A Cross-Sectional Serosurvey of Anti-Orthopoxvirus Antibodies in Central and Western Africa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
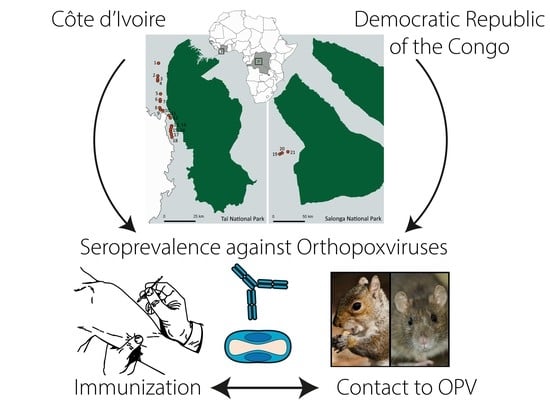
2.2. Study Populations and Sample Collection
2.3. Definition of the Smallpox Vaccination Status
2.4. Enzyme Linked Immunosorbent Assay (ELISA)
2.4.1. General Procedure
2.4.2. ELISA Data Processing and Normalization
2.4.3. Cross Validation by Method Comparison
2.4.4. Determination of the Seroprevalence
2.5. Stastistical Analyses
2.6. Longitudinal Study of Unvaccinated People in CIV
3. Results
3.1. Validation of the In-House ELISA
3.2. Serological Screening
3.2.1. Anti-OPV Seroprevalence in Smallpox-Vaccinated Participants
3.2.2. Anti-OPV Seroprevalence in Smallpox Non-Vaccinated Participants
3.2.3. Anti-OPV Seroprevalence in Participants with Unclear Smallpox Vaccination Status
3.2.4. Longitudinal Study of Unvaccinated Participants in CIV
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Damon, I.K. Poxviruses. In Field’s Virology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 2905–2975. [Google Scholar]
- Essbauer, S.; Pfeffer, M.; Meyer, H. Zoonotic poxviruses. Vet. Microbiol. 2010, 140, 229–236. [Google Scholar] [PubMed]
- Fenner, F.; Henderson, D.A.; Arita, I.; Ježek, Z.; Ladnyi, I.D. Smallpox and Its Eradication; World Health Organization: Geneva, Switzerland, 1988. [Google Scholar]
- Rimoin, A.W.; Mulembakani, P.M.; Johnston, S.C.; Lloyd Smith, J.O.; Kisalu, N.K.; Kinkela, T.L.; Blumberg, S.; Thomassen, H.A.; Pike, B.L.; Fair, J.N.; et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc. Natl. Acad. Sci. USA 2010, 107, 16262–16267. [Google Scholar] [PubMed]
- Shchelkunov, S.N. An increasing danger of zoonotic orthopoxvirus infections. PLoS Pathog. 2013, 9, e1003756. [Google Scholar]
- Nitsche, A.; Pauli, G. Sporadic human cases of cowpox in Germany. Euro Surveill. 2007, 12, E070419.3. [Google Scholar] [PubMed]
- Campe, H.; Zimmermann, P.; Glos, K.; Bayer, M.; Bergemann, H.; Dreweck, C.; Graf, P.; Weber, B.K.; Meyer, H.; Büttner, M.; et al. Cowpox virus transmission from pet rats to humans, Germany. Emerg. Infect. Dis. 2009, 15, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Silva-Fernandes, A.T.; Travassos, C.E.; Ferreira, J.M.; Abrahão, J.S.; Rocha, E.S.; Viana-Ferreira, F.; dos Santos, J.R.; Bonjardim, C.A.; Ferreira, P.C.; Kroon, E.G. Natural human infections with Vaccinia virus during bovine vaccinia outbreaks. J. Clin. Virol. 2009, 44, 308–313. [Google Scholar] [PubMed]
- McCollum, A.M.; Damon, I.K. Human monkeypox. Clin. Infect. Dis. 2014, 58, 260–267. [Google Scholar] [PubMed]
- Vora, S.; Damon, I.; Fulginiti, V.; Weber, S.G.; Kahana, M.; Stein, S.L.; Gerber, S.I.; Garcia-Houchins, S.; Lederman, E.; Hruby, D.; et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin. Infect. Dis. 2008, 46, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.G.; Damon, I.K. Outbreaks of human monkeypox after cessation of smallpox vaccination. Trends Microbiol. 2012, 20, 80–87. [Google Scholar] [PubMed]
- Parker, S.; Nuara, A.; Buller, R.M.; Schultz, D.A. Human monkeypox: An emerging zoonotic disease. Future Microbiol. 2007, 2, 17–34. [Google Scholar] [PubMed]
