Oral Insulin Delivery Using Poly (Styrene Co-Maleic Acid) Micelles in a Diabetic Mouse Model
Abstract
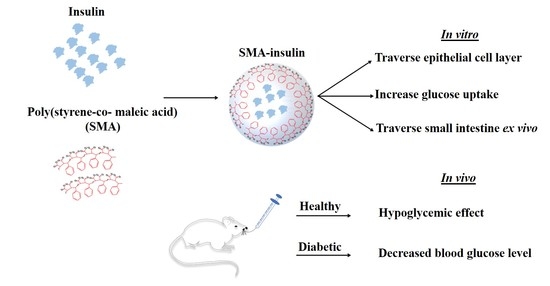
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of SMA-Insulin
2.2.2. Characterization of SMA-Insulin
Loading of the SMA Micelles
Size, Polydispersity Index (PDI), and Zeta Potential Determination of SMA Micelles
2.2.3. In Vitro Drug Release Profile at Physiological pH and in Simulated Gastric Fluid (SGF)
2.2.4. Cell Culture
2.2.5. In Vitro Model for Evaluating the Intestinal Transport of SMA-Insulin Micelles
2.2.6. In Vitro Model for Evaluating the Glucose Uptake of SMA-Insulin Micelles
2.2.7. Ex Vivo Model for Evaluating the Transport of SMA-Insulin Micelles
2.2.8. In Vivo Model to Evaluate the Effect of SMA-Insulin Micelles on Healthy and Diabetic C57BL/6 Mice
2.3. Statistical Analysis
3. Results
3.1. Characterization of SMA-Insulin
3.2. In Vitro Drug Release Profile at Physiological pH and in Simulated Gastric Fluid (SGF)
3.3. In Vitro Model for Evaluating the Transport of SMA-Insulin Micelles
3.4. In Vitro Model for Evaluating the Glucose Uptake of SMA-Insulin Micelles
3.5. Ex Vivo Model for Evaluating the Transport of SMA-Insulin Micelles
3.6. In Vivo Evaluation of Oral SMA-Insulin Micelles Effect on BGL of Healthy C57BL/6 Mice
3.7. In Vivo Evaluation of Subcutaneous Injection SMA-Insulin Micelles Effect on BGL of Healthy C57BL/6 Mice
3.8. In Vivo Evaluation of Oral SMA-Insulin Micelles Effect on BGL of Diabetic C57BL/6 Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2015; Technical Report; World Health Organization: Genev, Switzerland, 2015. [Google Scholar]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International diabetes federation Diabetes Atlas. IDF Diabete Atlas, 7th ed.; International Diabetes Federation: Brussels, Belgium, 2015. [Google Scholar]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes care 2014, 37, S81–S90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, D.; Mukherjee, K. Economic impact of type-2 diabetes mellitus on households in Hisar district of Haryana state. Health Agenda 2014, 2, 125–129. [Google Scholar]
- Ansari, M. Oral delivery of insulin for treatment of diabetes: Classical challenges and current opportunities. J. Med. Sci. 2015, 15, 209. [Google Scholar] [CrossRef] [Green Version]
- Amulya, C.; Gupta, M.E.; Babu, I.S. A review on alternative routes for insulin administration. World J. Pharm. Res. 2019, 8, 1471–1479. [Google Scholar]
- Nair, M. Diabetes mellitus, part 1: Physiology and complications. Br. J. Nurs. 2007, 16, 184–188. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, P.; Modica, J.A.; Drout, R.J.; Farha, O.K. Acid-resistant mesoporous metal–organic framework toward oral insulin delivery: Protein encapsulation, protection, and release. J. Am. Chem. Soc. 2018, 140, 5678–5681. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, D.; Liu, L.; Li, X. Development of poly (hydroxyethyl methacrylate) nanogel for effective oral insulin delivery. Pharm. Dev. Technol. 2018, 23, 351–357. [Google Scholar] [CrossRef]
- Ahmad, J.; Singhal, M.; Amin, S.; Rizwanullah, M.; Akhter, S.; Amjad Kamal, M.; Haider, N.; Midoux, P.; Pichon, C. Bile salt stabilized vesicles (bilosomes): A novel nano-pharmaceutical design for oral delivery of proteins and peptides. Curr. Pharm. Des. 2017, 23, 1575–1588. [Google Scholar] [CrossRef]
