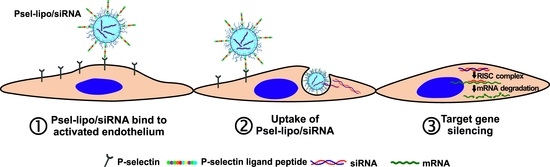
Targeted Transfection Using PEGylated Cationic Liposomes Directed Towards P-Selectin Increases siRNA Delivery into Activated Endothelial Cells
Abstract
:1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Preparation of P-Selectin Directed Cationic Liposomes
2.3. Formation of Lipoplexes
2.4. Characterization of Lipoplexes
2.4.1. Size and Zeta Potential
2.4.2. Gel Retardation Assay
2.5. Cell Culture
2.6. Evaluation of Lipoplexes Cytotoxicity
2.7. Uptake of Psel-Lipo/siRNA Lipoplexes by TNF-α Activated Endothelial Cells
2.7.1. Lipoplexes-EC Incubation in Static Conditions
2.7.2. Lipoplexes-EC Incubation in Dynamic Conditions
2.8. Intracellular Delivery of siRNA by Psel-Lipo/siRNA Lipoplexes
2.9. Knock-Down of GAPDH mRNA by Specific siRNA Delivered Intracellularly by Psel-Lipo/siRNA
2.10. Statistical Analysis
3. Results
3.1. Characterization of Psel-Lipo/siRNA Lipoplexes
3.2. Psel-Lipo Effectively Packs siRNA
3.3. Lipoplexes Cytotoxicity
3.4. Psel-Lipo/siRNA Lipoplexes Are Specifically Taken Up by Activated Endothelial Cells
3.4.1. P-Selectin Is Expressed Predominantly on the Surface of Activated Bend.3 Cells
3.4.2. Psel-Lipo/siRNA Lipoplexes Are Specifically Internalized by TNF-α Activated Endothelial Cells
3.4.3. Psel-Lipo/siRNA Lipoplexes Efficiently Deliver siRNA into TNF-α Activated Endothelial Cells
3.5. Psel-Lipo/siRNA-GAPDH Lipoplexes Knock-Down the Expression of GAPDH mRNA in Endothelial Cells
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Pradhan-Nabzdyk, L.; Huang, C.; LoGerfo, F.W.; Nabzdyk, C.S. Current siRNA targets in the prevention and treatment of intimal hyperplasia. Discov. Med. 2014, 18, 125–132. [Google Scholar] [PubMed]
- Leung, A.K.K.; Tam, Y.Y.C.; Cullis, P.R. Lipid Nanoparticles for Short Interfering RNA Delivery. Adv. Genet. 2014, 88, 71–110. [Google Scholar] [PubMed]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [Green Version]
- Weller, A.; Isenmann, S.; Vestweber, D. Cloning of the mouse endothelial selectins. Expression of both E- and P-selectin is inducible by tumor necrosis factor alpha. J. Biol. Chem. 1992, 267, 15176–15183. [Google Scholar] [PubMed]
- Gotsch, U.; Jäger, U.; Dominis, M.; Vestweber, D. Expression of P-selectin on endothelial cells is upregulated by LPS and TNF-alpha in vivo. Cell Adhes. Commun. 1994, 2, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Khew-Goodall, Y.; Butcher, C.M.; Litwin, M.S.; Newlands, S.; Korpelainen, E.I.; Noack, L.M.; Berndt, M.C.; Lopez, A.F.; Gamble, J.R.; Vadas, M.A. Chronic expression of P-selectin on endothelial cells stimulated by the T-cell cytokine, interleukin-3. Blood 1996, 87, 1432–1438. [Google Scholar] [PubMed]
- Kameda, H.; Morita, I.; Handa, M.; Kaburaki, J.; Yoshida, T.; Mimori, T.; Murota, S.; Ikeda, Y. Re-expression of functional P-selectin molecules on the endothelial cell surface by repeated stimulation with thrombin. Br. J. Haematol. 1997, 97, 348–355. [Google Scholar] [CrossRef]
- Manduteanu, I.; Pirvulescu, M.; Gan, A.M.; Stan, D.; Simion, V.; Dragomir, E.; Calin, M.; Manea, A.; Simionescu, M. Similar effects of resistin and high glucose on P-selectin and fractalkine expression and monocyte adhesion in human endothelial cells. Biochem. Biophys. Res. Commun. 2010, 391, 1443–1448. [Google Scholar] [CrossRef]
- Molenaar, T.J.M.; Twisk, J.; de Haas, S.A.M.; Peterse, N.; Vogelaar, B.J.C.P.; van Leeuwen, S.H.; Michon, I.N.; van Berkel, T.J.C.; Kuiper, J.; Biessen, E.A.L. P-selectin as a candidate target in atherosclerosis. Biochem. Pharmacol. 2003, 66, 859–866. [Google Scholar] [CrossRef]
- McAteer, M.A.; Schneider, J.E.; Ali, Z.A.; Warrick, N.; Bursill, C.A.; von zur Muhlen, C.; Greaves, D.R.; Neubauer, S.; Channon, K.M.; Choudhury, R.P. Magnetic resonance imaging of endothelial adhesion molecules in mouse atherosclerosis using dual-targeted microparticles of iron oxide. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 77–83. [Google Scholar] [CrossRef] [PubMed]
- McAteer, M.A.; Akhtar, A.M.; von Zur Muhlen, C.; Choudhury, R.P. An approach to molecular imaging of atherosclerosis, thrombosis, and vascular inflammation using microparticles of iron oxide. Atherosclerosis 2010, 209, 18–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobin-Valat, M.J.; Deramchia, K.; Mornet, S.; Hagemeyer, C.E.; Bonetto, S.; Robert, R.; Biran, M.; Massot, P.; Miraux, S.; Sanchez, S.; et al. MRI of inducible P-selectin expression in human activated platelets involved in the early stages of atherosclerosis. NMR Biomed. 2011, 24, 413–424. [Google Scholar] [CrossRef] [PubMed]
- McAteer, M.A.; Mankia, K.; Ruparelia, N.; Jefferson, A.; Nugent, H.B.; Stork, L.A.; Channon, K.M.; Schneider, J.E.; Choudhury, R.P. A leukocyte-mimetic magnetic resonance imaging contrast agent homes rapidly to activated endothelium and tracks with atherosclerotic lesion macrophage content. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bauer, W.; Israel, I.; Kreissl, M.C.; Weirather, J.; Richter, D.; Bauer, E.; Herold, V.; Jakob, P.; Buck, A.; et al. Targeting P-selectin by gallium-68-labeled fucoidan positron emission tomography for noninvasive characterization of vulnerable plaques: Correlation with in vivo 17.6T MRI. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Tidey, R.R.; McGregor, J.L.; Taylor, P.R.; Poston, R.N. Increase in the adhesion molecule P-selectin in endothelium overlying atherosclerotic plaques. Coexpression with intercellular adhesion molecule-1. Am. J. Pathol. 1994, 144, 952–961. [Google Scholar] [PubMed]
- Tenaglia, A.N.; Buda, A.J.; Wilkins, R.G.; Barron, M.K.; Jeffords, P.R.; Vo, K.; Jordan, M.O.; Kusnick, B.A.; Lefer, D.J. Levels of expression of P-selectin, E-selectin, and intercellular adhesion molecule-1 in coronary atherectomy specimens from patients with stable and unstable angina pectoris. Am. J. Cardiol. 1997, 79, 742–747. [Google Scholar] [CrossRef]
- Shamay, Y.; Elkabets, M.; Li, H.; Shah, J.; Brook, S.; Wang, F.; Adler, K.; Baut, E.; Scaltriti, M.; Jena, P.V.; et al. P-selectin is a nanotherapeutic delivery target in the tumor microenvironment. Sci. Transl. Med. 2016, 8, 345ra87. [Google Scholar] [CrossRef]
- Subramaniam, M.; Koedam, J.A.; Wagner, D.D. Divergent fates of P- and E-selectins after their expression on the plasma membrane. Mol. Biol. Cell. 1993, 4, 791–801. [Google Scholar] [CrossRef]
