Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix
Abstract
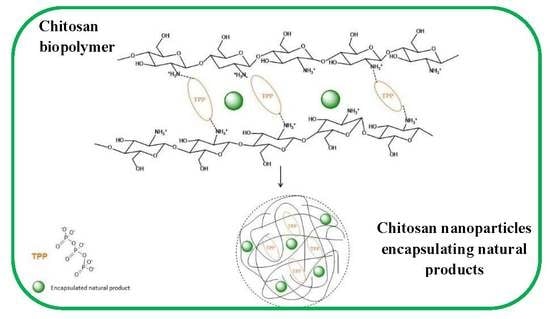
:1. Introduction
2. Chitosan as a Matrix for NanosystemsPreparation: Methods, Physicochemical Aspects, Modification Potential and Bioactivity
3. Chitosan as a Matrix for the Encapsulation of Pure Phytochemicals
4. Chitosan as a Matrix for the Encapsulation of Plant Extracts
5. Chitosan as a Matrix for the Encapsulation of EOs
6. Chitosan-Coated and Modified Chitosan Nanosystems Encapsulating Natural Products
7. Overview and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Singh, V.K.; Dwivedy, A.K.; Chaudhari, A.K.; Upadhyay, N.; Singh, P.; Sharma, S.; Dubey, N.K. Encapsulation in chitosan-based nanomatrix as an efficient green technology to boost the antimicrobial, antioxidant and in situ efficacy of Coriandrum sativum essential oil. Int. J. Biol. Macromol. 2019, 133, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Shetta, A.; Kegere, J.; Mamdouh, W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: Encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int. J. Biol. Macromol. 2019, 126, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Feyzioglu, G.C.; Tornuk, F. Development of chitosan nanoparticles loaded with summer savory (Saturejahortensis L.) essential oil for antimicrobial and antioxidant delivery applications. LWT 2016, 70, 104–110. [Google Scholar] [CrossRef]
- Ntohogian, S.; Gavriliadou, V.; Christodoulou, E.; Nanaki, S.; Lykidou, S.; Naidis, P.; Mischopoulou, L.; Barmpalexis, P.; Nikolaidis, N.; Bikiaris, D.N. Chitosan nanoparticles with encapsulated natural and uf-purified annatto and saffron for the preparation of uv protective cosmetic emulsions. Molecules 2018, 23, 2107. [Google Scholar] [CrossRef] [Green Version]
- Hussein, A.M.; Kamil, M.M.; Lotfy, S.N.; Mahmoud, K.F.; Mehaya, F.M.; Mohammad, A.A. Influence of nano-encapsulation on chemical composition, antioxidant activity and thermal stability of rosemary essential oil. Am. J. Food Technol. 2017, 12, 170–177. [Google Scholar] [CrossRef]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef] [Green Version]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; a Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [Green Version]
- Casanova, F.; Santos, L. Encapsulation of cosmetic active ingredients for topical application–A review. J. Microencapsul. 2016, 33, 1–17. [Google Scholar] [CrossRef]
- Jafari, S.M. An overview of nanoencapsulation techniques and their classification. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Academic Press: Cambridge, MA, USA, 2017; pp. 1–34. [Google Scholar] [CrossRef]
- Suganya, V.; Anuradha, V. Microencapsulation and Nanoencapsulation: A Review. Int. J. Pharm. Clin. Res. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Jyothi, N.V.; Prasanna, P.M.; Sakarkar, S.N.; Prabha, K.S.; Ramaiah, P.S.; Srawan, G.Y. Microencapsulation techniques, factors influencing encapsulation efficiency. J. Microencapsul. 2010, 27, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, B.F.; Kermasha, S.; Inteaz, A.; Catherine, N.; Mulligan, B. Encapsulation in the food industry: A review. Int. J. Food Sci. Nutr. 1999, 50, 213–224. [Google Scholar] [PubMed]
- Assadpour, E.; Jafari, S.M. Advances in Spray-Drying Encapsulation of Food Bioactive Ingredients: From Microcapsules to Nanocapsules. Annu. Rev. Food Sci. Technol. 2019, 10, 103–131. [Google Scholar] [CrossRef] [PubMed]
- Lohith Kumar, D.H.; Sarkar, P. Encapsulation of bioactive compounds using nanoemulsions. Environ. Chem. Lett. 2018, 16, 59–70. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Bandur, G.N.; David, I.; Hădărugă, D.I. A review on thermal analyses of cyclodextrins and cyclodextrin complexes. Environ. Chem. Lett. 2019, 17, 349–373. [Google Scholar] [CrossRef]
- Zarzycki, P.K.; Głód, B.K. Cyclodextrins-based nanocomplexes for encapsulation of bioactive compounds in food, cosmetics, and pharmaceutical products: Principles of supramolecular complexes formation, their influence on the antioxidative properties of target chemicals, and recent advances in selected industrial applications. In Encapsulations; Elsevier: Amsterdam, The Netherlands, 2016; pp. 717–767. [Google Scholar]
- Sengupta, P.K.; Bhattacharjee, S.; Chakraborty, S.; Bhowmik, S. Encapsulation of pharmaceutically active dietary polyphenols in cyclodextrin-based nanovehicles: Insights from spectroscopic studies. In Design of Nanostructures for Versatile Therapeutic Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 623–645. [Google Scholar]
- Guo, S. Encapsulation of curcumin into β-cyclodextrins inclusion: A review. In Proceedings of the E3S Web of Conferences, Guangzhou, China, 10–13 October 2019; p. 1100. [Google Scholar]
- Corina, D.; Bojin, F.; Ambrus, R.; Muntean, D.; Soica, C.; Paunescu, V.; Cristea, M.; Pinzaru, I.; Dehelean, C. Physico-chemical and biological evaluation of flavonols: Fisetin, quercetin and kaempferol alone and incorporated in beta cyclodextrins. Anti-Cancer Agents Med. Chem. 2017, 17, 615–626. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos Lima, B.; Shanmugam, S.; Quintans, J.D.S.S.; Quintans-Junior, L.J.; de Souza Araújo, A.A. Inclusion complex with cyclodextrins enhances the bioavailability of flavonoid compounds: A systematic review. Phytochem. Rev. 2019, 18, 1337–1359. [Google Scholar] [CrossRef]
- Asztemborska, M.; Ceborska, M.; Pietrzak, M. Complexation of tropane alkaloids by cyclodextrins. Carbohydr. Polym. 2019, 209, 74–81. [Google Scholar] [CrossRef]
- Ozdemir, N.; Pola, C.C.; Teixeira, B.N.; Hill, L.E.; Bayrak, A.; Gomes, C.L. Preparation of black pepper oleoresin inclusion complexes based on beta-cyclodextrin for antioxidant and antimicrobial delivery applications using kneading and freeze drying methods: A comparative study. LWT 2018, 91, 439–445. [Google Scholar] [CrossRef]
- Liu, K.; Liu, H.; Li, Z.; Li, W.; Li, L. In vitro dissolution study on inclusion complex of piperine with ethylenediamine-β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2020, 96, 1–11. [Google Scholar]
- Al-Nasiri, G.; Cran, M.J.; Smallridge, A.J.; Bigger, S.W. Optimisation of β-cyclodextrin inclusion complexes with natural antimicrobial agents: Thymol, carvacrol and linalool. J. Microencapsul. 2018, 35, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.S.; Carvalho, S.G.; Bertoli, L.D.; Villanova, J.C.O.; Pinheiro, P.F.; dos Santos, D.C.M.; Yoshida, M.I.; de Freitas, J.C.C.; Cipriano, D.F.; Bernardes, P.C. β-Cyclodextrin inclusion complexes with essential oils: Obtention, characterization, antimicrobial activity and potential application for food preservative sachets. Food Res. Int. 2019, 119, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Encapsulation in cyclodextrins to widen the applications of essential oils. Environ. Chem. Lett. 2019, 17, 129–143. [Google Scholar] [CrossRef]
- Shrestha, M.; Ho, T.M.; Bhandari, B.R. Encapsulation of tea tree oil by amorphous beta-cyclodextrin powder. Food Chem. 2017, 221, 1474–1483. [Google Scholar] [CrossRef]
- Kotronia, M.; Kavetsou, E.; Loupassaki, S.; Kikionis, S.; Vouyiouka, S.; Detsi, A. Encapsulation of Oregano (Origanumonites L.) essential oil in β-cyclodextrin (β-CD): Synthesis and characterization of the inclusion complexes. Bioengineering 2017, 4, 74. [Google Scholar] [CrossRef] [Green Version]
- Yuan, C.; Thomas, D.S.; Hook, J.M.; Qin, G.; Qi, K.; Zhao, J. Molecular Encapsulation of Eucalyptus staigeriana Essential Oil by Forming Inclusion Complexes with Hydroxypropyl-β-Cyclodextrin. Food Bioprocess. Technol. 2019, 12, 1264–1272. [Google Scholar] [CrossRef]
- Catchpole, O.; Mitchell, K.; Bloor, S.; Davis, P.; Suddes, A. Anti-gastrointestinal cancer activity of cyclodextrin-encapsulated propolis. J. Funct. Foods 2018, 41, 1–8. [Google Scholar] [CrossRef]
- Rimbach, G.; Fischer, A.; Schloesser, A.; Jerz, G.; Ikuta, N.; Ishida, Y.; Matsuzawa, R.; Matsugo, S.; Huebbe, P.; Terao, K. Anti-Inflammatory Properties of Brazilian Green Propolis Encapsulated in a γ-Cyclodextrin Complex in Mice Fed a Western-Type Diet. Int. J. Mol. Sci. 2017, 18, 1141. [Google Scholar] [CrossRef] [Green Version]
- Jaski, J.M.; Barão, C.E.; Liao, L.M.; Pinto, V.S.; Zanoelo, E.F.; Cardozo-Filho, L. β-Cyclodextrin complexation of extracts of olive leaves obtained by pressurized liquid extraction. Ind. Crops Prod. 2019, 129, 662–672. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.; Xu, Z.; Wu, M.; Xia, W.; Zhang, W. Chimonanthus praecox extract/cyclodextrin inclusion complexes: Selective inclusion, enhancement of antioxidant activity and thermal stability. Ind. Crops Prod. 2017, 95, 60–65. [Google Scholar] [CrossRef]
- Suvarna, V.; Gujar, P.; Murahari, M. Complexation of phytochemicals with cyclodextrin derivatives—An insight. Biomed. Pharmacother. 2017, 88, 1122–1144. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ye, F.; Dobretsov, S.; Dutta, J. Chitosan Nanocomposite Coatings for Food, Paints, and Water Treatment Applications. Appl. Sci. 2019, 9, 2409. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Kashdan, T.; Sterner, C.; Dombrowski, L.; Petrick, I.; Kröger, M.; Höfer, R. Chapter 2—Algal Biorefineries. In Industrial Biorefineries & White Biotechnology; Pandey, A., Höfer, R., Taherzadeh, M., Nampoothiri, K.M., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 35–90. [Google Scholar] [CrossRef]
- Lertsutthiwong, P.; Rojsitthisak, P.; Nimmannit, U. Preparation of turmeric oil-loaded chitosan-alginate biopolymeric nanocapsules. Mat. Sci. Eng. C 2009, 29, 856–860. [Google Scholar] [CrossRef]
- Ghayempour, S.; Montazer, M.; Mahmoudi Rad, M. Tragacanth gum as a natural polymeric wall for producing antimicrobial nanocapsules loaded with plant extract. Int. J. Biol. Macromol. 2015, 81, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.; Sarmah, M.; Khatun, B.; Maji, T. Encapsulation of active ingredients in polysaccharide-protein complex coacervates. Adv. Colloid Interface Sci. 2016, 239. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, F.M.; Carmona, O.G.; Carmona, C.G.; Lis, M.J.; de Moraes, F.F. Controlled release of microencapsulated citronella essential oil on cotton and polyester matrices. Cellulose 2016, 23, 1459–1470. [Google Scholar] [CrossRef]
- Taheri, A.; Jafari, S.M. Gum-based nanocarriers for the protection and delivery of food bioactive compounds. Adv. Colloid Interface Sci. 2019, 269, 277–295. [Google Scholar] [CrossRef]
- