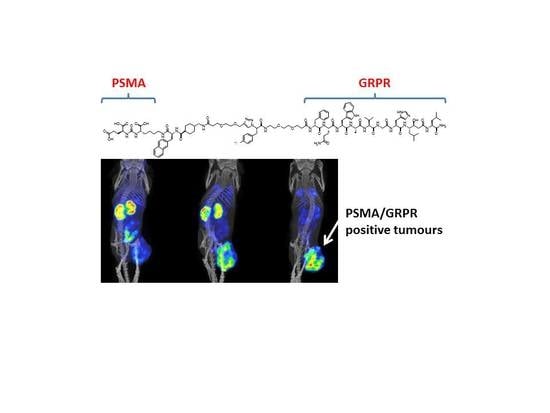
Synthesis and Preclinical Evaluation of Radio-Iodinated GRPR/PSMA Bispecific Heterodimers for the Theranostics Application in Prostate Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Syntheses of Heterodimers
2.1.1. 2-Azido-l-Tyr(tBu)-OH (1)
2.1.2. Bis(tert-butyl)-l-glutamyl 4-Nitrophenyl Carbamate
2.1.3. Solid Phase Peptide Synthesis (SPPS)
2.1.4. Azido-l-Tyr-(9-amino-4,7-dioxanonanoyl)-d-Phe-l-Gln-l-Trp-l-Ala-l-Val-l-Gly-l-His-(3S,4S)Sta-l-Leu-NH2) (3)
2.1.5. PSMA-617 alkyne (4)
2.1.6. PSMA-617 alkyne (5)
2.1.7. PSMA-617 alkyne (6)
2.1.8. BO530
2.1.9. BO535
2.1.10. BO536
2.2. Radiolabeling and Products’ Characterization
2.3. In Vitro Characterization
2.4. In Vivo Characterization
3. Results
3.1. Heterodimers Production, Radio-Iodination, and Characterization
3.2. In Vitro Characterization of Radio-Iodinated Heterodimers
3.3. In Vivo Characterization of Radio-Iodinated Heterodimers
4. Discussion
5. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Cell Lines, Chemicals and Equipment
References
- Attard, G.; Parker, C.; Eeles, R.A.; Schröder, F.; Tomlins, S.A.; Tannack, I.; Drake, C.G.; de Bono, J.S. Prostate Cancer. Lancet 2016, 387, 70–82. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, S. A Review of Imaging Methods for Prostate Cancer Detection. Biomed. Eng. Comput. Biol. 2016, 7, 1–15. [Google Scholar] [CrossRef]
- Sartor, O.; Bono, J.S. Metastatic Prostate Cancer. N. Engl. J. Med. 2018, 378, 645–657. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Heidenreich, A.; Bastian, P.J.; Bellmunt, J.; Bolla, M.; Joniau, S.; vad der Kwast, T.; Mason, M.; Matveev, V.; Wiegel, T.; Zattoni, F.; et al. EAU Guidelines on Prostate Cancer. Part II: Treatment of Advanced, Relapsing, and Castration-Resistant Prostate Cancer. Eur. Urol. 2014, 65, 467–479. [Google Scholar] [CrossRef]
- Barve, A.; Jin, W.; Cheng, K. Prostate Cancer Relevant Antigens and Enzymes for Targeted Drug Delivery. J. Control. Release 2014, 187, 118–132. [Google Scholar] [CrossRef]
- Cornelio, D.B.; Roesler, R.; Schwartsmann, G. Gastrin-releasing peptide receptor as a molecular target in experimental anticancer therapy. Ann. Oncol. 2007, 18, 1457–1466. [Google Scholar] [CrossRef]
- Körner, M.; Waser, B.; Rehmann, R.; Reubi, J.C. Early Over-Expression of GRP Receptors in Prostatic Carcinogenesis. Prostate 2014, 74, 217–224. [Google Scholar] [CrossRef]
- Bartholdi, M.F.; Wu, J.M.; Pu, H.; Troncoso, P.; Eden, P.A.; Feldman, R.I. In Situ Hybridization for Gastrin-Releasing Peptide Receptor (GRP Receptor) Expression in Prostatic Carcinoma. Int. J. Cancer 1998, 79, 82–90. [Google Scholar] [CrossRef]
