Cationic Niosomes as Non-Viral Vehicles for Nucleic Acids: Challenges and Opportunities in Gene Delivery
Abstract
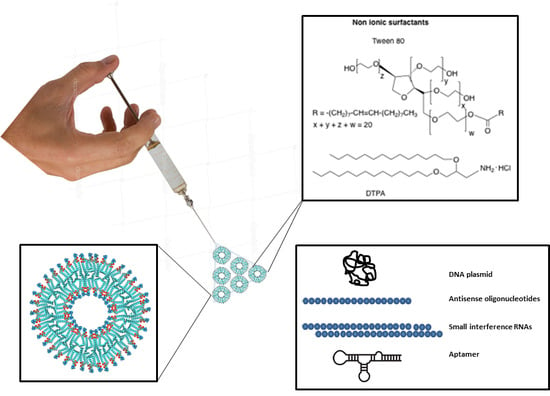
:1. Introduction
2. Composition, Preparation and Characterization of Niosomes
2.1. Composition of Niosomes
2.2. Niosome and Nioplexes Preparation
2.3. Characterization of Niosomes and Nioplexes
2.4. Small Angle X-ray Scattering (SAXS)
3. Applications of Niosomes in Gene Delivery
Plasmids
4. Use of Niosomes for Transfection of Oligonucleotides
4.1. Antisense Oligonucleotides
4.2. Aptamers
4.3. siRNA and microRNA
5. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Collins, M.; Thrasher, A. Gene therapy: Progress and predictions. Proc. R. Soc. B Biol. Sci. 2015, 282, 20143003. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.F.; Baker, B.F.; Pham, N.; Swayze, E.; Geary, R.S. Pharmacology of Antisense Drugs. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 81–105. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.A.; Castanotto, D. FDA-Approved Oligonucleotide Therapies in 2017. Mol. Ther. 2017, 25, 1069–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deleavey, G.F.; Damha, M.J. Designing chemically modified oligonucleotides for targeted gene silencing. Chem. Biol. 2012, 19, 937–954. [Google Scholar] [CrossRef]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.-C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, D.M.; Kim, K.S.; Jung, W.; Kim, D.E. Applications of cancer cell-specific aptamers in targeted delivery of anticancer therapeutic agents. Molecules 2018, 23, 830. [Google Scholar] [CrossRef] [PubMed]
- Grijalvo, S.; Alagia, A.; Jorge, F.A.; Eritja, R. Covalent Strategies for Targeting Messenger and Non-Coding RNAs: An Updated Review on siRNA, miRNA and antimiR Conjugates. Genes 2018, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.K.; Park, T.G. siRNA delivery systems for cancer treatment. Adv. Drug Deliv. Rev. 2009, 61, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Giacca, M.; Zacchigna, S. Virus-mediated gene delivery for human gene therapy. J. Control. Release 2012, 161, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Raouane, M.; Desmaële, D.; Urbinati, G.; Massaad-Massade, L.; Couvreur, P. Lipid Conjugated Oligonucleotides: A Useful Strategy for Delivery. Bioconjug. Chem. 2012, 23, 1091–1104. [Google Scholar] [CrossRef]
- Trabulo, S.; Cardoso, A.L.; Cardoso, A.M.S.; Morais, C.M.; Jurado, A.S.; de Lima, M.C. Cell-penetrating Peptides as Nucleic Acid Delivery Systems: From Biophysics to Biological Applications. Curr. Pharm. Des. 2013, 19, 2895–2923. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E. Polymers for siRNA delivery: Inspired by viruses to be targeted, dynamic and precise. Acc. Chem. Res. 2012, 45, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold nanoparticles in delivery applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, F.; Hu, R.G.; Becker, D.L.; Xu, C. Stimuli-responsive liposomes for the delivery of nucleic acid therapeutics. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Templeton, N.S. Liposomal delivery of nucleic acids in vivo. DNA Cell Biol. 2002, 21, 857–867. [Google Scholar] [CrossRef]
- Akinc, A.; Goldberg, M.; Qin, J.; Dorkin, J.R.; Gamba-Vitalo, C.; Maier, M.; Jayaprakash, K.N.; Jayaraman, M.; Rajeev, K.G.; Manoharan, M.; et al. Development of lipidoid-sirna formulations for systemic delivery to the liver. Mol. Ther. 2009, 17, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.K.; Tam, Y.Y.C.; Cullis, P.R. Lipid Nanoparticles for Short Interfering RNA Delivery; Elsevier: Amsterdam, The Netherlands, 2014; Volume 88, ISBN 9780128001486. [Google Scholar]
- Semple, S.C.; Akinc, A.; Chen, J.; Sandhu, A.P.; Mui, B.L.; Cho, C.K.; Sah, D.W.Y.; Stebbing, D.; Crosley, E.J.; Yaworski, E.; et al. Rational design of cationic lipids for siRNA delivery. Nat. Biotechnol. 2010, 28, 172. [Google Scholar] [CrossRef] [PubMed]