- Khodakevich, L.; Jezek, Z.; Kinzanzka, K. Isolation of monkeypox virus from wild squirrel infected in nature. Lancet 1986, 1, 98–99. [Google Scholar] [CrossRef]
- Radonic, A.; Metzger, S.; Dabrowski, P.W.; Couacy-Hymann, E.; Schuenadel, L.; Kurth, A.; Mätz-Rensing, K.; Boesch, C.; Leendertz, F.H.; Nitsche, A. Fatal monkeypox in wild-living sooty mangabey, Cote d’Ivoire, 2012. Emerg. Infect. Dis. 2014, 20, 1009–1011. [Google Scholar] [CrossRef] [PubMed]
- Hutin, Y.J.; Williams, R.J.; Malfait, P.; Pebody, R.; Loparev, V.N.; Ropp, S.L.; Rodriguez, M.; Knight, J.C.; Tshioko, F.K.; Khan, A.S.; et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 2001, 7, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.S.; Peterson, A.T.; Yorita, K.L.; Carroll, D.; Damon, I.K.; Reynolds, M.G. Ecological niche and geographic distribution of human monkeypox in Africa. PLoS ONE 2007, 2, e176. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, H.A.; Fuller, T.; Asefi-Najafabady, S.; Shiplacoff, J.A.; Mulembakani, P.M.; Blumberg, S.; Johnston, S.C.; Kisalu, N.K.; Kinkela, T.L.; Fair, J.N.; et al. Pathogen-host associations and predicted range shifts of human monkeypox in response to climate change in central Africa. Emerg. Infect. Dis. 2013, 8, e66071. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, Y.; Lash, R.R.; Carroll, D.S.; Damon, I.K.; Karem, K.L.; Reynolds, M.G.; Osorio, J.E.; Rocke, T.E.; Malekani, J.M.; Muyembe, J.J.; et al. Mapping monkeypox transmission risk through time and space in the Congo Basin. Emerg. Infect. Dis. 2013, 8, e74816. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.L.; Langland, J.O.; Kibler, K.V.; Denzler, K.L.; White, S.D.; Holechek, S.A.; Wong, S.; Huynh, T.; Baskin, C.R. Vaccinia virus vaccines: Past, present and future. Antivir. Res. 2009, 84, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jordan, R.; Leeds, J.M.; Tyavanagimatt, S.; Hruby, D.E. Development of ST-246(R) for treatment of poxvirus infections. Viruses 2010, 2, 2409–2435. [Google Scholar] [CrossRef] [PubMed]
- Lanier, R.; Trost, L.; Tippin, T.; Lampert, B.; Robertson, A.; Foster, S.; Rose, M.; Painter, W.; O’Mahony, R.; Almond, M.; et al. Development of CMX001 for the treatment of poxvirus infections. Viruses 2010, 2, 2740–2762. [Google Scholar] [CrossRef] [PubMed]
- Kurth, A.; Straube, M.; Kuczka, A.; Dunsche, A.J.; Meyer, H.; Nitsche, A. Cowpox virus outbreak in banded mongooses (Mungos mungo) and jaguarundis (Herpailurus yagouaroundi) with a time-delayed infection to humans. Emerg. Infect. Dis. 2009, 4, e6883. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, P.T.; Schuenadel, L.; Wiethaus, J.; Bourquain, D.R.; Kurth, A.; Nitsche, A. Cellular impedance measurement as a new tool for poxvirus titration, antibody neutralization testing and evaluation of antiviral substances. Biochem. Biophys. Res. Commun. 2010, 401, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Bayeen, R.H. Analyzing Linguistic Data. A Practical Introduction to Statistics Using R; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Lme4: Linear Mixed-Effects Models Using Eigen and S4. R Package Version. 2014, Volume 1. Available online: http://lme4.r-forge.r-project.org/ (accessed on 28 September 2017).
