Towards a Quantitative Mechanistic Understanding of Localized Pulmonary Tissue Retention—A Combined In Vivo/In Silico Approach Based on Four Model Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Study Design
2.3. Animal Studies
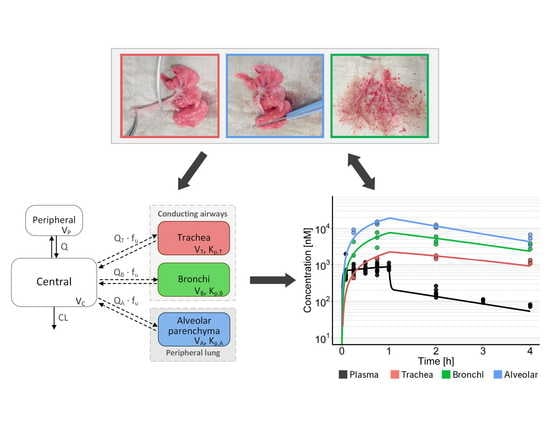
2.4. Tissue Preparation
2.5. Bioanalysis
2.6. Modelling and Simulation
3. Results
3.1. In Vivo PK Studies
3.2. Model-Based PK Analysis
3.3. Comparison with Total Lung Concentrations
3.4. Pulmonary Absorption Half-Lives
3.5. Time-Dependency of Tissue-to-Plasma Ratios
3.6. Effect of Distributional Delay on Plasma EC50,Free Estimates of SAL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kelly, H.W. Establishing a therapeutic index for the inhaled corticosteroids: Part i: Pharmacokinetic/pharmacodynamic comparison of the inhaled corticosteroids. J. Allergy Clin. Immunol. 1998, 102, S36–S51. [Google Scholar] [CrossRef]
- Lombardi, D.; Cuenoud, B.; Krämer, S.D. Lipid membrane interactions of indacaterol and salmeterol: Do they influence their pharmacological properties? Eur. J. Pharm. Sci. 2009, 38, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Begg, M.; Edwards, C.D.; Hamblin, J.N.; Pefani, E.; Wilson, R.; Gilbert, J.; Vitulli, G.; Mallett, D.; Morrell, J.; Hingle, M.I. Translation of inhaled drug optimization strategies into clinical pharmacokinetics and pharmacodynamics using gsk2292767a, a novel inhaled phosphoinositide 3-kinase δ inhibitor. J. Pharmacol. Exp. Ther. 2019, 369, 443–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghardt, J.M.; Kloft, C.; Sharma, A. Inhaled therapy in respiratory disease: The complex interplay of pulmonary kinetic processes. Can. Respir. J. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Mutlu, G.M.; Factor, P. Alveolar epithelial β2-adrenergic receptors. Am. J. Respir. Cell Mol. Biol. 2008, 38, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Backstrom, E.; Hamm, G.; Nilsson, A.; Fihn, B.M.; Strittmatter, N.; Andren, P.; Goodwin, R.J.A.; Friden, M. Uncovering the regional localization of inhaled salmeterol retention in the lung. Drug Deliv. 2018, 25, 838–845. [Google Scholar] [CrossRef]
- Hamm, G.R.; Bäckström, E.; Brülls, M.; Nilsson, A.; Strittmatter, N.; Andrén, P.E.; Grime, K.; Fridén, M.; Goodwin, R.J. Revealing the regional localization and differential lung retention of inhaled compounds by mass spectrometry imaging. J. Aerosol Med. Pulm. Drug Deliv. 2020, 33, 43–53. [Google Scholar] [CrossRef]
- Hendrickx, R.; Bergström, E.L.; Janzén, D.L.I.; Fridén, M.; Eriksson, U.; Grime, K.; Ferguson, D. Translational model to predict pulmonary pharmacokinetics and efficacy in man for inhaled bronchodilators. CPT Pharmacomet. Syst. Pharmacol. 2018, 7, 147–157. [Google Scholar] [CrossRef]
- Weber, B.; Hochhaus, G. A pharmacokinetic simulation tool for inhaled corticosteroids. AAPS J. 2013, 15, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, K.; Broeker, A.; Nowak, H.; Rahmel, T.; Nussbaumer-Pröll, A.; Österreicher, Z.; Zeitlinger, M.; Wicha, S. A pharmacometric approach to define target site-specific breakpoints for bacterial killing and resistance suppression integrating microdialysis, time-kill curves and heteroresistance data: A case study with moxifloxacin. Clin. Microbiol. Infect. 2020. [Google Scholar] [CrossRef]
