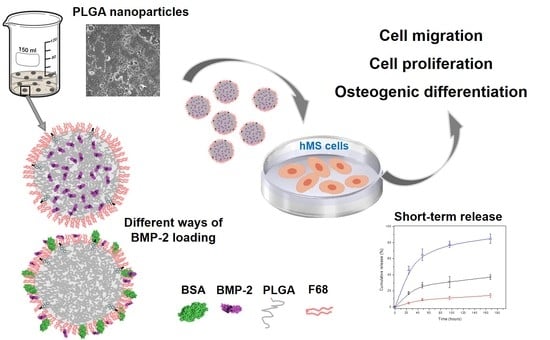
Formulation, Colloidal Characterization, and In Vitro Biological Effect of BMP-2 Loaded PLGA Nanoparticles for Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticle Synthesis
2.1.1. Formulation
2.1.2. Cleaning and Storage
2.1.3. Protein Loading and Encapsulation Efficiency
2.1.4. Physical Protein Adsorption
2.1.5. Protein Separation by Gel Electrophoresis, SDS-PAGE
2.2. Nanoparticle Characterization: Morphology, Size, Concentration, and Electrokinetic Mobility
2.3. Colloidal and Temporal Stability in Biological Media
2.4. Cell Interactions
2.4.1. Cell Migration
2.4.2. Cell Proliferation
2.4.3. Osteogenic Differentiation
2.4.4. Statistical Evaluation
3. Results and Discussion
3.1. Nanoparticle Formulation
3.2. Nanoparticle Characterization
3.2.1. Nanoparticle Size
3.2.2. Electrokinetic Mobility and Colloidal Stability
3.2.3. Protein Release
3.3. Biological Activity and Interactions
3.3.1. Cell Migration
3.3.2. Cell Proliferation
3.3.3. Osteogenic Differentiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Rijt, S.; Habibovic, P. Enhancing regenerative approaches with nanoparticles. J. R. Soc. Interface 2017, 14. [Google Scholar] [CrossRef]
- Kumar, B.; Jalodia, K.; Kumar, P.; Gautam, H.K. Recent advances in nanoparticle-mediated drug delivery. J. Drug Deliv. Sci. Technol. 2017, 41, 260–268. [Google Scholar] [CrossRef]
- Mir, M.; Ahmed, N.; Rehman, A.U.R. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf. B Biointerfaces 2017, 159, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Jana, S. Natural polymeric biodegradable nanoblend for macromolecules delivery. In Recent Developments in Polymer Macro, Micro and Nano Blends; Woodhead Publishing: Cambridge, UK, 2017; pp. 289–312. ISBN 9780081004081. [Google Scholar]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Zhu, Q. Recent advances of PLGA micro/nanoparticles for the delivery of biomacromolecular therapeutics. Mater. Sci. Eng. C 2018, 92, 1041–1060. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L.; Unciti-Broceta, J.D.; Maceira, J.; del Castillo, T.; Hernández-Quero, J.; Magez, S.; Soriano, M.; García-Salcedo, J.A. Nanobody conjugated PLGA nanoparticles for active targeting of African Trypanosomiasis. J. Control. Release 2015, 197, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Giteau, A.; Venier-Julienne, M.C.; Aubert-Pouëssel, A.; Benoit, J.P. How to achieve sustained and complete protein release from PLGA-based microparticles? Int. J. Pharm. 2008, 350, 14–26. [Google Scholar] [CrossRef] [Green Version]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—A review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef]
- White, L.J.; Kirby, G.T.S.; Cox, H.C.; Qodratnama, R.; Qutachi, O.; Rose, F.R.A.J.; Shakesheff, K.M. Accelerating protein release from microparticles for regenerative medicine applications. Mater. Sci. Eng. C 2013, 33, 2578–2583. [Google Scholar] [CrossRef]
- Ortega-Oller, I.; del Castillo-Santaella, T.; Padial-Molina, M.; Galindo-Moreno, P.; Jódar-Reyes, A.B.; Peula-García, J.M. Dual delivery nanosystem for biomolecules. Formulation, characterization, and in vitro release. Colloids Surf. B Biointerfaces 2017, 159, 586–595. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: A review. Adv. Colloid Interface Sci. 2018, 253, 1–22. [Google Scholar] [CrossRef]
- Ortega-Oller, I.; Padial-Molina, M.; Galindo-Moreno, P.; O’Valle, F.; Jódar-Reyes, A.B.; Peula-García, J.M. Bone Regeneration from PLGA Micro-Nanoparticles. BioMed Res. Int. 2015, 2015, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bapat, R.A.; Joshi, C.P.; Bapat, P.; Chaubal, T.V.; Pandurangappa, R.; Jnanendrappa, N.; Gorain, B.; Khurana, S.; Kesharwani, P. The use of nanoparticles as biomaterials in dentistry. Drug Discov. Today 2019, 24, 85–98. [Google Scholar] [CrossRef]
- Ji, Y.; Xu, G.P.; Zhang, Z.P.; Xia, J.J.; Yan, J.L.; Pan, S.H. BMP-2/PLGA delayed-release microspheres composite graft, selection of bone particulate diameters, and prevention of aseptic inflammation for bone tissue engineering. Ann. BioMed. Eng. 2010, 38, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Kirby, G.T.S.; White, L.J.; Rahman, C.V.; Cox, H.C.; Qutachi, O.; Rose, F.R.A.J.; Hutmacher, D.W.; Shakesheff, K.M.; Woodruff, M.A. PLGA-Based Microparticles for the Sustained Release of BMP-2. Polymers 2011, 3, 571–586. [Google Scholar] [CrossRef] [Green Version]
- Qutachi, O.; Shakesheff, K.M.; Buttery, L.D.K. Delivery of definable number of drug or growth factor loaded poly(dl-lactic acid-co-glycolic acid) microparticles within human embryonic stem cell derived aggregates. J. Control. Release 2013, 168, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, Y.; Zhang, X.; Xu, M.; Liu, F.; Ma, Q.; Cai, Q.; Deng, X. PLGA/PDLLA core-shell submicron spheres sequential release system: Preparation, characterization and promotion of bone regeneration in vitro and in vivo. Chem. Eng. J. 2015, 273, 490–501. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Zhang, X.-P.; Xiao, G.-Y.; Hou, -Y.; Cheng, L.; Si, M.; Wang, S.-S.; Li, Y.-H.; Nie, L. In vitro and in vivo evaluation of calcium phosphate composite scaffolds containing BMP-VEGF loaded PLGA microspheres for the treatment of avascular necrosis of the femoral head. Mater. Sci. Eng. C 2016, 60, 298–307. [Google Scholar] [CrossRef]
- Begam, H.; Nandi, S.K.; Kundu, B.; Chanda, A. Strategies for delivering bone morphogenetic protein for bone healing. Mater. Sci. Eng. C 2017, 70, 856–869. [Google Scholar] [CrossRef]
- Balmayor, E.R.; Feichtinger, G.A.; Azevedo, H.S.; Van Griensven, M.; Reis, R.L. Starch-poly-ε-caprolactone microparticles reduce the needed amount of BMP-2. Clin. Orthop. Relat. Res. 2009, 467, 3138–3148. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, C.S.; Saylor, D.M.; Koo, D. Polymer degradation and drug delivery in PLGA-based drug–polymer applications: A review of experiments and theories. J. BioMed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1692–1716. [Google Scholar] [CrossRef] [PubMed]
- Padial-Molina, M.; de Buitrago, J.G.; Sainz-Urruela, R.; Abril-Garcia, D.; Anderson, P.; O’Valle, F.; Galindo-Moreno, P. Expression of Musashi-1 during osteogenic differentiation of oral MSC: An in vitro study. Int. J. Mol. Sci. 2019, 20, 2171. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, I.; Garcia-Fuentes, M.; Parajó, Y.; Welle, A.; Vántus, T.; Horváth, A.; Bökönyi, G.; Kéri, G.; Alonso, M.J. Nanoparticles based on PLGA:poloxamer blends for the delivery of proangiogenic growth factors. Mol. Pharm. 2010, 7, 1724–1733. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-C.; Yang, C.; Feng, F.; Lin, F.-H.; Wang, C.-H.; Chang, P.-C. Bone morphogenetic protein-2 loaded poly(D,L-lactide-co-glycolide) microspheres enhance osteogenic potential of gelatin/hydroxyapatite/β-tricalcium phosphate cryogel composite for alveolar ridge augmentation. J. Formos. Med. Assoc. 2017, 116, 973–981. [Google Scholar] [CrossRef] [PubMed]
- Padial-Molina, M.; Volk, S.L.; Rios, H.F. Periostin increases migration and proliferation of human periodontal ligament fibroblasts challenged by tumor necrosis factor -α and Porphyromonas gingivalis lipopolysaccharides. J. Periodontal Res. 2014, 49, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Houghton, P.; Fang, R.; Techatanawat, I.; Steventon, G.; Hylands, P.J.; Lee, C.C. The sulphorhodamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activities related to reputed anticancer activity. Methods 2007, 42, 377–387. [Google Scholar] [CrossRef]
- Iqbal, M.; Zafar, N.; Fessi, H.; Elaissari, A. Double emulsion solvent evaporation techniques used for drug encapsulation. Int. J. Pharm. 2015, 496, 173–190. [Google Scholar] [CrossRef]
- Sánchez-Moreno, P.; Ortega-Vinuesa, J.L.; Boulaiz, H.; Marchal, J.A.; Peula-García, J.M. Synthesis and characterization of lipid immuno-nanocapsules for directed drug delivery: Selective antitumor activity against HER2 positive breast-cancer cells. Biomacromolecules 2013, 14, 4248–4259. [Google Scholar] [CrossRef]
- Lochmann, A.; Nitzsche, H.; von Einem, S.; Schwarz, E.; Mäder, K. The influence of covalently linked and free polyethylene glycol on the structural and release properties of rhBMP-2 loaded microspheres. J. Control. Release 2010, 147, 92–100. [Google Scholar] [CrossRef]
- Kempen, D.H.R.; Lu, L.; Hefferan, T.E.; Creemers, L.B.; Maran, A.; Classic, K.L.; Dhert, W.J.A.; Yaszemski, M.J. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials 2008, 29, 3245–3252. [Google Scholar] [CrossRef] [Green Version]
- Santander-Ortega, M.J.; Csaba, N.; González, L.; Bastos-González, D.; Ortega-Vinuesa, J.L.; Alonso, M.J. Protein-loaded PLGA–PEO blend nanoparticles: Encapsulation, release and degradation characteristics. Colloid Polym. Sci. 2010, 288, 141–150. [Google Scholar] [CrossRef]
- Chung, Y.I.; Ahn, K.M.; Jeon, S.H.; Lee, S.Y.; Lee, J.H.; Tae, G. Enhanced bone regeneration with BMP-2 loaded functional nanoparticle-hydrogel complex. J. Control. Release 2007, 121, 91–99. [Google Scholar] [CrossRef]
- La, W.-G.; Kang, S.-W.; Yang, H.S.; Bhang, S.H.; Lee, S.H.; Park, J.-H.; Kim, B.-S. The Efficacy of Bone Morphogenetic Protein-2 Depends on Its Mode of Delivery. Artif. Organs 2010, 34, 1150–1153. [Google Scholar] [CrossRef]
- Fu, Y.; Du, L.; Wang, Q.; Liao, W.; Jin, Y.; Dong, A.; Chen, C.; Li, Z. In vitro sustained release of recombinant human bone morphogenetic protein-2 microspheres embedded in thermosensitive hydrogels. Die Pharm. 2012, 67, 299–303. [Google Scholar] [CrossRef]
- Rahman, C.V.; Ben-David, D.; Dhillon, A.; Kuhn, G.; Gould, T.W.A.; Müller, R.; Rose, F.R.A.J.; Shakesheff, K.M.; Livne, E. Controlled release of BMP-2 from a sintered polymer scaffold enhances bone repair in a mouse calvarial defect model. J. Tissue Eng. Regen. Med. 2014, 8, 59–66. [Google Scholar] [CrossRef]
- Pakulska, M.M.; Elliott Donaghue, I.; Obermeyer, J.M.; Tuladhar, a.; McLaughlin, C.K.; Shendruk, T.N.; Shoichet, M.S. Encapsulation-free controlled release: Electrostatic adsorption eliminates the need for protein encapsulation in PLGA nanoparticles. Sci. Adv. 2016, 2, e1600519. [Google Scholar] [CrossRef]
- Fu, C.; Yang, X.; Tan, S.; Song, L. Enhancing Cell Proliferation and Osteogenic Differentiation of MC3T3-E1 Pre-osteoblasts by BMP-2 Delivery in Graphene Oxide-Incorporated PLGA/HA Biodegradable Microcarriers. Sci. Rep. 2017, 7, 12549. [Google Scholar] [CrossRef]
- Peula, J.M.; de las Nieves, F.J. Adsorption of monomeric bovine serum albumin on sulfonated polystyrene model colloids 1. Adsorption isotherms and effect of the surface charge density. Colloids Surf. A Physicochem. Eng. Asp. 1993, 77, 199–208. [Google Scholar] [CrossRef]
- Peula, J.M.; de las Nieves, F.J. Adsorption of monomeric bovine serum albumin on sulfonated polystyrene model colloids 3. Colloidal stability of latex—Protein complexes. Colloids Surf. A Physicochem. Eng. Asp. 1994, 90, 55–62. [Google Scholar] [CrossRef]
- Peula, J.M.; Hidalgo-Alvarez, R.; De Las Nieves, F.J. Coadsorption of IgG and BSA onto sulfonated polystyrene latex: I. Sequential and competitive coadsorption isotherms. J. Biomater. Sci. Polym. Ed. 1996, 7, 231–240. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Üstündağ Okur, N.; Karavas, E.; Bikiaris, D.N. Surface modified multifunctional and stimuli responsive nanoparticles for drug targeting: Current status and uses. Int. J. Mol. Sci. 2016, 17, 1440. [Google Scholar] [CrossRef]
- Peula-García, J.M.; Hidalgo-Alvarez, R.; De Las Nieves, F.J. Colloid stability and electrokinetic characterization of polymer colloids prepared by different methods. Colloids Surf. A Physicochem. Eng. Asp. 1997, 127, 19–24. [Google Scholar] [CrossRef]
- Santander-Ortega, M.J.; Lozano-López, M.V.; Bastos-González, D.; Peula-García, J.M.; Ortega-Vinuesa, J.L. Novel core-shell lipid-chitosan and lipid-poloxamer nanocapsules: Stability by hydration forces. Colloid Polym. Sci. 2010, 288, 159–172. [Google Scholar] [CrossRef]
- Peula-Garcia, J.M.; Hidaldo-Alvarez, R.; De las Nieves, F.J. Protein co-adsorption on different polystyrene latexes: Electrokinetic characterization and colloidal stability. Colloid Polym. Sci. 1997, 275, 198–202. [Google Scholar] [CrossRef]
- Santander-Ortega, M.J.; Bastos-González, D.; Ortega-Vinuesa, J.L. Electrophoretic mobility and colloidal stability of PLGA particles coated with IgG. Colloids Surf. B Biointerfaces 2007, 60, 80–88. [Google Scholar] [CrossRef]
- Peula, J.M.; Callejas, J.; de las NIeves, F.J. Adsorption of Monomeric Bovine Serum Albumin on Sulfonated Polystyrene Model Colloids. II. Electrokinetic Characterization of Latex-Protein Complexes. In Surface Properties of Biomaterials; Butterworth and Heinemann: Oxford, UK, 1994; pp. 61–69. [Google Scholar]
- Sun, D. Effect of Zeta Potential and Particle Size on the Stability of SiO2 Nanospheres as Carrier for Ultrasound Imaging Contrast Agents. Int. J. Electrochem. Sci. 2016, 8520–8529. [Google Scholar] [CrossRef]
- del Castillo-Santaella, T.; Peula-García, J.M.; Maldonado-Valderrama, J.; Jódar-Reyes, A.B. Interaction of surfactant and protein at the O/W interface and its effect on colloidal and biological properties of polymeric nanocarriers. Colloids Surf. B Biointerfaces 2019, 173, 295–302. [Google Scholar] [CrossRef]
- Schrier, J.A.; DeLuca, P.P. Porous bone morphogenetic protein-2 microspheres: Polymer binding and in vitro release. AAPS PharmSciTech 2001, 2, 66–72. [Google Scholar] [CrossRef]
- Padial-Molina, M.; O’Valle, F.; Lanis, A.; Mesa, F.; Dohan Ehrenfest, D.M.; Wang, H.-L.; Galindo-Moreno, P. Clinical application of mesenchymal stem cells and novel supportive therapies for oral bone regeneration. BioMed Res. Int. 2015, 2015. [Google Scholar] [CrossRef]
- Inai, K.; Norris, R.A.; Hoffman, S.; Markwald, R.R.; Sugi, Y. BMP-2 induces cell migration and periostin expression during atrioventricular valvulogenesis. Dev. Biol. 2008, 315, 383–396. [Google Scholar] [CrossRef] [Green Version]
- Gamell, C.; Osses, N.; Bartrons, R.; Rückle, T.; Camps, M.; Rosa, J.L.; Ventura, F.; Imamura, T. BMP2 induction of actin cytoskeleton reorganization and cell migration requires PI3-kinase and Cdc42 activity. J. Cell Sci. 2008, 121, 3960–3970. [Google Scholar] [CrossRef] [Green Version]
- Friedrichs, M.; Wirsdöerfer, F.; Flohé, S.B.; Schneider, S.; Wuelling, M.; Vortkamp, A. BMP signaling balances proliferation and differentiation of muscle satellite cell descendants. BMC Cell Biol. 2011, 12, 26. [Google Scholar] [CrossRef]
- Hrubi, E.; Imre, L.; Robaszkiewicz, A.; Virág, L.; Kerényi, F.; Nagy, K.; Varga, G.; Jenei, A.; Hegedüs, C. Diverse effect of BMP-2 homodimer on mesenchymal progenitors of different origin. Hum. Cell 2018, 31, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K.W.; Oxendine, I.; Kamiya, N. High-concentration of BMP2 reduces cell proliferation and increases apoptosis via DKK1 and SOST in human primary periosteal cells. Bone 2013, 54, 141–150. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Castillo-Santaella, T.; Ortega-Oller, I.; Padial-Molina, M.; O’Valle, F.; Galindo-Moreno, P.; Jódar-Reyes, A.B.; Peula-García, J.M. Formulation, Colloidal Characterization, and In Vitro Biological Effect of BMP-2 Loaded PLGA Nanoparticles for Bone Regeneration. Pharmaceutics 2019, 11, 388. https://doi.org/10.3390/pharmaceutics11080388
del Castillo-Santaella T, Ortega-Oller I, Padial-Molina M, O’Valle F, Galindo-Moreno P, Jódar-Reyes AB, Peula-García JM. Formulation, Colloidal Characterization, and In Vitro Biological Effect of BMP-2 Loaded PLGA Nanoparticles for Bone Regeneration. Pharmaceutics. 2019; 11(8):388. https://doi.org/10.3390/pharmaceutics11080388
Chicago/Turabian Styledel Castillo-Santaella, Teresa, Inmaculada Ortega-Oller, Miguel Padial-Molina, Francisco O’Valle, Pablo Galindo-Moreno, Ana Belén Jódar-Reyes, and José Manuel Peula-García. 2019. "Formulation, Colloidal Characterization, and In Vitro Biological Effect of BMP-2 Loaded PLGA Nanoparticles for Bone Regeneration" Pharmaceutics 11, no. 8: 388. https://doi.org/10.3390/pharmaceutics11080388
APA Styledel Castillo-Santaella, T., Ortega-Oller, I., Padial-Molina, M., O’Valle, F., Galindo-Moreno, P., Jódar-Reyes, A. B., & Peula-García, J. M. (2019). Formulation, Colloidal Characterization, and In Vitro Biological Effect of BMP-2 Loaded PLGA Nanoparticles for Bone Regeneration. Pharmaceutics, 11(8), 388. https://doi.org/10.3390/pharmaceutics11080388