- Field, A.P. Discovering Statistics Using SPSS: (And Sex, Drugs and Rock ‘n’ Roll), 2nd ed.; ISM Introducing Statistical Methods; Sage Publications: London, UK; Thousand Oaks, CA, USA, 2005; Volume xxxiv, 779p. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; Sage Publications: London, UK; Thousand Oaks, CA, USA, 2010. [Google Scholar]
- Bolker, B.M. Ecological Models and Data in R; Princeton University Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Barr, D.J. Random effects structure for testing interactions in linear mixed-effects models. Front. Psychol. 2013, 4, 328. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.; Richter, M.; Hapke, C.; Stern, D.; Nitsche, A. Genomic expression libraries for the identification of cross-reactive orthopoxvirus antigens. Emerg. Infect. Dis. 2011, 6, e21950. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.H.; Molina, D.M.; Wrammert, J.; Miller, J.; Hirst, S.; Mu, Y.; Pablo, J.; Unal, B.; Nakajima-Sasaki, R.; Liang, X.; et al. Proteome-wide analysis of the serological response to vaccinia and smallpox. Proteomics 2007, 7, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.G.; Carroll, D.S.; Olson, V.A.; Hughes, C.; Galley, J.; Likos, A.; Montgomery, J.M.; Suu-Ire, R.; Kwasi, M.O.; Jeffrey Root, J.; et al. A silent enzootic of an orthopoxvirus in Ghana, West Africa: Evidence for multi-species involvement in the absence of widespread human disease. Am. J. Trop. Med. Hyg. 2010, 82, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Lederman, E.R.; Reynolds, M.G.; Karem, K.; Braden, Z.; Learned-Orozco, L.A.; Wassa-Wassa, D.; Moundeli, O.; Hughes, C.; Harvey, J.; Regnery, R.; et al. Prevalence of antibodies against orthopoxviruses among residents of Likouala region, Republic of Congo: Evidence for monkeypox virus exposure. Am. J. Trop. Med. Hyg. 2007, 77, 1150–1156. [Google Scholar] [PubMed]
- Jezek, Z.; Nakano, J.H.; Arita, I.; Mutombo, M.; Szczeniowski, M.; Dunn, C. Serological survey for human monkeypox infections in a selected population in Zaire. J. Trop. Med. Hyg. 1987, 90, 31–38. [Google Scholar] [PubMed]
- Talani, P.; Maniane-Nanga, J.; Konongo, J.D.; Gromyko, A.I.; Yala, F. Prevalence des anticorps specifiques du monkeypox au Congo-Brazza Ville. Médecine d’Afrique Noire 1999, 46, 421–423. [Google Scholar]
- Taub, D.D.; Ershler, W.B.; Janowski, M.; Artz, A.; Key, M.L.; McKelvey, J.; Muller, D.; Moss, B.; Ferrucci, L.; Duffey, P.L.; et al. Immunity from smallpox vaccine persists for decades: A longitudinal study. Am. J. Med. 2008, 121, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S.; Felgner, P.; Davies, H.; Glidewell, J.; Villarreal, L.; Ahmed, R. Cutting edge: Long-term B cell memory in humans after smallpox vaccination. J. Immunol. 2003, 171, 4969–4973. [Google Scholar] [CrossRef] [PubMed]
- Putz, M.M.; Alberini, I.; Midgley, C.M.; Manini, I.; Montomoli, E.; Smith, G.L. Prevalence of antibodies to Vaccinia virus after smallpox vaccination in Italy. J. Gen. Virol. 2005, 86 Pt 11, 2955–2960. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.E.; Slifka, M.K. Retrospective analysis of monkeypox infection. Emerg. Infect. Dis. 2008, 14, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Gilchuk, I.; Gilchuk, P.; Sapparapu, G.; Lampley, R.; Singh, V.; Kose, N.; Blum, D.L.; Hughes, L.J.; Satheshkumar, P.S.; Townsend, M.B.; et al. Cross-neutralizing and protective human antibody specificities to poxvirus infections. Cell 2016, 167, 684–694.e9. [Google Scholar] [CrossRef] [PubMed]