- Straley, K.S.; Green, S.A. Rapid transport of internalized P-selectin to late endosomes and the TGN: Roles in regulating cell surface expression and recycling to secretory granules. J. Cell Biol. 2000, 151, 107–116. [Google Scholar] [CrossRef]
- Burch, E.E.; Shinde Patil, V.R.; Camphausen, R.T.; Kiani, M.F.; Goetz, D.J. The N-terminal peptide of PSGL-1 can mediate adhesion to trauma-activated endothelium via P-selectin in vivo. Blood 2002, 100, 531–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eniola, A.O.; Rodgers, S.D.; Hammer, D.A. Characterization of biodegradable drug delivery vehicles with the adhesive properties of leukocytes. Biomaterials 2002, 23, 2167–2177. [Google Scholar] [CrossRef]
- Sakhalkar, H.S.; Dalal, M.K.; Salem, A.K.; Ansari, R.; Fu, J.; Kiani, M.F.; Kurjiaka, D.T.; Hanes, J.; Shakesheff, K.M.; Goetz, D.J. Leukocyte-inspired biodegradable particles that selectively and avidly adhere to inflamed endothelium in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2003, 100, 15895–15900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eniola, A.O.; Hammer, D.A. In vitro characterization of leukocyte mimetic for targeting therapeutics to the endothelium using two receptors. Biomaterials 2005, 26, 7136–7144. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.M.; Crosdale, D.J.; Fisher, K.D.; Briggs, S.S.; Norman, K.E.; Seymour, L.W.; Hellewell, P.G. P-selectin dependent targeting to inflamed endothelium of recombinant P-selectin glycoprotein ligand-1 immunoglobulin chimera-coated poly[N-(2-hydroxypropyl) methacrylamide]-DNA polyplexes in vivo visualised by intravital microscopy. J. Gene Med. 2009, 11, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Sabnis, A.; Kona, S.; Nattama, S.; Patel, H.; Dong, J.F.; Nguyen, K.T. Shear-regulated uptake of nanoparticles by endothelial cells and development of endothelial-targeting nanoparticles. J. Biomed. Mater. Res. A 2010, 93, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Simion, V.; Constantinescu, C.A.; Stan, D.; Deleanu, M.; Tucureanu, M.M.; Butoi, E.; Manduteanu, I.; Simionescu, M.; Calin, M. P-Selectin Targeted Dexamethasone-Loaded Lipid Nanoemulsions: A Novel Therapy to Reduce Vascular Inflammation. Mediat. Inflamm. 2016, 2016, 1625149. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, P.S.; Lintermans, L.L.; Morselt, H.W.; Leus, N.G.; Ruiters, M.H.; Molema, G.; Kamps, J.A. Anti-VCAM-1 and anti-E-selectin SAINT-O-Somes for selective delivery of siRNA into inflammation-activated primary endothelial cells. Mol. Pharm. 2013, 10, 3033–3044. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, P.S.; Zwiers, P.J.; Morselt, H.W.; Kuldo, J.M.; Leus, N.G.; Ruiters, M.H.; Molema, G.; Kamps, J.A. Anti-VCAM-1 SAINT-O-Somes enable endothelial-specific delivery of siRNA and downregulation of inflammatory genes in activated endothelium in vivo. J. Control. Release 2014, 176, 64–75. [Google Scholar] [CrossRef]