- Fukuoka, Y.; Khafagy, E.-S.; Goto, T.; Kamei, N.; Takayama, K.; Peppas, N.A.; Takeda-Morishita, M. Combination strategy with complexation hydrogels and cell-penetrating peptides for oral delivery of insulin. Biol. Pharm. Bull. 2018, 41, 811–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugihara, H.; Yamamoto, H.; Kawashima, Y.; Takeuchi, H. Effectiveness of submicronized chitosan-coated liposomes in oral absorption of indomethacin. J. Liposome Res. 2012, 22, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Alai, M.S.; Lin, W.J.; Pingale, S.S. Application of polymeric nanoparticles and micelles in insulin oral delivery. J. Food Drug Anal. 2015, 23, 351–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, N.; Mohd Amin, M.C.I.; Ismail, I.; Buang, F. Enhancement of oral insulin bioavailability: In vitro and in vivo assessment of nanoporous stimuli-responsive hydrogel microparticles. Expert Opin. Drug Deliv. 2016, 13, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Brayden, D.J.; Mrsny, R.J. Oral peptide delivery: Prioritizing the leading technologies. Ther. Deliv. 2011, 2, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, A.; Al Remawi, M.; Qinna, N.; Farouk, A.; Badwan, A. Formulation and characterization of an oily-based system for oral delivery of insulin. Eur. J. Pharm. Biopharm. 2009, 73, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, J.; Zhu, X.; Shan, W.; Li, L.; Zhong, J.; Zhang, Z.; Huang, Y. Efficient mucus permeation and tight junction opening by dissociable “mucus-inert” agent coated trimethyl chitosan nanoparticles for oral insulin delivery. J. Controlled Release 2016, 222, 67–77. [Google Scholar] [CrossRef]
- Thanou, M.; Verhoef, J.; Junginger, H. Oral drug absorption enhancement by chitosan and its derivatives. Adv. Drug Delivery Rev. 2001, 52, 117–126. [Google Scholar] [CrossRef]
- Wang, J.; Kong, M.; Zhou, Z.; Yan, D.; Yu, X.; Cheng, X.; Feng, C.; Liu, Y.; Chen, X. Mechanism of surface charge triggered intestinal epithelial tight junction opening upon chitosan nanoparticles for insulin oral delivery. Carbohydr. Polym. 2017, 157, 596–602. [Google Scholar] [CrossRef]
- Sarmento, B.; Ribeiro, A.; Veiga, F.; Sampaio, P.; Neufeld, R.; Ferreira, D. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm. Res. 2007, 24, 2198–2206. [Google Scholar] [CrossRef] [Green Version]
- Chalasani, K.B.; Russell-Jones, G.J.; Jain, A.K.; Diwan, P.V.; Jain, S.K. Effective oral delivery of insulin in animal models using vitamin B12-coated dextran nanoparticles. J. Controlled Release 2007, 122, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Li, Y.; Liu, C.S.; Chen, Q.; Wang, G.H.; Guo, W.; Wu, X.E.; Li, D.H.; Wu, W.D.; Chen, X.D. Enteric-coated capsules filled with mono-disperse micro-particles containing PLGA-lipid-PEG nanoparticles for oral delivery of insulin. Int. J. Pharm. 2015, 484, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Zhu, X.; Tao, W.; Cui, Y.; Liu, M.; Wu, L.; Li, L.; Zheng, Y.; Huang, Y. Enhanced oral delivery of protein drugs using zwitterion-functionalized nanoparticles to overcome both the diffusion and absorption barriers. ACS Appl. Mater. Interfaces 2016, 8, 25444–25453. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Zhu, X.; Liu, M.; Li, L.; Zhong, J.; Sun, W.; Zhang, Z.; Huang, Y. Overcoming the diffusion barrier of mucus and absorption barrier of epithelium by self-assembled nanoparticles for oral delivery of insulin. ACS Nano 2015, 9, 2345–2356. [Google Scholar] [CrossRef]