- Boger, E.; Evans, N.; Chappell, M.; Lundqvist, A.; Ewing, P.; Wigenborg, A.; Fridén, M. Systems pharmacology approach for prediction of pulmonary and systemic pharmacokinetics and receptor occupancy of inhaled drugs. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.P.; Delp, M.D.; Lindstedt, S.L.; Rhomberg, L.R.; Beliles, R.P. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol. Ind. Health 1997, 13, 407–484. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.J.; Zhou, X.; Breen, P.; Gann, L.; Logsdon, T.W.; Compadre, C.M.; Hiller, F.C. Pharmacokinetics of (r, s)-albuterol after aerosol inhalation in healthy adult volunteers. J. Pharm. Sci. 1998, 87, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Minto, C.; Li, B.; Tattam, B.; Brown, K.; Seale, J.P.; Donnelly, R. Pharmacokinetics of epimeric budesonide and fluticasone propionate after repeat dose inhalation–intersubject variability in systemic absorption from the lung. Br. J. Clin. Pharmacol. 2000, 50, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Rohatagi, S.; Arya, V.; Zech, K.; Nave, R.; Hochhaus, G.; Jensen, B.; Barrett, J. Population pharmacokinetics and pharmacodynamics of ciclesonide. J. Clin. Pharmacol. 2003, 43, 365–378. [Google Scholar] [CrossRef]
- Ting, L.; Aksenov, S.; Bhansali, S.; Ramakrishna, R.; Tang, P.; Geller, D. Population pharmacokinetics of inhaled tobramycin powder in cystic fibrosis patients. CPT Pharmacomet. Syst. Pharmacol. 2014, 3, 1–7. [Google Scholar] [CrossRef]
- Wu, K.; Goyal, N.; Stark, J.G.; Hochhaus, G. Evaluation of the administration time effect on the cumulative cortisol suppression and cumulative lymphocytes suppression for once-daily inhaled corticosteroids: A population modeling/simulation approach. J. Clin. Pharmacol. 2008, 48, 1069–1080. [Google Scholar] [CrossRef]
- Rodgers, T.; Leahy, D.; Rowland, M. Physiologically based pharmacokinetic modeling 1: Predicting the tissue distribution of moderate-to-strong bases. J. Pharm. Sci. 2005, 94, 1259–1276. [Google Scholar] [CrossRef]
- Johnson, M.; Butchers, P.; Coleman, R.; Nials, A.; Strong, P.; Summer, M.; Vardey, C.; Whelan, C. The pharmacology of salmeterol. Life Sci. 1993, 52, 2131–2143. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing, 3.3.2; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Bhagwat, S.; Schilling, U.; Chen, M.J.; Wei, X.; Delvadia, R.; Absar, M.; Saluja, B.; Hochhaus, G. Predicting pulmonary pharmacokinetics from in vitro properties of dry powder inhalers. Pharm. Res. 2017, 34, 2541–2556. [Google Scholar] [CrossRef]
- Soulele, K.; Macheras, P.; Karalis, V. Pharmacokinetic analysis of inhaled salmeterol in asthma patients: Evidence from two dry powder inhalers. Biopharm. Drug Dispos. 2017, 38, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Slatter, J.G.; Adams, L.A.; Bush, E.C.; Chiba, K.; Daley-Yates, P.T.; Feenstra, K.L.; Koike, S.; Ozawa, N.; Peng, G.W.; Sams, J.P.; et al. Pharmacokinetics, toxicokinetics, distribution, metabolism and excretion of linezolid in mouse, rat and dog. Xenobiotica 2002, 32, 907–924. [Google Scholar] [CrossRef] [PubMed]
- Beringer, P.; Nguyen, M.; Hoem, N.; Louie, S.; Gill, M.; Gurevitch, M.; Wong-Beringer, A. Absolute bioavailability and pharmacokinetics of linezolid in hospitalized patients given enteral feedings. Antimicrob. Agents Chemother. 2005, 49, 3676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhalodi, A.A.; Papasavas, P.K.; Tishler, D.S.; Nicolau, D.P.; Kuti, J.L. Pharmacokinetics of intravenous linezolid in moderately to morbidly obese adults. Antimicrob. Agents Chemother. 2013, 57, 1144. [Google Scholar] [CrossRef] [Green Version]
- Plock, N.; Buerger, C.; Joukhadar, C.; Kljucar, S.; Kloft, C. Does linezolid inhibit its own metabolism?—population pharmacokinetics as a tool to explain the observed nonlinearity in both healthy volunteers and septic patients. Drug Metab. Dispos. 2007, 35, 1816–1823. [Google Scholar] [CrossRef] [Green Version]
- Pilari, S.; Huisinga, W. Lumping of physiologically-based pharmacokinetic models and a mechanistic derivation of classical compartmental models. J. Pharmacokinet. Pharmacodyn. 2010, 37, 365–405. [Google Scholar] [CrossRef]
- Kouzuki, H.; Suzuki, H.; Sugiyama, Y. Pharmacokinetic study of the hepatobiliary transport of indomethacin. Pharm. Res. 2000, 17, 432–438. [Google Scholar] [CrossRef]
- Rodgers, T.; Leahy, D.; Rowland, M. Tissue distribution of basic drugs: Accounting for enantiomeric, compound and regional differences amongst β-blocking drugs in rat. J. Pharm. Sci. 2005, 94, 1237–1248. [Google Scholar] [CrossRef]
- Bäckström, E.; Boger, E.; Lundqvist, A.; Hammarlund-Udenaes, M.; Fridén, M. Lung retention by lysosomal trapping of inhaled drugs can be predicted in vitro with lung slices. J. Pharm. Sci. 2016, 105, 3432–3439. [Google Scholar] [CrossRef]
- Kazmi, F.; Hensley, T.; Pope, C.; Funk, R.S.; Loewen, G.J.; Buckley, D.B.; Parkinson, A. Lysosomal sequestration (trapping) of lipophilic amine (cationic amphiphilic) drugs in immortalized human hepatocytes (fa2n-4 cells). Drug Metab. Dispos. 2013, 41, 897–905. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, T.; Rowland, M. Physiologically based pharmacokinetic modelling 2: Predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J. Pharm. Sci. 2006, 95, 1238–1257. [Google Scholar] [CrossRef] [PubMed]
- Chiang, P.-C.; Hu, Y. Simultaneous determination of logd, logp, and pka of drugs by using a reverse phase hplc coupled with a 96-well plate auto injector. Comb. Chem. High Throughput Screen. 2009, 12, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.; Sunderland, B.; Luna, G.; Czarniak, P. Evaluation of the stability of linezolid in aqueous solution and commonly used intravenous fluids. Drug Des. Dev. Ther. 2017, 11, 2087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inagi, T.; Muramatsu, T.; Nagai, H.; Terada, H. Mechanism of indomethacin partition between n-octanol and water. Chem. Pharm. Bull. 1981, 29, 2330–2337. [Google Scholar] [CrossRef] [Green Version]
- Bäckström, E.; Lundqvist, A.; Boger, E.; Svanberg, P.; Ewing, P.; Hammarlund-Udenaes, M.; Fridén, M. Development of a novel lung slice methodology for profiling of inhaled compounds. J. Pharm. Sci. 2016, 105, 838–845. [Google Scholar] [CrossRef] [Green Version]
- Coleman, T.G. Cardiac output by dye dilution in the conscious rat. J. Appl. Physiol. 1974, 37, 452–455. [Google Scholar] [CrossRef]
- Delp, M.; Manning, R.; Bruckner, J.; Armstrong, R. Distribution of cardiac output during diurnal changes of activity in rats. Am. J. Physiol.-Heart Circ. Physiol. 1991, 261, H1487–H1493. [Google Scholar] [CrossRef]
- Hachamovitch, R.; Wicker, P.; Capasso, J.M.; Anversa, P. Alterations of coronary blood flow and reserve with aging in fischer 344 rats. Am. J. Physiol.-Heart Circ. Physiol. 1989, 256, H66–H73. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Ferrone, R.A.; Walsh, G.M.; Frohlich, E.D. Regional blood flows measured in conscious rats by combined fick and microsphere methods. Am. J. Physiol.-Heart Circ. Physiol. 1978, 235, H357–H360. [Google Scholar] [CrossRef]
