [CrossRef]
- Varghese, J.N.; Laver, W.G.; Colman, P.M. Structure of the influenza virus glycoprotein antigen neuraminidase at 2.9 a resolution. Nature 1983, 303, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.J.; Haire, L.F.; Lin, Y.P.; Liu, J.; Ruseell, R.J.; Walker, P.A.; Skehel, J.J.; Martin, S.R.; Hay, A.J.; Gamblin, S. Crystal structures of oseltamivir-resistant influenza virus neuraminidase mutants. Nature 2008, 453, 1258–1261. [Google Scholar] [CrossRef]
- Vavricka, C.J.; Li, Q.; Wu, Y.; Qi, J.; Wang, M.; Liu, Y.; Gao, F.; Liu, J.; Feng, E.; He, J.; et al. Structural and functional analysis of laninamivir and itsoctanoate prodrug reveals group specificmechanisms for influenza NA inhibition. PLoS Pathog. 2011, 7, e1002249. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Bi, Y.H.; Vavricka, C.J.; Sun, X.M.; Zhang, Y.F.; Gao, F.; Zhao, M.; Xiao, H.X.; Qin, C.F.; He, J.H.; et al. Characterization of two distinct neuraminidases from avian-origin hu-man-infecting H7N9 influenza viruses. Cell Res. 2013, 23, 1347–1355. [Google Scholar] [CrossRef]
- Russell, R.; Haire, L.; Stevens, D.; Collins, P.J.; Lin, Y.P.; Blackburn, G.M.; Hay, A.J.; Gamblin, S.J.; Skehel, J.J. The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. Nature 2006, 443, 45–49. [Google Scholar] [CrossRef]
- Shoichet, B.K. Virtual screening of chemical libraries. Nature 2004, 432, 862–865. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.L.; Anderson, A.C. The Design and Docking of Virtual Compound Libraries to Structures of Drug Targets. Curr. Comput.-Aided Drug Des. 2005, 1, 103–127. [Google Scholar]
- Klebe, G.; Grädler, U.; Grüneberg, S.; Krämer, O.; Gohlke, H. Methods and Principles in Medicinal Chemistry; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008; pp. 207–227. [Google Scholar]
- Kapetanovic, I. Computer-Aided Drug Discovery and Development (CADDD): In sili-co-chemico-biological approach. Chem. Biol. Interact. 2008, 171, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Scior, T.; Bernard, P.; Medina-Franco, J.L.; Maggiora, G.M. Large compound databases for structure-activity relationships studies in drug discovery. Mini-Rev. Med. Chem. 2007, 7, 851–860. [Google Scholar] [CrossRef]
- Eichberg, J.; Maiworm, E.; Oberpaul, M.; Czudai-Matwich, V.; Lüddecke, T.; Vilcinskas, A.; Hardes, K. Antiviral Potential of Natural Resources against Influenza Virus Infections. Viruses 2022, 14, 2452. [Google Scholar] [CrossRef]
- Steindl, T.; Langer, T. Influenza virus neuraminidase inhibitors: Generation and comparison of structure-based and common feature pharmacophore hypotheses and their application in virtual screening. J. Chem. Inf. Comput. Sci. 2004, 44, 1849–1856. [Google Scholar] [CrossRef]
- Márquez-Domínguez, L.; Reyes-Leyva, J.; Herrera-Camacho, I.; Santos-López, G.; Scior, T. Five Novel Non-Sialic Acid-Like Scaffolds Inhibit In Vitro H1N1 and H5N2 Neuraminidase Activity of Influenza a Virus. Molecules 2020, 25, 4248. [Google Scholar] [CrossRef]
- Scior, T.; Medina-Franco, J.L.; Do, Q.T.; Martínez-Mayorga, K.; Yunes Rojas, J.A.; Bernard, P. How to recognize and worka-round pitfalls in QSAR studies: A critical review. Curr. Med. Chem. 2009, 16, 4297–4313. [Google Scholar] [CrossRef] [PubMed]
- Medina-Franco, J.L.; Martínez-Mayorga, K.; Bender, A.; Marín, R.M.; Giulianotti, M.A.; Pinilla, C.; Houghten, R.A. Characterization of activity landscapes using 2D and 3D similarity methods: Consensus activity cliffs. J. Chem. Inf. Model. 2009, 49, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Jones, J.C.; Wong, S.-S.; Zanin, M. Antivirals Targeting the Surface Glycoproteins of Influenza Virus: Mechanisms of Action and Resistance. Viruses 2021, 13, 624. [Google Scholar] [CrossRef] [PubMed]