- Durymanov, M.; Reineke, J. Non-viral delivery of nucleic acids: Insight into mechanisms of overcoming intracellular barriers. Front. Pharmacol. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Kim, S.I.; Shin, D.; Choi, T.H.; Lee, J.C.; Cheon, G.J.; Kim, K.Y.; Park, M.; Kim, M. Systemic and specific delivery of small interfering RNAs to the liver mediated by apolipoprotein A-I. Mol. Ther. 2007, 15, 1145–1152. [Google Scholar] [CrossRef]
- Paecharoenchai, O.; Teng, L.; Yung, B.C.; Opanasopit, P.; Lee, R.J. Non-ionic surfactant vesicles for delivery of RNAi therapeutics. Nanomedicine 2013, 8, 1865–1873. [Google Scholar] [CrossRef]
- Mahale, N.B.; Thakkar, P.D.; Mali, R.G.; Walunj, D.R.; Chaudhari, S.R. Niosomes: Novel sustained release nonionic stable vesicular systems—An overview. Adv. Colloid Interface Sci. 2012, 183–184, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, E.; Agirre, M.; Villate-Beitia, I.; Mashal, M.; Puras, G.; Zarate, J.; Pedraz, J.L. Elaboration and Physicochemical Characterization of Niosome-Based Nioplexes for Gene Delivery Purposes BT—Non-Viral Gene Delivery Vectors: Methods and Protocols; Candiani, G., Ed.; Springer: New York, NY, USA, 2016; pp. 63–75. ISBN 978-1-4939-3718-9. [Google Scholar]
- Kazi, K.M.; Mandal, A.S.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A future of targeted drug delivery systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, M.; Brijesh, S. Opportunities and Challenges for Niosomes as Drug Delivery Systems. Curr. Drug Deliv. 2016, 13, 1275–1289. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Mehta, S.K. Developments of Polysorbate (Tween) based microemulsions: Preclinical drug delivery, toxicity and antimicrobial applications. Int. J. Pharm. 2017, 529, 134–160. [Google Scholar] [CrossRef] [PubMed]
- Moghassemi, S.; Hadjizadeh, A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Control. Release 2014, 185, 22–36. [Google Scholar] [CrossRef]
- Yang, C.; Gao, S.; Song, P.; Dagnæs-Hansen, F.; Jakobsen, M.; Kjems, J. Theranostic Niosomes for Efficient siRNA/MicroRNA Delivery and Activatable Near-Infrared Fluorescent Tracking of Stem Cells. ACS Appl. Mater. Interfaces 2018, 10, 19494–19503. [Google Scholar] [CrossRef]
- Hui, S.W.; Langner, M.; Zhao, Y.L.; Ross, P.; Hurley, E.; Chan, K. The role of helper lipids in cationic liposome-mediated gene transfer. Biophys. J. 1996, 71, 590–599. [Google Scholar] [CrossRef] [Green Version]
- Ojeda, E.; Puras, G.; Agirre, M.; Zarate, J.; Grijalvo, S.; Eritja, R.; Digiacomo, L.; Caracciolo, G.; Pedraz, J.L. The role of helper lipids in the intracellular disposition and transfection efficiency of niosome formulations for gene delivery to retinal pigment epithelial cells. Int. J. Pharm. 2016, 503, 115–126. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Huang, L. A novel cationic liposome reagent for efficient transfection of mammalian cells. Biochem. Biophys. Res. Commun. 1991, 179, 280–285. [Google Scholar] [CrossRef]
- Regelin, A.E.; Fankhaenel, S.; Gürtesch, L.; Prinz, C.; von Kiedrowski, G.; Massing, U. Biophysical and lipofection studies of DOTAP analogs. Biochim. Biophys. Acta Biomembr. 2000, 1464, 151–164. [Google Scholar] [CrossRef] [Green Version]
- Dass, C.R. Lipoplex-mediated delivery of nucleic acids: Factors affecting in vivo transfection. J. Mol. Med. 2004, 82, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, G.P.; Sesma, J.Z.; Díez, M.A.; Díaz-Tahoces, A.; Avilés-Trigeros, M.; Grijalvo, S.; Eritja, R.; Fernández, E.; Pedraz, J.L. A novel formulation based on 2,3-di(tetradecyloxy)propan-1-amine cationic lipid combined with polysorbate 80 for efficient gene delivery to the retina. Pharm. Res. 2014, 31, 1665–1675. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, J.; Chen, X.; Gao, J.; Liang, W. PEGylated synthetic surfactant vesicles (Niosomes): Novel carriers for oligonucleotides. J. Mater. Sci. Mater. Med. 2008, 19, 607–614. [Google Scholar] [CrossRef]