- Leus, N.G.; Morselt, H.W.; Zwiers, P.J.; Kowalski, P.S.; Ruiters, M.H.; Molema, G.; Kamps, J.A. VCAM-1 specific PEGylated SAINT-based lipoplexes deliver siRNA to activated endothelium in vivo but do not attenuate target gene expression. Int. J. Pharm. 2014, 469, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, P.S.; Kuninty, P.R.; Bijlsma, K.T.; Stuart, M.C.; Leus, N.G.; Ruiters, M.H.; Molema, G.; Kamps, J.A. SAINT-liposome-polycation particles, a new carrier for improved delivery of siRNAs to inflamed endothelial cells. Eur. J. Pharm. Biopharm. 2015, 89, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Schreiber, A.; Eulenberg-Gustavus, C.; Scheidereit, C.; Kamps, J.; Kettritz, R. Endothelial NF-κB Blockade Abrogates ANCA-Induced GN. J. Am. Soc. Nephrol. 2017, 28, 3191–3204. [Google Scholar] [CrossRef] [PubMed]
- Byk, G.; Scherman, D.; Schwartz, B.; Dubertret, C. Lipopolyamines as Transfection Agents and Pharmaceutical Uses Thereof. U.S. Patent No. 6171612, 9 January 2001. [Google Scholar]
- Thompson, B.; Mignet, N.; Hofland, H.; Lamons, D.; Seguin, J.; Nicolazzi, C.; de la Figuera, N.; Kuen, R.L.; Meng, X.Y.; Scherman, D.; et al. Neutral postgrafted colloidal particles for gene delivery. Bioconjug. Chem. 2005, 16, 608–614. [Google Scholar] [CrossRef]
- Calin, M.; Stan, D.; Schlesinger, M.; Simion, V.; Deleanu, M.; Constantinescu, C.A.; Gan, A.M.; Tucureanu, M.M.; Butoi, E.; Manduteanu, I.; et al. VCAM-1 directed target-sensitive liposomes carrying CCR2 antagonists bind to activated endothelium and reduce adhesion and transmigration of monocytes. Eur. J. Pharm. Biopharm. 2015, 89, 18–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.J.; Kim, B.G.; Eum, J.Y.; Park, S.H.; Choi, S.E.; An, J.J.; Jang, S.H.; Eum, W.S.; Lee, J.; Lee, M.W.; et al. Design of a Pep-1 peptide-modified liposomal nanocarrier system for intracellular drug delivery: Conformational characterization and cellular uptake evaluation. J. Drug Target. 2011, 19, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.; Louis-Plence, P.; Escriou, V.; Noel, D.; Largeau, C.; Cantos, C.; Scherman, D.; Jorgensen, C. Efficient new cationic liposome formulation for systemic delivery of small interfering RNA silencing tumor necrosis factor alpha in experimental arthritis. Arthritis Rheum. 2006, 54, 1867–1877. [Google Scholar] [CrossRef]
- Uritu, C.M.; Varganici, C.D.; Ursu, L.; Coroaba, A.; Nicolescu, A.; Dascalu, A.I.; Peptanariu, D.; Stan, D.; Constantinescu, C.A.; Simion, V.; et al. Hybrid fullerene conjugates as vectors for DNA cell-delivery. J. Mater. Chem. B 2015, 3, 2433–2446. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Mathur, D. Selection of suitable housekeeping genes for expression analysis in glioblastoma using quantitative RT-PCR. Ann. Neurosci. 2014, 21, 62–63. [Google Scholar]
- Kaufmann, J.; Ahrens, K.; Santel, A. RNA interference for therapy in the vascular endothelium. Microvasc. Res. 2010, 80, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Zatsepin, T.S.; Kotelevtsev, Y.V.; Koteliansky, V. Lipid nanoparticles for targeted siRNA delivery—Going from bench to bedside. Int. J. Nanomed. 2016, 11, 3077–3086. [Google Scholar]
