Determining Thermal Conductivity of Small Molecule Amorphous Drugs with Modulated Differential Scanning Calorimetry and Vacuum Molding Sample Preparation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
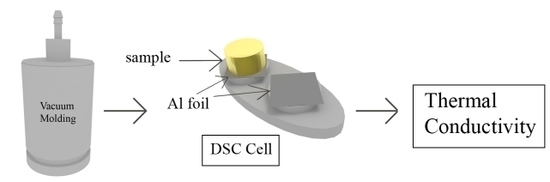
2.2. Sample Preparation
2.3. Modulated Differential Scanning Calorimetry
- The heat capacity of the “thin sample” (Cp,s) was measured in a standard run with the sample inside a DSC pan and an empty pan on the reference side.
- The “thick sample” was weighed and its length and diameter were measured with a caliper. The apparent heat capacity of the “thick sample” (Cp,app) was measured by placing the sample on the sample side of the DSC cell. A piece of aluminium foil with a small amount of silicone oil (wetted cotton swab to apply) was placed in between the sample and cell. A similar foil was placed on the reference side. The mass of the “thick sample” was entered in the DSC software as the sample mass.
- The thermal conductivity was calculated with the help of Cp,s, Cp,app, as well as mass, length and diameter of the “thick sample”. The equations that were used have been supplied in Section 2.4.
2.4. Equations Used to Calculate the Sample’s Thermal Conductivity
2.5. X-ray Powder Diffraction (XRPD)
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Anderson, D.R. Thermal Conductivity of Polymers. Chem. Rev. 1966, 66, 677–690. [Google Scholar] [CrossRef]
- Babu, N.J.; Nangia, A. Solubility Advantage of Amorphous Drugs and Pharmaceutical Cocrystals. Cryst. Growth Des. 2011, 11, 2662–2679. [Google Scholar] [CrossRef]
- Hancock, B.C.; Zografi, G. Characteristics and Significance of the Amorphous State in Pharmaceutical Systems. J. Pharm. Sci. 1997, 86, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wildfong, P.L.D.; Hancock, B.C.; Moore, M.D.; Morris, K.R. Towards an Understanding of the Structurally Based Potential for Mechanically Activated Disordering of Small Molecule Organic Crystals. J. Pharm. Sci. 2006, 95, 2645–2656. [Google Scholar] [CrossRef]
- Doreth, M.; Hussein, M.A.; Priemel, P.A.; Grohganz, H.; Holm, R.; Lopez de Diego, H.; Rades, T.; Löbmann, K. Amorphization within the tablet: Using microwave irradiation to form a glass solution in situ. Int. J. Pharm. 2017, 519, 343–351. [Google Scholar] [CrossRef]
- Eitzlmayr, A.; Koscher, G.; Reynolds, G.; Huang, Z.; Booth, J.; Shering, P.; Khinast, J. Mechanistic modeling of modular co-rotating twin-screw extruders. Int. J. Pharm. 2014, 474, 157–176. [Google Scholar] [CrossRef]
- Bochmann, E.S.; Gryczke, A.; Wagner, K.G. Validation of Model-Based Melt Viscosity in Hot-Melt Extrusion Numerical Simulation. Pharmaceutics 2018, 10, 132. [Google Scholar] [CrossRef] [Green Version]
- Karl, M.; Larsen, P.E.; Rangacharya, V.P.; Hwu, E.T.; Rantanen, J.; Boisen, A.; Rades, T. Ultrasensitive Microstring Resonators for Solid State Thermomechanical Analysis of Small and Large Molecules. J. Am. Chem. Soc. 2018, 140, 17522–17531. [Google Scholar] [CrossRef] [Green Version]
- Marcus, S.M.; Blaine, R.L. Thermal conductivity of polymers, glasses and ceramics by modulated DSC. Thermochim. Acta 1994, 243, 231–239. [Google Scholar] [CrossRef]
- Kollmetz, T.; Georgopanos, P.; Handge, U.A. Rheology in shear and elongation and dielectric spectroscopy of polystyrene-block-poly(4-vinylpyridine) diblock copolymers. Polymer 2017, 129, 68–82. [Google Scholar] [CrossRef]
- Shmool, T.A.; Zeitler, J.A. Insights into the structural dynamics of poly lactic-co-glycolic acid at terahertz frequencies. Polym. Chem. 2019, 10, 351–361. [Google Scholar] [CrossRef] [Green Version]
- Eder, S.; Beretta, M.; Witschnigg, A.; Koutsamanis, I.; Eggenreich, K.; Khinast, J.G.; Koscher, G.; Paudel, A.; Nickisch, K.; Friedrich, M.; et al. Establishment of a Molding Procedure to Facilitate Formulation Development for Co-extrudates. AAPS PharmSciTech 2017, 18, 2971–2976. [Google Scholar] [CrossRef] [PubMed]
- Marcus, S.M.; Blaine, R.L. Thermal Conductivity of Polymers, Glasses and Ceramics by Modulated DSC; TA Instruments Application Note 86; TA Instruments: New Castle, DE, USA, 1994. [Google Scholar]
- Hu, M.; Yu, D.; Wei, J. Thermal conductivity determination of small polymer samples by differential scanning calorimetry. Polym. Test. 2007, 26, 333–337. [Google Scholar] [CrossRef]
- Merzlyakov, M.; Schick, C. Thermal conductivity from dynamic response of DSC. Thermochim. Acta 2001, 377, 183–191. [Google Scholar] [CrossRef]
- Toledo, M. Simple Determination of the Thermal Conductivity of Polymers by DSC; Thermal Analysis Application UC 226; Mettler Toledo: Columbus, OH, USA, 2002. [Google Scholar]
- Camirand, C.P. Measurement of thermal conductivity by differential scanning calorimetry. Thermochim. Acta 2004, 417, 1–4. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, Z.; Wildfong, P.L.D. Thermal conductivity measurements for small molecule organic solid materials using modulated differential scanning calorimetry (MDSC) and data corrections for sample porosity. J. Pharm. Biomed. Anal. 2010, 51, 979–984. [Google Scholar] [CrossRef]
- Baird, J.A.; Santiago-Quinonez, D.; Rinaldi, C.; Taylor, L.S. Role of Viscosity in Influencing the Glass-Forming Ability of Organic Molecules from the Undercooled Melt State. Pharm. Res. 2012, 29, 271–284. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Thermal Conductivity and Thermal Diffusivity by Modulated Temperature Differential Scanning Calorimetry; Standard Test Method ASTM E1952-17; ASTM: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Hill, V.L.; Craig, D.Q.M.; Feely, L.C. The effects of experimental parameters and calibration on MTDSC data. Int. J. Pharm. 1999, 192, 21–32. [Google Scholar] [CrossRef]
- Assael, M.J.; Botsios, S.; GialouI, K.; Metaxa, I.N. Thermal Conductivity of Polymethyl Methacrylate (PMMA) and Borosilicate Crown Glass BK7. Int. J. Thermophys. 2005, 26, 1595–1605. [Google Scholar] [CrossRef]
- Tye, R.; Salmon, D.R. Thermal Conductivity Certified Reference Materials: Pyrex 7740 and Polymethylmethacrylate; DEStech Publications: Lancaster, PA, USA, 2005. [Google Scholar]
- Ho, C.Y.; Desai, P.D.; Wu, K.Y.; Havill, T.N.; Lee, T.Y. Thermal Properties of Polystyrene and poly(vinylchloride); NBS Publication GCR-7783, Order from NTIS (ntis.gov) as PB265228; National Technical Information Service: Alexandria, VA, USA, 1977. [Google Scholar]
- Graeser, K.A.; Patterson, J.E.; Zeitler, J.A.; Rades, T. The Role of Configurational Entropy in Amorphous Systems. Pharmaceutics 2010, 2, 224–244. [Google Scholar] [CrossRef]
- Standards, N.B.O. Thermal Conductivity of Solids at Room Temperature and Below. NBS Monogr. 1973, 131, 525–543. [Google Scholar]
- Cecil, O.B.; Munch, R.H. Thermal Conductivity of Some Organic Liquids. Ind. Eng. Chem. 1956, 48, 437–440. [Google Scholar] [CrossRef]



| Samples | Tg, DSC [°C] | Molding Temperature [°C] | Measurement Temperature [°C] a | ĸDSC [W/m °C] | Literature Values for ĸ [W/m °C] |
|---|---|---|---|---|---|
| PMMA | 104 | 142 | +17 | 0.1985 ± 0.0008 | 0.190 b |
| Polystyrene | 105 | 135 | −65 −25 +20 | 0.134 ± 0.008 0.144 ± 0.008 0.152 ± 0.09 | 0.1432 c 0.1474 c 0.1529 c |
| Amorphous Celecoxib | 57 | 68 | −65 −20 +20 | 0.147 ± 0.003 0.151 ± 0.005 0.153 ± 0.003 | N.A. N.A. N.A. |
| Amorphous Indomethacin | 45 | 75 | −56 −36 −16 +6 +16 +26 | 0.173 ± 0.006 0.174 ± 0.004 0.175 ± 0.005 0.176 ± 0.004 0.183 ± 0.002 0.181 ± 0.003 | N.A. N.A. N.A. N.A. N.A. N.A. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karl, M.; Rantanen, J.; Rades, T. Determining Thermal Conductivity of Small Molecule Amorphous Drugs with Modulated Differential Scanning Calorimetry and Vacuum Molding Sample Preparation. Pharmaceutics 2019, 11, 670. https://doi.org/10.3390/pharmaceutics11120670
Karl M, Rantanen J, Rades T. Determining Thermal Conductivity of Small Molecule Amorphous Drugs with Modulated Differential Scanning Calorimetry and Vacuum Molding Sample Preparation. Pharmaceutics. 2019; 11(12):670. https://doi.org/10.3390/pharmaceutics11120670
Chicago/Turabian StyleKarl, Maximilian, Jukka Rantanen, and Thomas Rades. 2019. "Determining Thermal Conductivity of Small Molecule Amorphous Drugs with Modulated Differential Scanning Calorimetry and Vacuum Molding Sample Preparation" Pharmaceutics 11, no. 12: 670. https://doi.org/10.3390/pharmaceutics11120670
APA StyleKarl, M., Rantanen, J., & Rades, T. (2019). Determining Thermal Conductivity of Small Molecule Amorphous Drugs with Modulated Differential Scanning Calorimetry and Vacuum Molding Sample Preparation. Pharmaceutics, 11(12), 670. https://doi.org/10.3390/pharmaceutics11120670