- Sorgi, F.L.; Bhattacharya, S.; Huang, L. Protamine sulphate enhances lipid-mediated gene transfer. Gene Ther. 1997, 4, 961. [Google Scholar] [CrossRef] [PubMed]
- Attia, N.; Soto, C.; Martínez, G.; Eritja, R.; Puras, G.; Luis, J. Gene transfer to rat cerebral cortex mediated by polysorbate 80 and poloxamer 188 nonionic surfactant vesicles. Drug Des. Dev. Ther. 2018, 2018, 3937–3949. [Google Scholar] [CrossRef]
- Baillie, A.J.; Florence, A.T.; Hume, L.R.; Muirhead, G.T.; Rogerson, A. The preparation and properties of niosomes—Non-ionic surfactant vesicles. J. Pharm. Pharmacol. 1985, 37, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Manosroi, A.; Wongtrakul, P.; Manosroi, J.; Sakai, H.; Sugawara, F.; Yuasa, M.; Abe, M. Characterization of vesicles prepared with various non-ionic surfactants mixed with cholesterol. Colloids Surf. B Biointerfaces 2003, 30, 129–138. [Google Scholar] [CrossRef]
- Garcia-Salinas, S.; Himawan, E.; Mendoza, G.; Arruebo, M.; Sebastian, V. Rapid on-Chip Assembly of Niosomes: Batch versus Continuous Flow Reactors. ACS Appl. Mater. Interfaces 2018, 10, 19197–19207. [Google Scholar] [CrossRef]
- Lo, C.T.; Jahn, A.; Locascio, L.E.; Vreeland, W.N. Controlled Self-Assembly of Monodisperse Niosomes by Microfluidic Hydrodynamic Focusing. Langmuir 2010, 26, 8559–8566. [Google Scholar] [CrossRef]
- Alatorre-Meda, M.; Taboada, P.; Hartl, F.; Wagner, T.; Freis, M.; Rodríguez, J.R. The influence of chitosan valence on the complexation and transfection of DNA: The weaker the DNA–chitosan binding the higher the transfection efficiency. Colloids Surf. B Biointerfaces 2011, 82, 54–62. [Google Scholar] [CrossRef]
- Pozharski, E.; MacDonald, R.C. Thermodynamics of cationic lipid-DNA complex formation as studied by isothermal titration calorimetry. Biophys. J. 2002, 83, 556–565. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, H.; Levorse, D.A.; Crocker, L.S. Ionization behaviour of amino lipids for siRNA delivery: Determination of ionization constants, SAR and the impact of lipid p K a on cationic lipid-biomembrane interactions. Langmuir 2011, 27, 1907–1914. [Google Scholar] [CrossRef]
- Jayaraman, M.; Ansell, S.M.; Mui, B.L.; Tam, Y.K.; Chen, J.; Du, X.; Butler, D.; Eltepu, L.; Matsuda, S.; Narayanannair, J.K.; et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. Int. Ed. 2012, 51, 8529–8533. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, E.; Puras, G.; Agirre, M.; Zárate, J.; Grijalvo, S.; Pons, R.; Eritja, R.; Martinez-Navarrete, G.; Soto-Sanchez, C.; Fernández, E.; et al. Niosomes based on synthetic cationic lipids for gene delivery: The influence of polar head-groups on the transfection efficiency in HEK-293, ARPE-19 and MSC-D1 cells. Org. Biomol. Chem. 2015, 13, 1068–1081. [Google Scholar] [CrossRef]
- Tristram-Nagle, S.; Nagle, J.F. Lipid bilayers: Thermodynamics, structure, fluctuations and interactions. Chem. Phys. Lipids 2004, 127, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Pack, D.W.; Hoffman, A.S.; Pun, S.; Stayton, P.S. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005, 4, 581–593. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular Carcinoma: Epidemiology and Molecular Carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Nacher, A.; Vassallo, A.; Armentano, M.F.; Pons, R.; Fernàndez-Busquets, X.; Carbone, C.; Valenti, D.; Fadda, A.M.; Manconi, M. Effect of quercetin and resveratrol co-incorporated in liposomes against inflammatory/oxidative response associated with skin cancer. Int. J. Pharm. 2016, 513, 153–163. [Google Scholar] [CrossRef]