- Agamennone, M.; Fantacuzzi, M.; Vivenzio, G.; Scala, M.C.; Campiglia, P.; Superti, F.; Sala, M. Antiviral Peptides as Anti-Influenza Agents. Int. J. Mol. Sci. 2022, 23, 11433. [Google Scholar] [CrossRef]
- Sartori, A.; Dell’Amico, L.; Battistini, L.; Curti, C.; Rivara, S.; Pala, D.; Kerry, P.S.; Pelosi, G.; Casiraghi, G.; Rassu, G.; et al. Synthesis, structure and inhibitory activity of a stereoisomer of oseltamivir carboxylate. Org. Biomol. Chem. 2014, 12, 1561–1569. [Google Scholar] [CrossRef] [PubMed]
- Scior, T.; Bender, A.; Tresadern, G.; Medina-Franco, J.L.; Martínez-Mayorga, K. Recognizing pitfalls in virtual screening: A critical review. J. Chem. Inf. Model. 2012, 52, 867–881. [Google Scholar] [CrossRef]
- Quiroga, I.; Scior, T. Induced fit for cytochrome P450 3A4 based on molecular dynamics. ADMET DMPK 2019, 11, 252–266. [Google Scholar] [CrossRef]
- Nabuurs, S.B.; Wagener, M.; De Vlieg, J. A flexible approach to induced fit docking. J. Med. Chem. 2007, 50, 6507–6518. [Google Scholar] [CrossRef]
- Sano, E.; Li, W.; Yuki, H.; Liu, X.; Furihata, V.; Kobayashi, K.; Chiba, K.; Neya, S.; Hoshino, T. Mechanism of the decrease in catalytic activity of human cytochrome P450 2C9 polymorphic variants investigated by computational analysis. J. Comput. Chem. 2010, 15, 2746–2758. [Google Scholar] [CrossRef]
- Sheng, Y.; Chen, Y.; Wang, L.; Liu, G.; Li, W.; Tang, Y. Effects of protein flexibility on the site of metabolism prediction for CYP2A6 substrates. J. Mol. Graph. Model. 2014, 54, 90–99. [Google Scholar] [CrossRef]
- Naceri, S.; Marc, D.; Camproux, A.C.; Flatters, D. Influenza a Virus NS1 Protein Structural Flexibility Analysis According to Its Structural Polymorphism Using Computational Approaches. Int. J. Mol. Sci. 2022, 4, 1805. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, D.; Shen, Z.; Li, S.; Li, H. Multi-Body Interactions in Molecular Docking Program Devised with Key Water Molecules in Protein Binding Sites. Molecules 2018, 23, 2321. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, I.; Meléndez, F.; Atonal-Salvador, K.; Kammerer, B.; Scior, T. Identification a New Site of Metabolism for Phenprocoumon by Modeling it’s CYP2C9 Hydroxylation Pattern. SAJ Pharm. Pharmacol. 2018, 5, 1–12. [Google Scholar]
- Kirchmair, J.; Wolber, G.; Laggner, C.; Langer, T. Comparative performance assessment of the conformational model generators 397 omega and catalyst: A large-scale survey on the retrieval of protein-bound ligand conformations. J. Chem. Inf. Model. 2006, 46, 1848–1861. [Google Scholar]
- Shaillay Kumar, D. Script for Computing Tanimoto Coefficient. QSARWorld—Free Online Resource for QSAR Modeling. Available online: http://www.qsarworld.com/virtual-workshop.php (accessed on 10 April 2023).







| Extracted 3D-Structure | PDB Code | Types of Molecules and Viruses | Resolution (Å); Year | Ref. |
|---|---|---|---|---|
| Neuraminidase (target protein) | 5NZ4 | OS—liganded neuraminidase N1; unidentified strain; (*) | 2.2; 2018 | [21] |
| Sialic Acid (SA) | 2BAT | SA—N2 complex; influenza A virus; A/Tokyo/3/1967 (H2N2) | 2; 1992 | [22] |
| Oseltamivir (OS) | 3CL0 | OS—N1 complex; influenza A virus; influenza A virus; A/Viet Nam/1203/2004 (H5N1) (**) | 2.2; 2008 | [23] |
| Zanamivir (ZA) | 3TI5 | ZA—N1 complex; influenza A virus; A/California/04/2009 (H1N1) | 1.9; 2011 | [24] |
| Peramivir (PE) | 4MWV | PE—N9 complex; influenza H7N9 virus; human-infecting variant from avian origin | 2.0; 2013 | [25] |
| Laninamivir (LA) | 3TI4 | LA—N1 complex; influenza A virus (A/California/04/2009 (H1N1) | 1.6; 2011 | [24] |
| DANA | 2HTR | DANA—N8 complex; influenza A virus (unspecified strain) | 2.5; 2006 | [26] |