- Nalca, A.; Rimoin, A.W.; Bavari, S.; Whitehouse, C.A. Reemergence of monkeypox: Prevalence, diagnostics, and countermeasures. Clin. Infect. Dis. 2005, 41, 1765–1771. [Google Scholar] [PubMed]
- Dubois, M.E.; Hammarlund, E.; Slifka, M.K. Optimization of peptide-based ELISA for serological diagnostics: A retrospective study of human monkeypox infection. Vector Borne Zoonotic Dis. 2012, 12, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, Y.; Mauldin, M.R.; Emerson, G.L.; Reynolds, M.G.; Lash, R.R.; Gao, J.; Zhao, H.; Li, Y.; Muyembe, J.J.; Kingebeni, P.M.; et al. A phylogeographic investigation of African monkeypox. Viruses 2015, 7, 2168–2184. [Google Scholar] [CrossRef] [PubMed]
- Learned, L.A.; Reynolds, M.G.; Wassa, D.W.; Li, Y.; Olson, V.A.; Karem, K.; Stempora, L.L.; Braden, Z.H.; Kline, R.; Likos, A.; et al. Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am. J. Trop. Med. Hyg. 2005, 73, 428–434. [Google Scholar] [PubMed]
- Chen, N.; Li, G.; Liszewski, M.K.; Atkinson, J.P.; Jahrling, P.B.; Feng, Z.; Schriewer, J.; Buck, C.; Wang, C.; Lefkowitz, E.J.; et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology 2005, 340, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.D.; Melski, J.W.; Graham, M.B.; Regnery, R.L.; Sotir, M.J.; Wegner, M.V.; Kazmierczak, J.J.; Stratman, E.J.; Li, Y.; Fairley, J.A.; et al. The detection of monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 2004, 350, 342–350. [Google Scholar] [CrossRef] [PubMed]




| Sample | Côte D’Ivoire | Democratic Rep. of the Congo | ||
|---|---|---|---|---|
| Prevalence (% (95% CIV)) | n | Prevalence (% (95% CIV)) | n | |
| Overall | 51 (48–55) | 737 | 60 (53–65) | 267 |
| Vaccinated | 80 (75–84) | 357 | 96 (91–99) | 112 |
| Females | 79 (73–85) | 180 | 98 (90–100) | 50 |
| Males | 80 (74–85) | 177 | 95 (87–98) | 62 |
| Unvaccinated | 19 (14–26) | 202 | 26 (18–35) | 113 |
| Females | 15 (9–23) | 101 | 23 (15–34) | 69 |
| Males | 24 (17–33) | 101 | 30 (18–44) | 44 |
| Resampling unvaccinated | 25 (16–37) | 75 | Not done | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leendertz, S.A.J.; Stern, D.; Theophil, D.; Anoh, E.; Mossoun, A.; Schubert, G.; Wiersma, L.; Akoua-Koffi, C.; Couacy-Hymann, E.; Muyembe-Tamfum, J.-J.; et al. A Cross-Sectional Serosurvey of Anti-Orthopoxvirus Antibodies in Central and Western Africa. Viruses 2017, 9, 278. https://doi.org/10.3390/v9100278
Leendertz SAJ, Stern D, Theophil D, Anoh E, Mossoun A, Schubert G, Wiersma L, Akoua-Koffi C, Couacy-Hymann E, Muyembe-Tamfum J-J, et al. A Cross-Sectional Serosurvey of Anti-Orthopoxvirus Antibodies in Central and Western Africa. Viruses. 2017; 9(10):278. https://doi.org/10.3390/v9100278
Chicago/Turabian StyleLeendertz, Siv Aina J., Daniel Stern, Dennis Theophil, Etile Anoh, Arsène Mossoun, Grit Schubert, Lidewij Wiersma, Chantal Akoua-Koffi, Emmanuel Couacy-Hymann, Jean-Jacques Muyembe-Tamfum, and et al. 2017. "A Cross-Sectional Serosurvey of Anti-Orthopoxvirus Antibodies in Central and Western Africa" Viruses 9, no. 10: 278. https://doi.org/10.3390/v9100278
APA StyleLeendertz, S. A. J., Stern, D., Theophil, D., Anoh, E., Mossoun, A., Schubert, G., Wiersma, L., Akoua-Koffi, C., Couacy-Hymann, E., Muyembe-Tamfum, J. -J., Karhemere, S., Pauly, M., Schrick, L., Leendertz, F. H., & Nitsche, A. (2017). A Cross-Sectional Serosurvey of Anti-Orthopoxvirus Antibodies in Central and Western Africa. Viruses, 9(10), 278. https://doi.org/10.3390/v9100278