- Pabst, G.; Kučerka, N.; Nieh, M.-P.; Rheinstädter, M.C.; Katsaras, J. Applications of neutron and X-ray scattering to the study of biologically relevant model membranes. Chem. Phys. Lipids 2010, 163, 460–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucerka, N.; Nieh, M.P.; Katsaras, J. Asymmetric distribution of cholesterol in unilamellar vesicles of monounsaturated phospholipids. Langmuir 2009, 25, 13522–13527. [Google Scholar] [CrossRef]
- Handjani-Vila, R.M.; Ribier, A.; Rondot, B.; Vanlerberghie, G. Dispersions of lamellar phases of non-ionic lipids in cosmetic products. Int. J. Cosmet. Sci. 1979, 1, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Irchhaiya, R. Niosomes: A potential tool for novel drug delivery. J. Pharm. Investig. 2016, 46, 195–204. [Google Scholar] [CrossRef]
- Khan, R.; Irchhaiya, R. An overview on niosomes as efficient drug carriers. Int. J. Pharm. Biosci. 2017, 8, 106–116. [Google Scholar] [CrossRef]
- Rajera, R.; Nagpal, K.; Singh, S.K.; Mishra, D.N. Niosomes: A Controlled and Novel Drug Delivery System. Biol. Pharm. Bull. 2011, 34, 945–953. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.H.P.; Lim, S.; Wong, W.S.F. Antisense oligonucleotides: From design to therapeutic application. Clin. Exp. Pharmacol. Physiol. 2006, 33, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.; Rossi, J. RNA-Based Therapeutics: Current Progress and Future Prospects. Chem. Biol. 2012, 19, 60–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajj, K.A.; Whitehead, K.A. Tools for translation: Non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017, 2, 17056. [Google Scholar] [CrossRef]
- Baxter, R.; Hastings, N.; Law, A.; Glass, E.J. Plasmids for Therapy and Vaccination; Animal Genetics; Wiley-VCH Verlag GmbH: D-69469, Weinheim, Germany, 2008; Volume 39, ISBN 3527302697. [Google Scholar]
- Li, S.D.; Huang, L. Gene therapy progress and prospects: Non-viral gene therapy by systemic delivery. Gene Ther. 2006, 13, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Zhang, S.; Cui, S.; Zhao, Y.; Wang, Y.; Zhao, D. The Headgroup Evolution of Cationic Lipids for Gene Delivery. Bioconjug. Chem. 2013, 24, 487–519. [Google Scholar] [CrossRef]
- Teixeira, H.F.; Bruxel, F.; Fraga, M.; Schuh, R.S.; Zorzi, G.K.; Matte, U.; Fattal, E. Cationic nanoemulsions as nucleic acids delivery systems. Int. J. Pharm. 2017, 534, 356–367. [Google Scholar] [CrossRef]
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.D. Cationic Liposomes for Gene Therapy. Angew. Chem. Int. Ed. 1998, 37, 1768–1785. [Google Scholar] [CrossRef]
- Lewis, J.G.; Lin, K.-Y.; Kothavale, A.; Flanagan, W.M.; Matteucci, M.D.; DePrince, R.B.; Mook, R.A.; Hendren, R.W.; Wagner, R.W. A serum-resistant cytofectin for cellular delivery of antisense oligodeoxynucleotides and plasmid DNA. Proc. Natl. Acad. Sci. USA 1996, 93, 3176–3181. [Google Scholar] [CrossRef] [PubMed]
- Massing, U.; Kley, J.T.; Gürtesch, L.; Fankhaenel, S. A simple approach to DOTAP and its analogs bearing different fatty acids. Chem. Phys. Lipids 2000, 105, 189–191. [Google Scholar] [CrossRef]
- Ronsin, G.; Perrin, C.; Guédat, P.; Kremer, A.; Camilleri, P.; Kirby, A.J. Novel spermine-based cationic gemini surfactants for gene delivery. Chem. Commun. 2001, 21, 2234–2235. [Google Scholar] [CrossRef]
- Paecharoenchai, O.; Niyomtham, N.; Leksantikul, L.; Ngawhirunpat, T.; Rojanarata, T.; Yingyongnarongkul, B.; Opanasopit, P. Non-ionic Surfactant Vesicles Composed of Novel Spermine-Derivative Cationic Lipids as an Effective Gene Carrier In Vitro. AAPS PharmSciTech 2014, 15, 722–730. [Google Scholar] [CrossRef]
- Paecharoenchai, O.; Niyomtham, N.; Ngawhirunpat, T.; Rojanarata, T.; Yingyongnarongkul, B.E.; Opanasopit, P. Cationic niosomes composed of spermine-based cationic lipids mediate high gene transfection efficiency. J. Drug Target. 2012, 20, 783–792. [Google Scholar] [CrossRef]
- Opanasopit, P.; Leksantikul, L.; Niyomtham, N.; Ngawhirunpat, T.; Yingyongnarongkul, B.; Opanasopit, P.; Leksantikul, L.; Niyomtham, N.; Rojanarata, T.; Ngawhirunpat, T. Cationic niosomes an effective gene carrier composed of novel spermine-derivative cationic lipids: Effect of central core structures Cationic niosomes an effective gene carrier composed of novel spermine-derivative cationic lipids: Effect of central cor. Pharm. Dev. Technol. 2015, 7450, 1–10. [Google Scholar] [CrossRef]
- Pamornpathomkul, B.; Niyomtham, N.; Yingyongnarongkul, B.-E.; Prasitpuriprecha, C.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Cationic Niosomes for Enhanced Skin Immunization of Plasmid DNA-Encoding Ovalbumin via Hollow Microneedles. AAPS PharmSciTech 2018, 19, 481–488. [Google Scholar] [CrossRef]
- Rose, J.K.; Buonocore, L.; Whitt, M.A. A new cationic liposome reagent mediating nearly quantitative transfection of animal cells. Biotechniques 1991, 10, 520–525. [Google Scholar]
- Manosroi, J.; Khositsuntiwong, N.; Manosroi, W.; Götz, F.; Werner, R.G.; Manosroi, A. Enhancement of Transdermal Absorption, Gene Expression and Stability of Tyrosinase Plasmid (pMEL34)-Loaded Elastic Cationic Niosomes: Potential Application in Vitiligo Treatment. J. Pharm. Sci. 2010, 99, 3533–3541. [Google Scholar] [CrossRef] [PubMed]
- Manosroi, A.; Thathang, K.; Werner, R.G.; Schubert, R.; Manosroi, J. Stability of luciferase plasmid entrapped in cationic bilayer vesicles. Int. J. Pharm. 2008, 356, 291–299. [Google Scholar] [CrossRef]
- Conley, S.M.; Naash, M.I. Nanoparticles for retinal gene therapy. Prog. Retin. Eye Res. 2010, 29, 376–397. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541. [Google Scholar] [CrossRef] [PubMed]
- Mashal, M.; Attia, N.; Puras, G.; Martínez-Navarrete, G.; Fernández, E.; Pedraz, J.L. Retinal gene delivery enhancement by lycopene incorporation into cationic niosomes based on DOTMA and polysorbate 60. J. Control. Release 2017, 254, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Mashal, M.; Attia, N.; Soto-Sánchez, C.; Martínez-Navarrete, G.; Fernández, E.; Puras, G.; Pedraz, J.L. Non-viral vectors based on cationic niosomes as efficient gene delivery vehicles to central nervous system cells into the brain. Int. J. Pharm. 2018, 552, 48–55. [Google Scholar] [CrossRef]
- Cheng, X.; Lee, R.J. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv. Drug Deliv. Rev. 2016, 99, 129–137. [Google Scholar] [CrossRef]
- Ojeda, E.; Puras, G.; Agirre, M.; Zarate, J.; Grijalvo, S.; Eritja, R.; Fern, E. The influence of the polar head-group of synthetic cationic lipids on the transfection ef fi ciency mediated by niosomes in rat retina and brain. Biomaterials 2016, 77, 267–279. [Google Scholar] [CrossRef]
- Attia, N.; Mashal, M.; Grijalvo, S.; Eritja, R.; Zárate, J.; Puras, G.; Pedraz, J.L. Stem cell-based gene delivery mediated by cationic niosomes for bone regeneration. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 521–531. [Google Scholar] [CrossRef]
- Dias, N.; Stein, C.A. Antisense oligonucleotides: Basic concepts and mechanisms. Mol. Cancer Ther. 2002, 1, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, J.C.; Kowalski, P.S.; Anderson, D.G. Advances in the delivery of RNA therapeutics: From concept to clinical reality. Genome Med. 2017, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.E.; Kim, C.K. Long-term stable cationic solid lipid nanoparticles for the enhanced intracellular delivery of SMAD3 antisense oligonucleotides in activated murine macrophages. J. Pharm. Pharm. Sci. 2012, 15, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Quan, J.; Zhang, M.; Yung, B.C.; Cheng, X.; Liu, Y.; Lee, Y.B.; Ahn, C.H.; Kim, D.J.; Lee, R.J. Lipid-albumin nanoparticles (LAN) for therapeutic delivery of antisense oligonucleotide against HIF-1α. Mol. Pharm. 2016, 13, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.; Gupta, V.; Liu, Y.Y.; Nazzal, S. Doxorubicin and MBO-asGCS oligonucleotide loaded lipid nanoparticles overcome multidrug resistance in adriamycin resistant ovarian cancer cells (NCI/ADR-RES). Int. J. Pharm. 2012, 431, 222–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fattal, E.; Couvreur, P.; Dubernet, C. “Smart” delivery of antisense oligonucleotides by anionic pH-sensitive liposomes. Adv. Drug Deliv. Rev. 2004, 56, 931–946. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Gao, J.Q.; Chen, J.L.; Liang, W.Q. Cationic liposomes modified with non-ionic surfactants as effective non-viral carrier for gene transfer. Colloids Surf. B Biointerfaces 2006, 49, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Timko, B.P.; Dvir, T.; Kohane, D.S. Remotely triggerable drug delivery systems. Adv. Mater. 2010, 22, 4925–4943. [Google Scholar] [CrossRef] [PubMed]
- Grijalvo, S.; Alagia, A.; Puras, G.; Zárate, J.; Pedraz, J.L.; Eritja, R. Cationic vesicles based on non-ionic surfactant and synthetic aminolipids mediate delivery of antisense oligonucleotides into mammalian cells. Colloids Surf. B Biointerfaces 2014, 119, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-Y. mRNA accessible site tagging (MAST): A novel high throughput method for selecting effective antisense oligonucleotides. Nucleic Acids Res. 2003, 31, 72e. [Google Scholar] [CrossRef]
- Grijalvo, S.; Alagia, A.; Puras, G.; Zárate, J.; Mayr, J.; Pedraz, J.L.; Eritja, R.; Díaz, D.D. Cationic nioplexes-in-polysaccharide-based hydrogels as versatile biodegradable hybrid materials to deliver nucleic acids. J. Mater. Chem. B 2017, 5, 7756–7767. [Google Scholar] [CrossRef]
- Grijalvo, S.; Puras, G.; Zárate, J.; Pons, R.; Pedraz, J.L.; Eritja, R.; Díaz, D.D. Nioplexes encapsulated in supramolecular hybrid biohydrogels as versatile delivery platforms for nucleic acids. RSC Adv. 2016, 6, 39688–39699. [Google Scholar] [CrossRef] [Green Version]
- Krebs, M.D.; Jeon, O.; Alsberg, E. Localized and sustained delivery of silencing RNA from macroscopic biopolymer hydrogels. J. Am. Chem. Soc. 2009, 131, 9204–9206. [Google Scholar] [CrossRef]
- Grijalvo, S.; Mayr, J.; Eritja, R.; Díaz, D.D. Biodegradable liposome-encapsulated hydrogels for biomedical applications: A marriage of convenience. Biomater. Sci. 2016, 4, 555–574. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2016, 16, 181. [Google Scholar] [CrossRef] [PubMed]
- Cerchia, L.; de Franciscis, V. Targeting cancer cells with nucleic acid aptamers. Trends Biotechnol. 2010, 28, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.S.M.; Matthews, C.S.; Missailidis, S. DNA Aptamers That Bind to MUC1 Tumour Marker: Design and Characterization of MUC1-Binding Single-Stranded DNA Aptamers. Tumor Biol. 2006, 27, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Duan, J.; Zhan, Q.; Wang, F.; Lu, X.; Yang, X.-D. Novel MUC1 Aptamer Selectively Delivers Cytotoxic Agent to Cancer Cells In Vitro. PLoS ONE 2012, 7, e31970. [Google Scholar] [CrossRef]
- Seleci, D.A.; Seleci, M.; Jochums, A.; Walter, J.G.; Stahl, F.; Scheper, T. Aptamer mediated niosomal drug delivery. RSC Adv. 2016, 6, 87910–87918. [Google Scholar] [CrossRef] [Green Version]
- Bates, P.J.; Reyes-Reyes, E.M.; Malik, M.T.; Murphy, E.M.; O’Toole, M.G.; Trent, J.O. G-quadruplex oligonucleotide AS1411 as a cancer-targeting agent: Uses and mechanisms. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1414–1428. [Google Scholar] [CrossRef]
- Mangiapia, G.; D’Errico, G.; Simeone, L.; Irace, C.; Radulescu, A.; Di Pascale, A.; Colonna, A.; Montesarchio, D.; Paduano, L. Ruthenium-based complex nanocarriers for cancer therapy. Biomaterials 2012, 33, 3770–3782. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, C.; Fàbrega, C.; Grijalvo, S.; Vitiello, G.; D’Errico, G.; Eritja, R.; Montesarchio, D. AS1411-decorated niosomes as effective nanocarriers for Ru(iii)-based drugs in anticancer strategies. J. Mater. Chem. B 2018, 6, 5368–5384. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806. [Google Scholar] [CrossRef] [PubMed]
- Wittrup, A.; Lieberman, J. Knocking down disease: A progress report on siRNA therapeutics. Nat. Rev. Genet. 2015, 16, 543. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Mao, Y.; Sugimoto, Y.; Zhang, Y.; Kanthamneni, N.; Yu, B.; Brueggemeier, R.W.; Lee, L.J.; Lee, R.J. SPANosomes as delivery vehicles for small interfering RNA (siRNA). Mol. Pharm. 2012, 9, 201–210. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Yu, B.; Phelps, M.A.; Lee, L.J.; Lee, R.J. Comparative cellular pharmacokinetics and pharmacodynamics of siRNA delivery by SPANosomes and by cationic liposomes. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 504–513. [Google Scholar] [CrossRef] [Green Version]
- Obeid, M.A.; Elburi, A.; Young, L.C.; Mullen, A.B.; Tate, R.J.; Ferro, V.A. Formulation of Non-ionic Surfactant Vesicles (NISV) Prepared by Microfluidics for Therapeutic Delivery of siRNA into Cancer Cells. Mol. Pharm. 2017, 14, 2450–2458. [Google Scholar] [CrossRef]
- Obeid, M.A.; Dufès, C.; Somani, S.; Mullen, A.B.; Tate, R.J.; Ferro, V.A. Proof of concept studies for siRNA delivery by non-ionic surfactant vesicles: In vitro and in vivo evaluation of protein knockdown. J. Liposome Res. 2018, 1–27. [Google Scholar] [CrossRef]
- Sun, M.; Yang, C.; Zheng, J.; Wang, M.; Chen, M.; Le, D.Q.S.; Kjems, J.; Bünger, C.E. Enhanced efficacy of chemotherapy for breast cancer stem cells by simultaneous suppression of multidrug resistance and antiapoptotic cellular defense. Acta Biomater. 2015, 28, 171–182. [Google Scholar] [CrossRef]
- Rajput, S.; Puvvada, N.; Kumar, B.N.P.; Sarkar, S.; Konar, S.; Bharti, R.; Dey, G.; Mazumdar, A.; Pathak, A.; Fisher, P.B.; et al. Overcoming Akt Induced Therapeutic Resistance in Breast Cancer through siRNA and Thymoquinone Encapsulated Multilamellar Gold Niosomes. Mol. Pharm. 2015, 12, 4214–4225. [Google Scholar] [CrossRef]
- Hemati, M.; Haghiralsadat, F.; Yazdian, F.; Jafari, F.; Moradi, A.; Malekpour-Dehkordi, Z. Development and characterization of a novel cationic PEGylated niosome-encapsulated forms of doxorubicin, quercetin and siRNA for the treatment of cancer by using combination therapy. Artif. Cells Nanomed. Biotechnol. 2018, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Yu, X.; Hu, S.; Yu, J. A Brief Review on the Mechanisms of miRNA Regulation. Genom. Proteom. Bioinform. 2009, 7, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Gambari, R.; Brognara, E.; Spandidos, D.A.; Fabbri, E. Targeting oncomiRNAs and mimicking tumour suppressor miRNAs: Ew trends in the development of miRNA therapeutic strategies in oncology. Int. J. Oncol. 2016, 49, 5–32. [Google Scholar] [CrossRef] [PubMed]
- Wolfrum, C.; Shi, S.; Jayaprakash, K.N.; Jayaraman, M.; Wang, G.; Pandey, R.K.; Rajeev, K.G.; Nakayama, T.; Charrise, K.; Ndungo, E.M.; et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat. Biotechnol. 2007, 25, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, C.; Hadwiger, P.; John, M.; Vornlocher, H.P.; Unverzagt, C. Steroid and lipid conjugates of siRNAs to enhance cellular uptake and gene silencing in liver cells. Bioorgan. Med. Chem. Lett. 2004, 14, 4975–4977. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.K.; Willoughby, J.L.S.; Chan, A.; Charisse, K.; Alam, M.R.; Wang, Q.; Hoekstra, M.; Kandasamy, P.; Kel’in, A.V.; Milstein, S.; et al. Multivalent N-Acetylgalactosamine-Conjugated siRNA Localizes in Hepatocytes and Elicits Robust RNAi-Mediated Gene Silencing. J. Am. Chem. Soc. 2014, 136, 16958–16961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parmar, R.G.; Poslusney, M.; Busuek, M.; Williams, J.M.; Garbaccio, R.; Leander, K.; Walsh, E.; Howell, B.; Sepp-Lorenzino, L.; Riley, S.; et al. Novel Endosomolytic Poly(amido amine) Polymer Conjugates for Systemic Delivery of siRNA to Hepatocytes in Rodents and Nonhuman Primates. Bioconjug. Chem. 2014, 25, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Duggan, S.T.; Keating, G.M. Pegylated Liposomal Doxorubicin. Drugs 2011, 71, 2531–2558. [Google Scholar] [CrossRef] [PubMed]