- Sun, B.; Halmos, G.; Schally, A.V.; Wang, X.; Martinez, M. Presence of Receptors for Bombesin/Gastrin-Releasing Peptide and mRNA for Three Receptor Subtypes in Human Prostate Cancers. Prostate 1999, 42, 295–303. [Google Scholar] [CrossRef]
- Reubi, J.C.; Wenger, S.; Schmuckli-Maurer, J.; Schaer, J.C.; Gugger, M. Bombesin Receptor Subtypes in Human Cancers: Detection with the Universal Radioligand 125I-[d-Tyr6, -ALA11, PHE13, NLE14] Bombesin(6-14). Clin. Cancer Res. 2002, 8, 1139–1146. [Google Scholar]
- de Visser, M.; van Weerden, W.M.; de Ridder, C.M.A.; Reneman, S.; Melis, M.; Krenning, E.P.; de Jong, M. Androgen-Dependent Expression of the Gastrin-Releasing Peptide Receptor in Human Prostate Tumor Xenografts. J. Nucl. Med. 2007, 48, 88–93. [Google Scholar]
- Silver, D.A.; Pellicer, I.; Fair, W.R.; Heston, W.D.W.; Cordon-Cardo, C. Prostate-specific Membrane Antigen Expression in Normal and Malignant Human Tissues. Clin. Cancer Res. 1997, 3, 81–85. [Google Scholar]
- Sweat, S.D.; Pacelli, A.; Murphy, G.P.; Bostwick, D.G. Prostate-Specific Membrane Antigen Expression is Greatest in Prostate Adenocarcinoma and Lymph Node Metastases. Urology 1998, 52, 637–640. [Google Scholar] [CrossRef]
- Schally, A.V.; Comaru-Schally, A.M.; Nagy, A.; Kovacs, M.; Szepeshazi, K.; Plonowski, A.; Varga, J.L.; Halmos, G. Hypothalamic Hormones and Cancer. Front. Neuroendocrinol. 2001, 22, 248–291. [Google Scholar] [CrossRef]
- Wright, G.L.; Haley, C.; Beckett, M.L.; Schellhammer, P.F. Expression of Prostate-Specific Membrane Antigen in Normal, Benign, and Malignant Prostate Tissues. Urol. Oncol. 1995, 1, 18–28. [Google Scholar] [CrossRef]
- Wüstemann, T.; Haberkorn, U.; Babich, J.; Mier, W. Targeting prostate cancer: Prostate-specific membrane antigen based diagnosis and therapy. Med. Res. Rev. 2019, 39, 40–69. [Google Scholar] [CrossRef]
- Chang, S.S.; Reuter, V.E.; Heston, W.D.W.; Bander, N.H.; Grauer, L.S.; Gaudin, P.B. Five Different Anti-Prostate-specific Membrane Antigen (PSMA) Antibodies Confirm PSMA Expression in Tumor-associated Neovasculature. Cancer Res. 1999, 59, 3192–3198. [Google Scholar]
- Nicolas, G.P.; Morgenstern, A.; Schottelius, M.; Fani, M. New Developments in Peptide Receptor Radionuclide Therapy. J. Nucl. Med. 2019, 60, 167–171. [Google Scholar] [CrossRef]
- Dorff, T.B.; Fanti, A.; Reiter, R.E.; Sadun, T.Y.; Sartor, O. The Evolving Role of Prostate-Specific Membrane Antigen-Based Diagnostics and Therapeutics in Prostate Cancer. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 321–330. [Google Scholar] [CrossRef]
- Minamimoto, R.; Hancock, S.; Schneider, B.; Chin, F.T.; Jamali, M.; Loening, A.; Varga, J.L.; Iagaru, A. Pilot Comparison of 68Ga-RM2 PET and 68Ga-PSMA-11 PET in Patients with Biochemically Recurrent Prostate Cancer. J. Nucl. Med. 2015, 2015. 57, 557–562. [Google Scholar] [CrossRef]