| Residue | Sial ac. | DANA | Osel | Zana | Pera | Lani | AAmol | Fmol |
|---|---|---|---|---|---|---|---|---|
| Arg118 | -COO (-) | -COO (-) | -COO (-) | -COO (-) | -COO (-) | -COO (-) | BB amide | BB amide |
| Arg292 | -COO (-) | -COO (-) | -COO (-) | -COO (-) | -COO (-) | -COO (-) | -COO (-) | -COO (-) |
| Arg371 | -COO (-) | -COO (-) | -COO (-) | -COO (-) | -COO (-) | -COO (-) | acetamido | BB amide |
| Arg152 | acetamido | acetamido | acetamido | acetamido | acetamido | acetamido | -COO (-) | -COO (-) |
| Arg224 | - | tri- hydroxy- propyl | - | - | - | - | -COO (-) | -COO (-) |
| Glu276 | tri- hydroxy- propyl | tri- hydroxy- propyl | - | tri- hydroxy- propyl | - | - | - | - |
| Glu277 | - | - | - | guanidino | Guanidino | guanidino | - | - |
| Glu119 | - | - | -NH3 (+) | guanidino | Guanidino | guanidino | - | - |
| Asp151; -CH- not OO | 2-hydroxy on oxane ring; | - | -NH3 (+); none | guanidino; none | guanidino; 2-hydroxy on cyclopentane | guanidino; none | amido; -S-CH3 | piper- azinyl; none |
| Ser246 | - | - | [no IA with alkyl ] | tri- hydroxy- propyl | - | 2,3-dihydroxy- 1-methoxy propyl | - | - |
| Asn294 | tri- hydroxy- propyl | same as left but Gly294 on N2 prot. | - | tri- hydroxy- propyl | - | 2,3-dihydroxy −1-methoxy propyl | - | - |
| Tyr347 | -COO (-) | - | - | - | acetamido | - | ||
| Tyr406 | ether-O- in oxane | ether-O- in pyran | -- | ether-O- in pyran | - | ether-O- in pyran | - | - |
| Val149 Ile 427 Pro431 | - | - | - | - | - | - | phenyl | di- methyl- phenyl |
| Ala248 | - | [no IA tri- hydroxy- propyl ] | -alkyl | - | 2-ethylbutyl | - | - | - |
| Name | Acidic pKa | MlogP (Neutral Form) | logD (ionized) | Perm Skin | Solu w |
|---|---|---|---|---|---|
| AAmol | 3.8 | 1.0 | −1.6 | 6.85 | 0.9 |
| Fmol | 11.3; 4.0 | −1.6 (*) | −1.4 (*) | 0.01 | 4.6 |
| Name | pH in w | BBB_Filter | Vd in L/Kg | RuleOf5 | CYP_1A2 |
| AAmol | 3.24 | Low | 0.22 | 0 | No (96%) |
| Fmol | 6.89 | Low | 0.54 | 0 | No (96%) |
| Name | CYP_2C8 | CYP_2C8 (id) | CYP_2C9 | CYP_risk | TOX_MRTD |
| AAmol | Yes (73%) | S19(992); C20(869); C4(828) | No (56%) | 0 | Above_3.16 |
| Fmol | No (92%) | NonSubstrate | No (98%) | 0 | Above_3.16 |
| Name | TOX_hERG | TOX_ER | TOX_rat | TOX_skin | TOX_biodeg |
| AAmol | No (95%) | Nontoxic | 2066.07 | Nonsensit. (75%) | No (63%) |
| Fmol | No (95%) | Nontoxic | 941.78 | Nonsensit. (85%) | No (96%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scior, T.; Cuanalo-Contreras, K.; Islas, A.A.; Martinez-Laguna, Y. Targeting the Human Influenza a Virus: The Methods, Limitations, and Pitfalls of Virtual Screening for Drug-like Candidates Including Scaffold Hopping and Compound Profiling. Viruses 2023, 15, 1056. https://doi.org/10.3390/v15051056
Scior T, Cuanalo-Contreras K, Islas AA, Martinez-Laguna Y. Targeting the Human Influenza a Virus: The Methods, Limitations, and Pitfalls of Virtual Screening for Drug-like Candidates Including Scaffold Hopping and Compound Profiling. Viruses. 2023; 15(5):1056. https://doi.org/10.3390/v15051056
Chicago/Turabian StyleScior, Thomas, Karina Cuanalo-Contreras, Angel A. Islas, and Ygnacio Martinez-Laguna. 2023. "Targeting the Human Influenza a Virus: The Methods, Limitations, and Pitfalls of Virtual Screening for Drug-like Candidates Including Scaffold Hopping and Compound Profiling" Viruses 15, no. 5: 1056. https://doi.org/10.3390/v15051056
APA StyleScior, T., Cuanalo-Contreras, K., Islas, A. A., & Martinez-Laguna, Y. (2023). Targeting the Human Influenza a Virus: The Methods, Limitations, and Pitfalls of Virtual Screening for Drug-like Candidates Including Scaffold Hopping and Compound Profiling. Viruses, 15(5), 1056. https://doi.org/10.3390/v15051056