- Sarmento, B.; Martins, S.; Ferreira, D.; Souto, E.B. Oral insulin delivery by means of solid lipid nanoparticles. Int. J. Nanomed. 2007, 2, 743. [Google Scholar]
- Malathi, S.; Nandhakumar, P.; Pandiyan, V.; Webster, T.J.; Balasubramanian, S. Novel PLGA-based nanoparticles for the oral delivery of insulin. Int. J. Nanomed. 2015, 10, 2207. [Google Scholar]
- Cammas-Marion, S.; Okano, T.; Kataoka, K. Functional and site-specific macromolecular micelles as high potential drug carriers. Colloids Surf., B 1999, 16, 207–215. [Google Scholar] [CrossRef]
- Lavasanifar, A.; Samuel, J.; Kwon, G.S. Poly (ethylene oxide)-block-poly (L-amino acid) micelles for drug delivery. Adv. Drug Deliv. Rev. 2002, 54, 169–190. [Google Scholar] [CrossRef]
- Greish, K.; Nagamitsu, A.; Fang, J.; Maeda, H. Copoly (styrene-maleic acid)− Pirarubicin Micelles: High Tumor-Targeting Efficiency with Little Toxicity1. Bioconjugate Chem. 2005, 16, 230–236. [Google Scholar] [CrossRef]
- Parayath, N.N.; Nehoff, H.; Müller, P.; Taurin, S.; Greish, K. Styrene maleic acid micelles as a nanocarrier system for oral anticancer drug delivery–dual uptake through enterocytes and M-cells. Int. J. Nanomed. 2015, 10, 4653. [Google Scholar]
- Nehoff, H.; Parayath, N.N.; Taurin, S.; Greish, K. The influence of drug loading on caveolin-1 mediated intracellular internalization of doxorubicin nanomicelles in vitro. J. Nanomed. Nanatechnol. 2014, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Greish, K.; Sawa, T.; Fang, J.; Akaike, T.; Maeda, H. SMA–doxorubicin, a new polymeric micellar drug for effective targeting to solid tumours. J. Controlled Release 2004, 97, 219–230. [Google Scholar] [CrossRef]
- Najjar, A.; Alawi, M.; AbuHeshmeh, N.; Sallam, A. A rapid, isocratic HPLC method for determination of insulin and its degradation product. Adv. Pharm. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ho, G.T.T.; Nguyen, T.K.Y.; Kase, E.T.; Tadesse, M.; Barsett, H.; Wangensteen, H. Enhanced glucose uptake in human liver cells and inhibition of carbohydrate hydrolyzing enzymes by nordic berry extracts. Molecules 2017, 22, 1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barthe, L.; Woodley, J.; Kenworthy, S.; Houin, G. An improved everted gut sac as a simple and accurate technique to measure paracellular transport across the small intestine. Eur. J. Drug Metab. Pharmacokinet. 1998, 23, 313–323. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2015, 70, 5–47. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Simões, S.; Veiga, F.; Seiça, R.; Ribeiro, A. Why most oral insulin formulations do not reach clinical trials. Ther. Deliv. 2015, 6, 973–987. [Google Scholar] [CrossRef]
- Bahman, F.; Greish, K.; Taurin, S. Nanotechnology in Insulin Delivery for Management of Diabetes. Pharm. Nanotechnol. 2019, 7, 113–128. [Google Scholar] [CrossRef]
- Kanzarkar, M.; Pathak, P.P.; Vaidya, M.; Brumlik, C.; Choudhury, A. Oral insulin-delivery system for diabetes mellitus. Pharm. Pat. Anal. 2015, 4, 29–36. [Google Scholar] [CrossRef]