- Eder, M.; Schäfer, M.; Bauder-Wüst, U.; Haberkorn, U.; Eisenhut, M.; Kopka, K. Preclinical Evaluation of a Bispecific Low-Molecular Heterodimer Targeting Both PSMA and GRPR for Improved PET Imaging and Therapy of Prostate Cancer. Prostate 2014, 74, 659–668. [Google Scholar] [CrossRef]
- Bandari, R.P.; Jiang, Z.; Reynolds, T.S.; Berskoetter, N.E.; Szczodroski, A.F.; Bassuner, K.J.; Kirkpatrick, D.L.; Rold, T.L.; Sieckman, G.L.; Hoffman, T.J.; et al. Synthesis and biological evaluation of copper-64 radiolabeled [DUPA-6-Ahx-(NODAGA)-5-Ava-BBN(7-14)NH2], a novel bivalent targeting vector having affinity for two distinct biomarkers (GRPr/PSMA) of prostate cancer. Nucl. Med. Biol. 2014, 41, 355–363. [Google Scholar] [CrossRef]
- Liolios, C.; Schäfer, M.; Haberkorn, U.; Eder, M.; Kopka, K. Novel Bispecific PSMA/GRPr Targeting Radioligands with Optimized Pharmacokinetics for Improved PET Imaging of Prostate Cancer. Bioconjug. Chem. 2016, 27, 737–751. [Google Scholar] [CrossRef]
- Millar, J.B.; Rozengurt, E. Chronic desensitization to bombesin by progressive down-regulation of bombesin receptors in Swiss 3T3 cells. Distinction from acute desensitization. J. Biol. Chem. 1990, 265, 12052–12058. [Google Scholar]
- Ginj, M.; Zhang, H.; Waser, B.; Cescato, R.; Wild, D.; Wang, X.; Erchegyi, J.; Rivier, J.; Mäcke, H.R.; Reubi, J.C. Radiolabeled somatostatin receptor antagonists are preferable to agonists for in vivo peptide receptor targeting of tumors. Proc. Natl. Acad. Sci. USA 2006, 103, 16436–16441. [Google Scholar] [CrossRef]
- Varasteh, Z.; Velikyan, I.; Lindeberg, G.; Sörensen, J.; Larhed, M.; Sandström, M.; Selvaraju, R.K.; Malmberg, J.; Tolmachev, V.; Orlova, A. Synthesis and Characterization of a High-Affinity NOTA-Conjugated Bombesin Antagonist for GRPR-Targeted Tumor Imaging. Bioconjug. Chem. 2013, 24, 1144–1153. [Google Scholar] [CrossRef] [Green Version]
- Mitran, B.; Thisgaard, H.; Rosenström, U.; Dam, J.H.; Larhed, M.; Tolmachev, V.; Orlova, A. High Contrast PET Imaging of GRPR Expression in Prostate Cancer Using Cobalt-Labeled Bombesin Antagonist RM26. Contrast Media Mol. Imaging 2017, 1–10. [Google Scholar] [CrossRef]
- Mitran, B.; Rinne, S.S.; Konijnenberg, M.; Maina, T.; Nock, B.A.; Altai, M.; Vorobyeva, A.; Larhed, M.; Tolmachev, V.; de Jong, M.; et al. Trastuzumab co-treatment improves survival of mice with PC-3 prostate cancer xenografts treated with GRPR antagonist [177Lu]Lu-DOTAGA-PEG2-RM26. Int. J. Cancer 2019. [Google Scholar] [CrossRef]
