A Liquid Chromatography-Quadrupole-Time-of-Flight Mass Spectrometric Assay for the Quantification of Fabry Disease Biomarker Globotriaosylceramide (GB3) in Fabry Model Mouse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Stock Solution
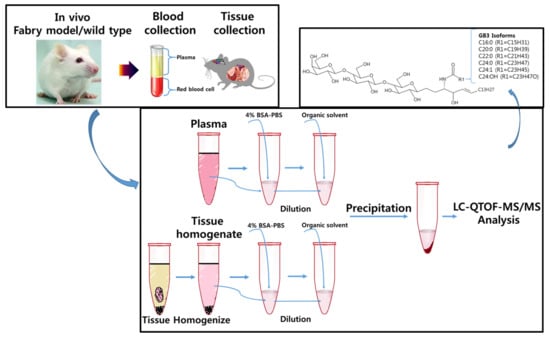
2.3. Sample Preparation-Plasma
2.4. Sample Preparation–Tissues (Heart, Liver, Spleen, Kidney, Brain)
2.5. Liquid Chromatographic Mass Spectrometry (LC-MS/MS) Condition
2.6. Method Qualification
2.7. Software
2.8. Application for Animal Study
3. Results
3.1. Method Development: Sample Preparation and LC-MS/MS Analysis
3.2. Method Qualification
3.2.1. Calibration Curve, Accuracy, and Precision
3.2.2. Preliminary Stability
3.3. Application for Animal Study
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brady, R.O. Enzymatic abnormalities in diseases of sphingolipid metabolism. Clin. Chem. 1967, 13, 565–577. [Google Scholar] [PubMed]
- Fabry, H. An historical overview of Fabry disease. J. Inherit. Metab. Dis. 2001, 24 (Suppl. 2), 3–7. [Google Scholar] [CrossRef] [PubMed]
- Desnick, R.J.; Allen, K.Y.; Simmons, R.L.; Woods, J.E.; Anderson, C.F.; Najarian, J.S.; Krivit, W. Fabry disease: Correction of the enzymatic deficiency by renal transplantation. Birth Defects Orig. Artic. Ser. 1973, 9, 88–96. [Google Scholar] [PubMed]
- Desnick, R.J.; Brady, R.; Barranger, J.; Collins, A.J.; Germain, D.P.; Goldman, M.; Grabowski, G.; Packman, S.; Wilcox, W.R. Fabry disease, an under-recognized multisystemic disorder: Expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann. Intern. Med. 2003, 138, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Thurberg, B.L.; Rennke, H.; Colvin, R.B.; Dikman, S.; Gordon, R.E.; Collins, A.B.; Desnick, R.J.; O’Callaghan, M. Globotriaosylceramide accumulation in the Fabry kidney is cleared from multiple cell types after enzyme replacement therapy. Kidney Int. 2002, 62, 1933–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDermot, K.D.; Holmes, A.; Miners, A.H. Anderson-Fabry disease: Clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J. Med. Genet. 2001, 38, 750–760. [Google Scholar] [CrossRef] [PubMed]
- MacDermot, K.D.; Holmes, A.; Miners, A.H. Anderson-Fabry disease: Clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J. Med. Genet. 2001, 38, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.O.; Tallman, J.F.; Johnson, W.G.; Gal, A.E.; Leahy, W.R.; Quirk, J.M.; Dekaban, A.S. Replacement therapy for inherited enzyme deficiency. Use of purified ceramidetrihexosidase in Fabry’s disease. N. Engl. J. Med. 1973, 289, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Eng, C.M.; Guffon, N.; Wilcox, W.R.; Germain, D.P.; Lee, P.; Waldek, S.; Caplan, L.; Linthorst, G.E.; Desnick, R.J.; International Collaborative Fabry Disease Study Group. Safety and efficacy of recombinant human α-galactosidase A replacement therapy in Fabry’s disease. N. Engl. J. Med. 2001, 345, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann, R.; Ries, M.; Timmons, M.; Flaherty, J.T.; Brady, R.O. Long-term therapy with agalsidase alfa for Fabry disease: Safety and effects on renal function in a home infusion setting. Nephrol. Dial. Transplant. 2006, 21, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Valenzano, K.J.; Khanna, R.; Powe, A.C.; Boyd, R.; Lee, G.; Flanagan, J.J.; Benjamin, E.R. Identification and characterization of pharmacological chaperones to correct enzyme deficiencies in lysosomal storage disorders. Assay Drug Dev. Technol. 2011, 9, 213–235. [Google Scholar] [CrossRef] [PubMed]
- Yam, G.H.; Bosshard, N.; Zuber, C.; Steinmann, B.; Roth, J. Pharmacological chaperone corrects lysosomal storage in Fabry disease caused by trafficking-incompetent variants. Am. J. Physiol. Cell Physiol. 2006, 290, C1076–C1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young-Gqamana, B.; Brignol, N.; Chang, H.H.; Khanna, R.; Soska, R.; Fuller, M.; Sitaraman, S.A.; Germain, D.P.; Giugliani, R.; Hughes, D.A.; et al. Migalastat HCl reduces globotriaosylsphingosine (lyso-Gb3) in Fabry transgenic mice and in the plasma of Fabry patients. PLoS ONE 2013, 8, e57631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitagawa, T.; Ishige, N.; Suzuki, K.; Owada, M.; Ohashi, T.; Kobayashi, M.; Eto, Y.; Tanaka, A.; Mills, K.; Winchester, B.; et al. Non-invasive screening method for Fabry disease by measuring globotriaosylceramide in whole urine samples using tandem mass spectrometry. Mol. Genet. Metab. 2005, 85, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, P.D.; Calvin, J.; Hogg, S.; O’Driscoll, E.; Halsall, D.; Burling, K.; Maguire, G.; Wright, N.; Cox, T.M.; Meikle, P.J.; et al. Monitoring enzyme replacement therapy in Fabry disease—Role of urine globotriaosylceramide. J. Inherit. Metab. Dis. 2005, 28, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Auray-Blais, C.; Cyr, D.; Ntwari, A.; West, M.L.; Cox-Brinkman, J.; Bichet, D.G.; Germain, D.P.; Laframboise, R.; Melancon, S.B.; Stockley, T.; et al. Urinary globotriaosylceramide excretion correlates with the genotype in children and adults with Fabry disease. Mol. Genet. Metab. 2008, 93, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.C.; Roddy, T.; Araghi, S.; Wilkens, D.; Thomas, J.J.; Zhang, K.; Sung, C.C.; Richards, S.M. Globotriaosylceramide isoform profiles in human plasma by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 805, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, I.; Nishizawa, M.; Ariga, T.; Miyatake, T. Biochemical and clinical analysis of accumulated glycolipids in symptomatic heterozygotes of angiokeratoma corporis diffusum (Fabry’s disease) in comparison with hemizygotes. J. Lipid Res. 1990, 31, 335–340. [Google Scholar] [PubMed]
- Kniep, B.; Muhlradt, P.F. Immunochemical detection of glycosphingolipids on thin-layer chromatograms. Anal. Biochem. 1990, 188, 5–8. [Google Scholar] [CrossRef]