- Daruwalla, J.; Nikfarjam, M.; Greish, K.; Malcontenti-Wilson, C.; Muralidharan, V.; Christophi, C.; Maeda, H. In vitro and in vivo evaluation of tumor targeting styrene-maleic acid copolymer-pirarubicin micelles: Survival improvement and inhibition of liver metastases. Cancer Sci. 2010, 101, 1866–1874. [Google Scholar] [CrossRef]
- Iyer, A.K.; Greish, K.; Fang, J.; Murakami, R.; Maeda, H. High-loading nanosized micelles of copoly (styrene–maleic acid)–zinc protoporphyrin for targeted delivery of a potent heme oxygenase inhibitor. Biomaterials 2007, 28, 1871–1881. [Google Scholar] [CrossRef]
- Whittingham, J.L.; Scott, D.J.; Chance, K.; Wilson, A.; Finch, J.; Brange, J.; Guy Dodson, G. Insulin at pH 2: Structural analysis of the conditions promoting insulin fibre formation. J. Mol. Biol. 2002, 318, 479–490. [Google Scholar] [CrossRef]
- Kaszuba, M.; McKnight, D.; Connah, M.T.; McNeil-Watson, F.K.; Nobbmann, U. Measuring sub nanometre sizes using dynamic light scattering. J. Nanopart. Res. 2008, 10, 823–829. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A.; Qi, J.; Gogoi, R.; Wong, J.; Mitragotri, S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J. Control Release 2016, 238, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Win, K.Y.; Feng, S.S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 2713–2722. [Google Scholar] [CrossRef]
- He, C.; Yin, L.; Tang, C.; Yin, C. Size-dependent absorption mechanism of polymeric nanoparticles for oral delivery of protein drugs. Biomaterials 2012, 33, 8569–8578. [Google Scholar] [CrossRef]
- Dorr, J.M.; Scheidelaar, S.; Koorengevel, M.C.; Dominguez, J.J.; Schafer, M.; van Walree, C.A.; Killian, J.A. The styrene-maleic acid copolymer: A versatile tool in membrane research. Eur. Biophys. J. 2016, 45, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.Y.; Xiong, X.Y.; Tian, Y.; Li, Z.L.; Gong, Y.C.; Li, Y.P. A review of biodegradable polymeric systems for oral insulin delivery. Drug Deliv. 2016, 23, 1882–1891. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Musyanovych, A.; Rocker, C.; Landfester, K.; Mailander, V.; Nienhaus, G.U. Specific effects of surface carboxyl groups on anionic polystyrene particles in their interactions with mesenchymal stem cells. Nanoscale 2011, 3, 2028–2035. [Google Scholar] [CrossRef]
- Kou, L.; Sun, J.; Zhai, Y.; He, Z. The endocytosis and intracellular fate of nanomedicines: Implication for rational design. Asian J. Pharma. Sci. 2013, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Abdulkarim, M.; Agulló, N.; Cattoz, B.; Griffiths, P.; Bernkop-Schnürch, A.; Borros, S.G.; Gumbleton, M. Nanoparticle diffusion within intestinal mucus: Three-dimensional response analysis dissecting the impact of particle surface charge, size and heterogeneity across polyelectrolyte, pegylated and viral particles. Eur. J. Pharm. Biopharm. 2015, 97, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Hellmig, S.; Von Schöning, F.; Gadow, C.; Katsoulis, S.; Hedderich, J.; Fölsch, U.R.; Stüber, E. Gastric emptying time of fluids and solids in healthy subjects determined by 13C breath tests: Influence of age, sex and body mass index. J. Gastroenterol. Hepatol. 2006, 21, 1832–1838. [Google Scholar] [CrossRef]
- Lane, M.E.; Corrigan, O.I. Paracellular and transcellular pathways facilitate insulin permeability in rat gut. J. Pharm. Pharmacol. 2006, 58, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Pan, H.; Zhang, C.; Zhao, L.; Zhao, R.; Zhu, Y.; Pan, W. Developments in methods for measuring the intestinal absorption of nanoparticle-bound drugs. Int. J. Mol. Sci. 2016, 17, 1171. [Google Scholar] [CrossRef]
- Jung, C.; Hugot, J.-P.; Barreau, F. Peyer’s patches: The immune sensors of the intestine. Int. J. Inflam. 2010, 2010, 823710. [Google Scholar] [CrossRef] [Green Version]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef]
- Pabst, O.; Mowat, A. Oral tolerance to food protein. Mucosal Immunol. 2012, 5, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Shantha, T.; Shantha, J.G. Inhalation Insulin and Oral and Nasal Insulin Sprays for Diabetics: Panacea or Evolving Future Health Disaster, Part I. Townsend Lett. J. 2008, 106–110. [Google Scholar]
- Lin, Y.-H.; Chen, C.-T.; Liang, H.-F.; Kulkarni, A.R.; Lee, P.-W.; Chen, C.-H.; Sung, H.-W. Novel nanoparticles for oral insulin delivery via the paracellular pathway. Nanotechnology 2007, 18, 105102. [Google Scholar] [CrossRef]
- Sun, S.; Liang, N.; Piao, H.; Yamamoto, H.; Kawashima, Y.; Cui, F. Insulin-SO (sodium oleate) complex-loaded PLGA nanoparticles: Formulation, characterization and in vivo evaluation. J. Microencapsul. 2010, 27, 471–478. [Google Scholar] [CrossRef]
- Cui, F.; Shi, K.; Zhang, L.; Tao, A.; Kawashima, Y. Biodegradable nanoparticles loaded with insulin–phospholipid complex for oral delivery: Preparation, in vitro characterization and in vivo evaluation. J. Control. Release 2006, 114, 242–250. [Google Scholar] [CrossRef]








| Group No. | Treatment/Dose | Administration Route |
|---|---|---|
| 1-Healthy mice | DW | Oral gavage |
| 2-Healthy mice | Free Insulin 15 mg/kg | Oral gavage |
| 3-Healthy mice | SMA-insulin 3.3 mg/kg | Oral gavage |
| 4-Healthy mice | SMA-insulin 6.6 mg/kg | Oral gavage |
| 5-Healthy mice | Free insulin 2 mg/kg | SC injections |
| 6-Healthy mice | SMA-insulin 2 mg/kg | SC injections |
| Group No. | Treatment/Dose | Administration Route |
|---|---|---|
| 1-Diabetic mice | DW | Oral gavage |
| 2-Diabetic mice | SMA-insulin 36 mg/kg | Oral gavage |
| 3-Diabetic mice | SMA-insulin 72 mg/kg | Oral gavage |
| Recovery | Loading (wt/wt) | Size (nm) | PDI | Zeta Potential (mV) | |
|---|---|---|---|---|---|
| SMA-insulin | 78% | 18% | 179.7 ± 38.9 | 0.27 | −0.979 ± 0.0346 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahman, F.; Taurin, S.; Altayeb, D.; Taha, S.; Bakhiet, M.; Greish, K. Oral Insulin Delivery Using Poly (Styrene Co-Maleic Acid) Micelles in a Diabetic Mouse Model. Pharmaceutics 2020, 12, 1026. https://doi.org/10.3390/pharmaceutics12111026
Bahman F, Taurin S, Altayeb D, Taha S, Bakhiet M, Greish K. Oral Insulin Delivery Using Poly (Styrene Co-Maleic Acid) Micelles in a Diabetic Mouse Model. Pharmaceutics. 2020; 12(11):1026. https://doi.org/10.3390/pharmaceutics12111026
Chicago/Turabian StyleBahman, Fatemah, Sebastien Taurin, Diab Altayeb, Safa Taha, Moiz Bakhiet, and Khaled Greish. 2020. "Oral Insulin Delivery Using Poly (Styrene Co-Maleic Acid) Micelles in a Diabetic Mouse Model" Pharmaceutics 12, no. 11: 1026. https://doi.org/10.3390/pharmaceutics12111026
APA StyleBahman, F., Taurin, S., Altayeb, D., Taha, S., Bakhiet, M., & Greish, K. (2020). Oral Insulin Delivery Using Poly (Styrene Co-Maleic Acid) Micelles in a Diabetic Mouse Model. Pharmaceutics, 12(11), 1026. https://doi.org/10.3390/pharmaceutics12111026