- Benešová, M.; Schäfer, M.; Bauder-Wüst, U.; Afshar-Oromieh, A.; Kratochwil, C.; Mier, W.; Eder, M. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J. Nucl. Med. 2015, 56, 914–920. [Google Scholar] [CrossRef] [Green Version]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Ravi Kumar, A.; Murphy, D.G.; et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Bräuer, A.; Grubert, L.S.; Roll, W.; Schrader, A.J.; Schäfers, M.; Bögemann, M.; Rahbar, K. 177Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1663–16670. [Google Scholar] [CrossRef]
- Pandiakumar, A.K.; Sarma, S.P.; Samuelson, A.G. Mechanistic studies on the diazo transfer reaction. Tetrahedron Lett. 2014, 55, 2917–2920. [Google Scholar] [CrossRef]
- Potter, G.T.; Jayson, G.C.; Miller, G.J.; Gardiner, J.M. An Updated Synthesis of the Diazo-Transfer Reagent Imidazole-1-sulfonyl Azide Hydrogen Sulfate. J. Org. Chem. 2016, 81, 3443–3446. [Google Scholar] [CrossRef]
- Boeijen, A.; Liskamp, R.M.J. Solid-Phase Synthesis of Oligourea Peptidomimetics. Eur. J. Org. Chem. 1999, 9, 2127–2135. [Google Scholar]
- Kim, J.M.; Bi, Y.; Paikoff, S.J.; Schultz, P.G. The Solid Phase Synthesis of Oligoureas. Tetrahedron Lett. 1996, 37, 5305–5308. [Google Scholar] [CrossRef]
- Fields, G.B.; Noble, R.L. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int. J. Pept. Protein Res. 1990, 35, 161–214. [Google Scholar] [CrossRef]
- Llinares, M.; Devin, C.; Chaloin, O.; Azay, J.; Noel-Artis, A.M.; Bernad, N.; Fehrentz, J.A.; Martinez, J. Syntheses and biological activities of potent bombesin receptor antagonists. J. Pept. Res. 1999, 53, 275–283. [Google Scholar] [CrossRef]
- Eder, M.; Schäfer, M.; Bauder-Wüst, U.; Hull, W.E.; Wangler, C.; Mier, W.; Haberkorn, U.; Eisenhut, M. 68Ga-Complex Lipophilicity and the Targetting Property of a Urea-Based PSMA Inhibitor for PET Imaging. Bioconjug. Chem. 2012, 23, 688–697. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. Engl. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Salacinski, P.R.; McLean, C.; Sykes, J.E.; Clement-Jones, V.V.; Lowry, P.J. Iodination of proteins, glycoproteins, and peptides using a solid-phase oxidizing agent, 1,3,4,6-tetrachloro-3α,6α-diphenyl glycoluril (Iodogen). Anal. Biochem. 1981, 117, 136–146. [Google Scholar] [CrossRef]
- Stafford, R.G.; Mehta, M.; Kemppainen, B.W. Comparison of the partition coefficient and skin penetration of a marine algal toxin (lyngbyatoxin A). Food Chem. Toxicol. 1992, 30, 795–801. [Google Scholar] [CrossRef]
- Mendoza-Figueroa, M.J.; Escudero-Castellanos, A.; Ramirez-Nava, G.J.; Ocampo-García, B.E.; Santos-Cuevas, C.L.; Ferro-Flores, G.; Pedraza-Lopez, M.; Avila-Rodriguez, M.A. Preparation and preclinical evaluation of 68Ga-iPSMA-BN as a potential heterodimeric radiotracer for PET-imaging of prostate cancer. J. Radioanal. Nucl. Chem. 2018, 318, 2097–2105. [Google Scholar] [CrossRef]