- Groener, J.E.; Poorthuis, B.J.; Kuiper, S.; Helmond, M.T.; Hollak, C.E.; Aerts, J.M. HPLC for simultaneous quantification of total ceramide, glucosylceramide, and ceramide trihexoside concentrations in plasma. Clin. Chem. 2007, 53, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Fauler, G.; Rechberger, G.N.; Devrnja, D.; Erwa, W.; Plecko, B.; Kotanko, P.; Breunig, F.; Paschke, E. Rapid determination of urinary globotriaosylceramide isoform profiles by electrospray ionization mass spectrometry using stearoyl-d35-globotriaosylceramide as internal standard. Rapid Commun. Mass Spectrom. 2005, 19, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Auray-Blais, C.; Boutin, M. Novel Gb(3) isoforms detected in urine of fabry disease patients: A metabolomic study. Curr. Med. Chem. 2012, 19, 3241–3252. [Google Scholar] [CrossRef] [PubMed]
- Sueoka, H.; Aoki, M.; Tsukimura, T.; Togawa, T.; Sakuraba, H. Distributions of Globotriaosylceramide Isoforms, and Globotriaosylsphingosine and Its Analogues in an α-Galactosidase A Knockout Mouse, a Model of Fabry Disease. PLoS ONE 2015, 10, e0144958. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, F.J.; Geberhiwot, T.; Hughes, D.A.; Ward, D.G. A Novel Rapid MALDI-TOF-MS-Based Method for Measuring Urinary Globotriaosylceramide in Fabry Patients. J. Am. Soc. Mass Spectrom. 2016, 27, 719–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruger, R.; Bruns, K.; Grunhage, S.; Rossmann, H.; Reinke, J.; Beck, M.; Lackner, K.J. Determination of globotriaosylceramide in plasma and urine by mass spectrometry. Clin. Chem. Lab. Med. 2010, 48, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Boutin, M.; Menkovic, I.; Martineau, T.; Vaillancourt-Lavigueur, V.; Toupin, A.; Auray-Blais, C. Separation and Analysis of Lactosylceramide, Galabiosylceramide, and Globotriaosylceramide by LC-MS/MS in Urine of Fabry Disease Patients. Anal. Chem. 2017, 89, 13382–13390. [Google Scholar] [CrossRef] [PubMed]
- Polo, G.; Burlina, A.P.; Kolamunnage, T.B.; Zampieri, M.; Dionisi-Vici, C.; Strisciuglio, P.; Zaninotto, M.; Plebani, M.; Burlina, A.B. Diagnosis of sphingolipidoses: A new simultaneous measurement of lysosphingolipids by LC-MS/MS. Clin. Chem. Lab. Med. 2017, 55, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Boutin, M.; Gagnon, R.; Lavoie, P.; Auray-Blais, C. LC-MS/MS analysis of plasma lyso-Gb3 in Fabry disease. Clin. Chim. Acta 2012, 414, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, Y.A.; Zeidner, K.M.; Gordon, R.E.; Desnick, R.J. Fabry disease: Preclinical studies demonstrate the effectiveness of α-galactosidase A replacement in enzyme-deficient mice. Am. J. Hum. Genet. 2001, 68, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, T.; Murray, G.J.; Swaim, W.D.; Longenecker, G.; Quirk, J.M.; Cardarelli, C.O.; Sugimoto, Y.; Pastan, I.; Gottesman, M.M.; Brady, R.O.; et al. α-Galactosidase A deficient mice: A model of Fabry disease. Proc. Natl. Acad. Sci. USA 1997, 94, 2540–2544. [Google Scholar] [CrossRef] [PubMed]