- Ozcan, G.; Ozpolat, B.; Coleman, R.L.; Sood, A.K.; Lopez-Berestein, G. Preclinical and clinical development of siRNA-based therapeutics. Adv. Drug Deliv. Rev. 2015, 87, 108–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallego, J.; Garcia-Pras, E.; Mejias, M.; Pell, N.; Schaeper, U.; Fernandez, M. Therapeutic siRNA targeting endothelial KDR decreases portosystemic collateralization in portal hypertension. Sci. Rep. 2017, 7, 14791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, K. The role of selectins in inflammation and disease. Trends Mol. Med. 2003, 9, 263–268. [Google Scholar] [CrossRef] [Green Version]
- Hahne, M.; Jäger, U.; Isenmann, S.; Hallmann, R.; Vestweber, D. Five tumor necrosis factor-inducible cell adhesion mechanisms on the surface of mouse endothelioma cells mediate the binding of leukocytes. J. Cell Biol. 1993, 121, 655–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Córdova, L.A.; Trichet, V.; Escriou, V.; Rosset, P.; Amiaud, J.; Battaglia, S.; Charrier, C.; Berreur, M.; Brion, R.; Gouin, F. Inhibition of osteolysis and increase of bone formation after local administration of siRNA-targeting RANK in a polyethylene particle-induced osteolysis model. Acta Biomater. 2015, 13, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Asgeirsdóttir, S.A.; Talman, E.G.; de Graaf, I.A.; Kamps, J.A.; Satchell, S.C.; Mathieson, P.W.; Ruiters, M.H.; Molema, G. Targeted transfection increases siRNA uptake and gene silencing of primary endothelial cells in vitro—A quantitative study. J. Control. Release 2010, 141, 241–251. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 28–51. [Google Scholar] [CrossRef] [Green Version]
- Cicha, I. Strategies to enhance nanoparticle-endothelial interactions under flow. J. Cell. Biotechnol. 2016, 1, 191–208. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantinescu, C.A.; Fuior, E.V.; Rebleanu, D.; Deleanu, M.; Simion, V.; Voicu, G.; Escriou, V.; Manduteanu, I.; Simionescu, M.; Calin, M. Targeted Transfection Using PEGylated Cationic Liposomes Directed Towards P-Selectin Increases siRNA Delivery into Activated Endothelial Cells. Pharmaceutics 2019, 11, 47. https://doi.org/10.3390/pharmaceutics11010047
Constantinescu CA, Fuior EV, Rebleanu D, Deleanu M, Simion V, Voicu G, Escriou V, Manduteanu I, Simionescu M, Calin M. Targeted Transfection Using PEGylated Cationic Liposomes Directed Towards P-Selectin Increases siRNA Delivery into Activated Endothelial Cells. Pharmaceutics. 2019; 11(1):47. https://doi.org/10.3390/pharmaceutics11010047
Chicago/Turabian StyleConstantinescu, Cristina Ana, Elena Valeria Fuior, Daniela Rebleanu, Mariana Deleanu, Viorel Simion, Geanina Voicu, Virginie Escriou, Ileana Manduteanu, Maya Simionescu, and Manuela Calin. 2019. "Targeted Transfection Using PEGylated Cationic Liposomes Directed Towards P-Selectin Increases siRNA Delivery into Activated Endothelial Cells" Pharmaceutics 11, no. 1: 47. https://doi.org/10.3390/pharmaceutics11010047
APA StyleConstantinescu, C. A., Fuior, E. V., Rebleanu, D., Deleanu, M., Simion, V., Voicu, G., Escriou, V., Manduteanu, I., Simionescu, M., & Calin, M. (2019). Targeted Transfection Using PEGylated Cationic Liposomes Directed Towards P-Selectin Increases siRNA Delivery into Activated Endothelial Cells. Pharmaceutics, 11(1), 47. https://doi.org/10.3390/pharmaceutics11010047