- Escudero-Castellanos, A.; Ocampo-García, B.; Ferro-Flores, G.; Santos-Cuevas, C.; Morales-Ávila, E.; Luna-Gutiérrez, M.; Isaac-Olivé, K. Synthesis and preclinical evaluation of the 177Lu-DOTA-PSMA(inhibitor)-Lys3-bombesin heterodimer designed as a radiotheranostic probe for prostate cancer. Nucl. Med. Commun. 2019, 40, 278–286. [Google Scholar] [CrossRef]
- Zechmann, C.M.; Afshar-Omorieh, A.; Armor, T.; Stubbs, J.B.; Mier, W.; Hadaschik, B.; Joyal, J.; Kopka, K.; Debus, J.; Babich, J.W.; et al. Radiation dosimetry and first therapy results with a 124I/131I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1280–1292. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Cai, W.; Cao, F.; Schreibmann, E.; Wu, Y.; Wu, J.C.; Xing, L.; Chen, X. 18F-Labeled Bombesin Analogs for Targeting GRP Receptor-Expressing Prostate Cancer. J. Nucl. Med. 2006, 47, 492–501. [Google Scholar]
- Orlova, A.; Bruskin, A.; Sjöström, A.; Lundqvist, H.; Gedda, L.; Tolmachev, V. Cellular processing of 125I and 111In labeled epidermal growth factor (EGF) bound to cultured A431 tumor cells. Nucl. Med. Biol. 2000, 27, 827–835. [Google Scholar] [CrossRef]
- Vegt, E.; Melis, M.; Eek, A.; de Visser, M.; Brom, M.; Oyen, W.J.; Gotthardt, M.; de Jong, M.; Boerman, O.C. Renal uptake of different radiolabelled peptides is mediated by megalin: SPECT and biodistribution studies in megalin-deficient mice. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 623–632. [Google Scholar] [CrossRef]
- Gandaglia, G.; Abdollah, F.; Schiffmann, J.; Trudeau, V.; Shariat, S.F.; Kim, S.P.; Perrotte, P.; Montorsi, F.; Briganti, A.; Trinh, Q.D.; et al. Distribution of Metastatic Sites in Patients With Prostate Cancer: A Population-Based Analysis. Prostate 2014, 74, 210–216. [Google Scholar] [CrossRef]









| Heterodimer | [125I]I-BO530 | [125I]I-BO535 | [125I]I-BO536 | |||
|---|---|---|---|---|---|---|
| Organ | Non-blocked | Blocked | Non-blocked | Blocked | Non-blocked | Blocked |
| Blood | 5 ± 1 | 4.5 ± 0.6 | 8 ± 1 | 8.4 ± 0.7 | 5.0 ± 0.7 | 6 ± 2 |
| Tumor | 4.3 ± 0.6 | 2.3 ± 0.3 1 | 2.2 ± 0.6 | 2.2 ± 0.2 | 3.0 ± 0.3 | 2.6 ± 0.2 |
| Saliv. Gl. | 2.0 ± 0.3 | 1.7 ± 0.2 | 2.2 ± 0.5 | 2.4 ± 0.3 | 2.7 ± 0.3 | 2.8 ± 0.5 |
| Thyroid | 5 ± 1 | 3.1 ± 0.3 1 | 4.4 ± 0.9 | 4.3 ± 0.7 | 3.7 ± 0.6 | 3.8 ± 0.8 |
| Lung | 20 ± 7 | 35 ± 4 1 | 10 ± 2 | 10 ± 2 | 13 ± 4 | 15 ± 7 |
| Liver | 23 ± 3 | 23 ± 4 | 27 ± 4 | 32 ± 1 | 23 ± 3 | 26 ± 5 |
| Spleen | 8 ± 2 | 6.4 ± 0.4 | 3.2 ± 0.7 | 3.6 ± 0.4 | 12 ± 1 | 11.6 ± 0.8 |
| Pancreas | 19 ± 4 | 5 ± 1 1 | 4 ± 1 | 1.7 ± 0.1 1 | 8 ± 1 | 2.7 ± 0.4 1 |
| Stomach | 2.2 ± 0.2 | 1.4 ± 0.2 1 | 1.5 ± 0.3 | 1.6 ± 0.3 | 2.2 ± 0.5 | 1.8 ± 0.3 |
| Intestines | 4 ± 1 | 2.8 ± 0.7 | 3.2 ± 0.8 | 3.3 ± 0.6 | 3.8 ± 0.5 | 4 ± 1 |
| Kidneys | 66 ± 18 | 61 ± 16 | 12 ± 3 | 14 ± 2 | 51 ± 6 | 48 ± 9 |