- Ramagiri, S.; Garofolo, F. Large molecule bioanalysis using Q-TOF without predigestion and its data processing challenges. Bioanalysis 2012, 4, 529–540. [Google Scholar] [CrossRef] [PubMed]








| TOF-MS Condition | |||
| GS1 | 50 | CUR (Curtain Gas) | 30 |
| GS2 | 50 | ISVP (Ion Spray Voltage) | 5500 |
| SRM High Sensitive Scan Mode, Positive | |||
| GB3 Isoform | Parent-To-Parent Transition | Declustering Voltage (DP) | Collision Energy (CE) |
| C16:0-GB3 | 1046.7→1046.7 | 100 | 66 |
| C17:0-GB3 | 1060.7→1060.7 (ISTD) | 100 | 66 |
| C20:0-GB3 | 1102.7→1102.7 | 100 | 66 |
| C22:0-GB3 | 1130.8→1130.8 | 100 | 66 |
| C24:1-GB3 | 1156.8→1156.8 | 100 | 66 |
| C24:0-GB3 | 1158.8→1158.8 | 100 | 66 |
| C24:0-OH-GB3 | 1174.8→1174.8 | 100 | 66 |
| Matrix | QC Samples | Mean Concentration (ng/mL) | RSD (%) | Mean Accuracy (%) | n |
|---|---|---|---|---|---|
| Plasma | QC medium (400 ng/mL) | 314.28 | 15.06 | 78.57 | 3 |
| QC high (2000 ng/mL) | 2131.34 | 5.92 | 106.57 | 3 | |
| Heart | QC medium (800 ng/mL) | 779.48 | 11.96 | 99.02 | 3 |
| QC high (4000 ng/mL) | 3286.93 | 8.41 | 83.23 | 3 | |
| Liver | QC medium (800 ng/mL) | 823.21 | 14.53 | 102.90 | 3 |
| QC high (4000 ng/mL) | 3395.26 | 8.66 | 84.88 | 3 | |
| Spleen | QC medium (800 ng/mL) | 848.22 | 10.64 | 106.03 | 3 |
| QC high (4000 ng/mL) | 3861.45 | 25.49 | 96.54 | 3 | |
| Kidney | QC medium (800 ng/mL) | 839.15 | 26.16 | 104.89 | 3 |
| QC high (4000 ng/mL) | 4275.53 | 9.38 | 106.89 | 3 | |
| Brain | QC medium (800 ng/mL) | 740.97 | 11.60 | 92.62 | 3 |
| QC high (4000 ng/mL) | 3826.03 | 15.16 | 95.65 | 3 |
| Matrix | Time Point (min) | Mean Area Ratio | RSD (%) | Mean Accuracy (%) | n |
|---|---|---|---|---|---|
| Plasma | 0 | 3.78 | 6.54 | 100.00 | 3 |
| 60 | 3.27 | 6.02 | 86.66 | ||
| 120 | 3.64 | 9.04 | 96.49 | ||
| 180 | 4.12 | 13.53 | 109.20 | ||
| Heart | 0 | 3.06 | 12.10 | 100.00 | 3 |
| 60 | 3.09 | 1.70 | 101.09 | ||
| 120 | 2.88 | 9.98 | 94.34 | ||
| 180 | 2.53 | 12.23 | 82.83 | ||
| Liver | 0 | 3.14 | 9.73 | 100.00 | 3 |
| 60 | 3.07 | 12.30 | 97.80 | ||
| 120 | 3.17 | 12.58 | 100.97 | ||
| 180 | 3.01 | 3.92 | 95.85 | ||
| Spleen | 0 | 3.51 | 8.45 | 100.00 | 3 |
| 60 | 2.89 | 8.54 | 82.31 | ||
| 120 | 3.02 | 11.61 | 86.14 | ||
| 180 | 2.50 | 3.18 | 71.32 | ||
| Kidney | 0 | 2.42 | 10.26 | 100.00 | 3 |
| 60 | 3.03 | 2.88 | 125.16 | ||
| 120 | 2.95 | 14.21 | 121.72 | ||
| 180 | 2.69 | 5.63 | 111.00 | ||
| Brain | 0 | 1.48 | 3.70 | 100.00 | 3 |
| 60 | 1.37 | 16.01 | 92.77 | ||
| 120 | 1.37 | 16.97 | 92.42 | ||
| 180 | 1.55 | 16.97 | 105.07 |
| Matrix | Control/FT-3 Cycle | Mean Area Ratio | RSD (%) | Mean Accuracy (%) | n |
|---|---|---|---|---|---|
| Plasma | Control | 2.31 | 1.91 | 100.00 | 3 |