- Ginn, S.L.; Amaya, A.K.; Alexander, I.E.; Edelstein, M.; Abedi, M.R. Gene therapy clinical trials worldwide to 2017: An update. J. Gene Med. 2018, 20, 1–16. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281. [Google Scholar] [CrossRef] [PubMed]
- Rui, Y.; Wilson, D.R.; Green, J.J. Non-Viral Delivery To Enable Genome Editing. Trends Biotechnol. 2018, 1–13. [Google Scholar] [CrossRef] [PubMed]







| Spermine-Based Cationic Lipid | Particle Size (nm) | Optimal Weight Ratio | Transfection Efficiency (cells/cm2) |
|---|---|---|---|
| 1 | 213 | 10 | 7556 ± 92 |
| 2 | 315 | 10 | 6897 ± 292 |
| 3 | 487 | 5 | 5453 ± 36 |
| 4 | 876 | 20 | 2082 ± 63 |
| 5 | 462 | 30 | 5959 ± 197 |
| 6 | 385 | 10 | 7993 ± 94 |
| Cationic Lipid | Niosome Preparation | Cargo | Therapy | Testing Conditions | References |
|---|---|---|---|---|---|
| Polyamine derivatives | Thin-film | pEGFP-C2 | - | In vitro | [68,71] |
| Polyamine derivative | Thin-film | pOVA | Skin vaccination | In vivo | [72] |
| DODAB | Thin-film | pMEL34 and pLuc | Topical delivery | In vitro | [74,75] |
| DOTMA | Reverse-phase evaporation | pCMS-EGFP | Ocular delivery | In vitro and In vivo | [78] |
| 13 | Oil-in-water emulsion | pCMS-EGFP | - | In vitro | [30] |
| Glycerol-based amino lipid derivatives | Oil-in-water emulsion | pCMS-EGFP | Ocular delivery | In vitro and In vivo | [82] |
| DTPA | Emulsification-evaporation | pCMS-EGFP | Ocular delivery | In vitro and In vivo | [34] |
| DTPA | Reverse-phase evaporation | pUNO1-hBMP-7 | Bone regeneration | In vitro | [83] |
| Serinol-based amino lipid derivatives | Oil-in-water emulsion | pCMS-EGFP | - | In vitro | [46] |
| DC-Chol | Reverse-phase evaporation and thin-film | ASO | - | In vitro | [90,91] |
| DTPA | Thin-film | ASO | - | In vitro | [92] |
| PEGNIO | Thin-film | MUC1 Aptamer | Chemotherapy | In vitro | [102] |
| DTPA | Thin-film | AS1411 | Chemotherapy | In vitro | [105] |
| DOTAP | Ethanol injection | siLuc | - | In vitro | [108] |
| DDAB | Microfluidic | siRNA GFP | Chemotherapy | In vitro and In vivo | [110,111] |
| DOTAP | Ethanol injection | 2 siRNAs | Chemotherapy | In vivo | [112] |
| Gold niosomes (Nio-Au) | Ethanol evaporation | siRNA | Chemotherapy | In vivo | [113] |
| DOTAP | Thin-film | siRNA | Chemotherapy | In vitro | [114] |
| DOTAP | Ethanol injection | siRNA and miRNA | Chemotherapy | In vitro and In vivo | [28] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grijalvo, S.; Puras, G.; Zárate, J.; Sainz-Ramos, M.; Qtaish, N.A.L.; López, T.; Mashal, M.; Attia, N.; Díaz Díaz, D.; Pons, R.; et al. Cationic Niosomes as Non-Viral Vehicles for Nucleic Acids: Challenges and Opportunities in Gene Delivery. Pharmaceutics 2019, 11, 50. https://doi.org/10.3390/pharmaceutics11020050
Grijalvo S, Puras G, Zárate J, Sainz-Ramos M, Qtaish NAL, López T, Mashal M, Attia N, Díaz Díaz D, Pons R, et al. Cationic Niosomes as Non-Viral Vehicles for Nucleic Acids: Challenges and Opportunities in Gene Delivery. Pharmaceutics. 2019; 11(2):50. https://doi.org/10.3390/pharmaceutics11020050
Chicago/Turabian StyleGrijalvo, Santiago, Gustavo Puras, Jon Zárate, Myriam Sainz-Ramos, Nuseibah A. L. Qtaish, Tania López, Mohamed Mashal, Noha Attia, David Díaz Díaz, Ramon Pons, and et al. 2019. "Cationic Niosomes as Non-Viral Vehicles for Nucleic Acids: Challenges and Opportunities in Gene Delivery" Pharmaceutics 11, no. 2: 50. https://doi.org/10.3390/pharmaceutics11020050
APA StyleGrijalvo, S., Puras, G., Zárate, J., Sainz-Ramos, M., Qtaish, N. A. L., López, T., Mashal, M., Attia, N., Díaz Díaz, D., Pons, R., Fernández, E., Pedraz, J. L., & Eritja, R. (2019). Cationic Niosomes as Non-Viral Vehicles for Nucleic Acids: Challenges and Opportunities in Gene Delivery. Pharmaceutics, 11(2), 50. https://doi.org/10.3390/pharmaceutics11020050