- Walsh, G.M.; Tsuchiya, M.; Frohlich, E.D. Direct fick application for measurement of cardiac output in rat. J. Appl. Physiol. 1976, 40, 849–853. [Google Scholar] [CrossRef]
- Boger, E.; Fridén, M. Physiologically based pharmacokinetic/pharmacodynamic modeling accurately predicts the better bronchodilatory effect of inhaled versus oral salbutamol dosage forms. J. Aerosol. Med. Pulm. Drug Deliv. 2019, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.L.; Glenny, R.W.; Polissar, N.L.; Luchtel, D.L.; Lakshminarayan, S. Distribution of pulmonary and bronchial blood supply to airways measured by fluorescent microspheres. J. Appl. Physiol. 1996, 80, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Umland, J.P.; Chang, G.; Huang, Y.; Lin, Z.; Scott, D.O.; Troutman, M.D.; Liston, T.E. Species independence in brain tissue binding using brain homogenates. Drug Metab. Dispos. 2011, 39, 1270–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartels, C.; Looby, M.; Sechaud, R.; Kaiser, G. Determination of the pharmacokinetics of glycopyrronium in the lung using a population pharmacokinetic modelling approach. Br. J. Clin. Pharmacol. 2013, 76, 868–879. [Google Scholar] [CrossRef] [Green Version]
- Borghardt, J.M.; Weber, B.; Staab, A.; Kunz, C.; Formella, S.; Kloft, C. Investigating pulmonary and systemic pharmacokinetics of inhaled olodaterol in healthy volunteers using a population pharmacokinetic approach. Br. J. Clin. Pharmacol. 2016, 81, 538–552. [Google Scholar] [CrossRef] [Green Version]
- Melin, J.; Prothon, S.; Kloft, C.; Cleton, A.; Amilon, C.; Jorup, C.; Bäckman, P.; Olsson, B.; Hamrén, U.W. Pharmacokinetics of the inhaled selective glucocorticoid receptor modulator azd5423 following inhalation using different devices. AAPS J. 2017, 19, 865–874. [Google Scholar] [CrossRef]
- Soulele, K.; Macheras, P.; Silvestro, L.; Savu, S.R.; Karalis, V. Population pharmacokinetics of fluticasone propionate/salmeterol using two different dry powder inhalers. Eur. J. Pharm. Sci. 2015, 80, 33–42. [Google Scholar] [CrossRef]
- Krishnaswami, S.; Hochhaus, G.; Möllmann, H.; Barth, J.; Derendorf, H. Interpretation of absorption rate data for inhaled fluticasone propionate obtained in compartmental pharmacokinetic modeling. Int. J. Clin. Pharmacol. Ther. 2005, 43, 117–122. [Google Scholar] [CrossRef]
- Rohrschneider, M.; Bhagwat, S.; Krampe, R.; Michler, V.; Breitkreutz, J.r.; Hochhaus, G.n. Evaluation of the transwell system for characterization of dissolution behavior of inhalation drugs: Effects of membrane and surfactant. Mol. Pharm. 2015, 12, 2618–2624. [Google Scholar] [CrossRef]
- Bosquillon, C. Drug transporters in the lung--do they play a role in the biopharmaceutics of inhaled drugs? J. Pharm. Sci. 2010, 99, 2240–2255. [Google Scholar] [CrossRef]
- Sakamoto, A.; Matsumaru, T.; Yamamura, N.; Uchida, Y.; Tachikawa, M.; Ohtsuki, S.; Terasaki, T. Quantitative expression of human drug transporter proteins in lung tissues: Analysis of regional, gender, and interindividual differences by liquid chromatography–tandem mass spectrometry. J. Pharm. Sci. 2013, 102, 3395–3406. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.; Mendes, E.S.; Schmid, N.; Schmid, A.; Conner, G.E.; Salathe, M.; Wanner, A. The effect of corticosteroids on the disposal of long-acting β2-agonists by airway smooth muscle cells. J. Allergy Clin. Immunol. 2007, 120, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Hallifax, D.; Houston, J.B. Saturable uptake of lipophilic amine drugs into isolated hepatocytes: Mechanisms and consequences for quantitative clearance prediction. Drug Metab. Dispos. 