| FT-3 cycle | 2.24 | 7.97 | 97.14 | ||
| Heart | Control | 2.27 | 11.81 | 100.00 | 3 |
| FT-3 cycle | 2.19 | 7.70 | 96.63 | ||
| Liver | Control | 1.93 | 1.43 | 100.00 | 3 |
| FT-3 cycle | 1.97 | 21.40 | 102.16 | ||
| Spleen | Control | 2.23 | 6.15 | 100.00 | 3 |
| FT-3 cycle | 2.12 | 10.07 | 95.20 | ||
| Kidney | Control | 2.14 | 5.96 | 100.00 | 3 |
| FT-3 cycle | 2.11 | 14.91 | 98.69 | ||
| Brain | Control | 1.34 | 7.09 | 100.00 | 3 |
| FT-3 cycle | 1.32 | 5.68 | 98.21 |
| Organ | Week | Mean Area Ratio | RSD (%) | Mean Accuracy (%) | n |
|---|---|---|---|---|---|
| Plasma | 0 week | 2.26 | 3.36 | 100 | 3 |
| 1 week | 2.29 | 14.55 | 101.46 | 3 | |
| 2 week | 2.27 | 11.23 | 100.23 | 3 | |
| Heart | 0 week | 3.62 | 17.56 | 100 | 3 |
| 1 week | 3.81 | 8.19 | 105.32 | 3 | |
| 2 week | 3.94 | 12.43 | 108.84 | 3 | |
| Liver | 0 week | 2.76 | 12.83 | 100 | 3 |
| 1 week | 2.52 | 0.73 | 91.18 | 3 | |
| 2 week | 3.08 | 6.72 | 111.51 | 3 | |
| Spleen | 0 week | 4.05 | 4.44 | 100 | 3 |
| 1 week | 3.73 | 15.32 | 91.91 | 3 | |
| 2 week | 3.72 | 5.13 | 91.8 | 3 | |
| Kidney | 0 week | 2.63 | 14.47 | 100 | 3 |
| 1 week | 2.69 | 10.44 | 102.31 | 3 | |
| 2 week | 2.77 | 11.44 | 105.4 | 3 | |
| Brain | 0 week | 0.82 | 10.86 | 100 | 3 |
| 1 week | 0.8 | 7.71 | 97.46 | 3 | |
| 2 week | 0.77 | 21.72 | 93.86 | 3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.-H.; Park, M.-H.; Byeon, J.-J.; Lee, B.I.; Park, Y.; Ko, A.-r.; Seong, M.-r.; Lee, S.; Kim, M.R.; Seo, J.; et al. A Liquid Chromatography-Quadrupole-Time-of-Flight Mass Spectrometric Assay for the Quantification of Fabry Disease Biomarker Globotriaosylceramide (GB3) in Fabry Model Mouse. Pharmaceutics 2018, 10, 69. https://doi.org/10.3390/pharmaceutics10020069
Shin S-H, Park M-H, Byeon J-J, Lee BI, Park Y, Ko A-r, Seong M-r, Lee S, Kim MR, Seo J, et al. A Liquid Chromatography-Quadrupole-Time-of-Flight Mass Spectrometric Assay for the Quantification of Fabry Disease Biomarker Globotriaosylceramide (GB3) in Fabry Model Mouse. Pharmaceutics. 2018; 10(2):69. https://doi.org/10.3390/pharmaceutics10020069
Chicago/Turabian StyleShin, Seok-Ho, Min-Ho Park, Jin-Ju Byeon, Byeong Ill Lee, Yuri Park, Ah-ra Ko, Mi-ran Seong, Soyeon Lee, Mi Ra Kim, Jinwook Seo, and et al. 2018. "A Liquid Chromatography-Quadrupole-Time-of-Flight Mass Spectrometric Assay for the Quantification of Fabry Disease Biomarker Globotriaosylceramide (GB3) in Fabry Model Mouse" Pharmaceutics 10, no. 2: 69. https://doi.org/10.3390/pharmaceutics10020069
APA StyleShin, S.-H., Park, M.-H., Byeon, J.-J., Lee, B. I., Park, Y., Ko, A.-r., Seong, M.-r., Lee, S., Kim, M. R., Seo, J., Jung, M. E., Jin, D.-K., & Shin, Y. G. (2018). A Liquid Chromatography-Quadrupole-Time-of-Flight Mass Spectrometric Assay for the Quantification of Fabry Disease Biomarker Globotriaosylceramide (GB3) in Fabry Model Mouse. Pharmaceutics, 10(2), 69. https://doi.org/10.3390/pharmaceutics10020069