| Muscle | 0.7 ± 0.2 | 0.6 ± 0.1 | 0.7 ± 0.2 | 1.0 ± 0.3 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| Bone | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.9 ± 0.2 | 1.2 ± 0.3 | 0.8 ± 0.2 | 0.9 ± 0.3 |
| Heterodimer | [125I]I-BO530 | [125I]I-BO535 | [125I]I-BO536 | |||
|---|---|---|---|---|---|---|
| Organ | Non-blocked | Blocked | Non-blocked | Blocked | Non-blocked | Blocked |
| Blood | 4 ± 1 | 4 ± 1 | 6 ± 1 | 6 ± 1 | 8 ± 3 | 6.0 ± 0.9 |
| Tumor | 10 ± 3 | 2 ± 2 1 | 3 ± 2 | 2 ± 2 | 11 ± 3 | 1.4 ± 0.7 1 |
| Saliv. Gl. | 1.7 ± 0.7 | 1.5 ± 0.3 | 1.7 ± 0.4 | 1.5 ± 0.4 | 3.1 ± 0.8 | 1.9 ± 0.2 1 |
| Thyroid | 2.6 ± 0.5 | 3.5 ± 0.9 | 3.3 ± 0.5 | 3.4 ± 0.6 | 4 ± 1 | 3.4 ± 0.6 |
| Lung | 5.6 ± 0.9 1 | 46 ± 19 | 21.5 ± 0.8 | 14 ± 3 1 | 5 ± 1 | 8 ± 1 1 |
| Liver | 29 ± 3 | 27 ± 1 | 40 ± 5 | 37 ± 7 | 30 ± 9 | 29 ± 3 |
| Spleen | 9 ± 5 | 6 ± 2 | 4.5 ± 0.8 | 3.4 ± 0.8 | 18 ± 6 | 3.5 ± 0.8 1 |
| Pancreas | 28 ± 6 | 22 ± 3 | 7 ± 2 | 7 ± 2 | 10 ± 2 | 7.0 ± 0.7 1 |
| Stomach | 1.9 ± 0.4 | 2.3 ± 0.8 | 1.1 ± 0.2 | 1.2 ± 0.2 | 3 ± 2 | 3 ± 1 |
| Intestines | 7 ± 3 | 4.4 ± 0.4 | 3.2 ± 0.8 | 2.7 ± 0.7 | 5 ± 1 | 5 ± 2 |
| Kidneys | 56 ± 22 | 13.1 ± 0.7 1 | 6.8 ± 0.9 | 3.8 ± 0.5 1 | 63 ± 20 | 19 ± 3 1 |
| Muscle | 0.5 ± 0.1 | 0.6 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.8 ± 0.2 | 0.6 ± 0.1 |
| Bone | 0.6 ± 0.1 | 0.8 ± 0.4 | 0.7 ± 0.1 | 0.7 ± 0.3 | 0.8 ± 0.1 | 0.9 ± 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abouzayed, A.; Yim, C.-B.; Mitran, B.; Rinne, S.S.; Tolmachev, V.; Larhed, M.; Rosenström, U.; Orlova, A. Synthesis and Preclinical Evaluation of Radio-Iodinated GRPR/PSMA Bispecific Heterodimers for the Theranostics Application in Prostate Cancer. Pharmaceutics 2019, 11, 358. https://doi.org/10.3390/pharmaceutics11070358
Abouzayed A, Yim C-B, Mitran B, Rinne SS, Tolmachev V, Larhed M, Rosenström U, Orlova A. Synthesis and Preclinical Evaluation of Radio-Iodinated GRPR/PSMA Bispecific Heterodimers for the Theranostics Application in Prostate Cancer. Pharmaceutics. 2019; 11(7):358. https://doi.org/10.3390/pharmaceutics11070358
Chicago/Turabian StyleAbouzayed, Ayman, Cheng-Bin Yim, Bogdan Mitran, Sara S. Rinne, Vladimir Tolmachev, Mats Larhed, Ulrika Rosenström, and Anna Orlova. 2019. "Synthesis and Preclinical Evaluation of Radio-Iodinated GRPR/PSMA Bispecific Heterodimers for the Theranostics Application in Prostate Cancer" Pharmaceutics 11, no. 7: 358. https://doi.org/10.3390/pharmaceutics11070358
APA StyleAbouzayed, A., Yim, C.-B., Mitran, B., Rinne, S. S., Tolmachev, V., Larhed, M., Rosenström, U., & Orlova, A. (2019). Synthesis and Preclinical Evaluation of Radio-Iodinated GRPR/PSMA Bispecific Heterodimers for the Theranostics Application in Prostate Cancer. Pharmaceutics, 11(7), 358. https://doi.org/10.3390/pharmaceutics11070358