2007, 35, 1325–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oeff, K.; König, A. Das blutvolumen einiger rattenorgane und ihre restblutmenge nach entbluten bzw. Durchspülung. Bestimmung mit p32-markierten erythrocyten. Naunyn-Schmiedebergs Archiv für Experimentelle Pathologie und Pharmakologie 1955, 226, 98–102. [Google Scholar] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Koivusalo, M.; Haimi, P.; Heikinheimo, L.; Kostiainen, R.; Somerharju, P. Quantitative determination of phospholipid compositions by esi-ms: Effects of acyl chain length, unsaturation, and lipid concentration on instrument response. J. Lipid Res. 2001, 42, 663–672. [Google Scholar]
- Pulfer, M.; Murphy, R.C. Electrospray mass spectrometry of phospholipids. Mass. Spectrom. Rev. 2003, 22, 332–364. [Google Scholar] [CrossRef]






| Parameter | Unit | Salmeterol | Fluticasone Propionate | Linezolid | Indomethacin |
|---|---|---|---|---|---|
| CL | L·h−1·kg−1 | 3.86 (6.07) | 3.37 (3.42) | 0.279 (2.22) | 0.0691 (11.0) |
| VC | L·kg−1 | 0.123 (16.2) | 0.223 (56.0) | 0.320 (19.7) | 0.154 (5.27) |
| Q | L·h−1·kg−1 | 3.24 (21.1) | 4.72 (12.8) | 2.79 (34.9) | - |
| VP | L·kg−1 | 3.77 (14.6) | 2.41 (8.18) | 0.628 (9.96) | - |
| Kp,T | - | 6.52 (7.04) | 5.21 (16.9) | 0.404 (11.2) | 0.356 (20.2) |
| Kp,B | - | 18.6 (12.2) | 6.64 (13.2) | 0.534 (6.52) | 0.249 (16.0) |
| Kp,A | - | 39.3 (8.10) | 5.84 (10.6) | 0.785 (5.11) | 0.384 (35.5) |
| QT 1 | L·h−1·kg−1 | 0.054 (14.3) | |||
| QB 1 | L·h−1·kg−1 | 0.777 (21.5) | |||
| QA 1 | L·h−1·kg−1 | 10.6 (10.7) | |||
| Drug | Tissue | t½ (Rat) | t½ (Human) 2 |
|---|---|---|---|
| Salmeterol | Trachea | 1.2 h | 4.75 h |
| Bronchi | 57 min | 3.77 h | |
| Alveolar | 45 min | 2.91 h | |
| Full lung | 48.3 min | 3.20 h | |
| Fluticasone propionate | Trachea | 52.4 min | 3.48 h |
| Bronchi | 18.6 min | 1.23 h | |
| Alveolar | 5.99 min | 23.8 min | |
| Full lung | 7.28 min | 28.9 min | |
| Linezolid | Trachea | 0.5 s | 2.0 s |
| Bronchi | 0.2 s | 0.8 s | |
| Alveolar | 0.1 s | 0.4 s | |
| Full lung | 0.1 s | 0.5 s | |
| Indomethacin | Trachea | 14 s | 55 s |
| Bronchi | 2.7 s | 11 s | |
| Alveolar | 1.5 s | 6.0 s | |
| Full lung | 1.6 s | 6.2 s |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Himstedt, A.; Braun, C.; Wicha, S.G.; Borghardt, J.M. Towards a Quantitative Mechanistic Understanding of Localized Pulmonary Tissue Retention—A Combined In Vivo/In Silico Approach Based on Four Model Drugs. Pharmaceutics 2020, 12, 408. https://doi.org/10.3390/pharmaceutics12050408
Himstedt A, Braun C, Wicha SG, Borghardt JM. Towards a Quantitative Mechanistic Understanding of Localized Pulmonary Tissue Retention—A Combined In Vivo/In Silico Approach Based on Four Model Drugs. Pharmaceutics. 2020; 12(5):408. https://doi.org/10.3390/pharmaceutics12050408
Chicago/Turabian StyleHimstedt, Anneke, Clemens Braun, Sebastian Georg Wicha, and Jens Markus Borghardt. 2020. "Towards a Quantitative Mechanistic Understanding of Localized Pulmonary Tissue Retention—A Combined In Vivo/In Silico Approach Based on Four Model Drugs" Pharmaceutics 12, no. 5: 408. https://doi.org/10.3390/pharmaceutics12050408
APA StyleHimstedt, A., Braun, C., Wicha, S. G., & Borghardt, J. M. (2020). Towards a Quantitative Mechanistic Understanding of Localized Pulmonary Tissue Retention—A Combined In Vivo/In Silico Approach Based on Four Model Drugs. Pharmaceutics, 12(5), 408. https://doi.org/10.3390/pharmaceutics12050408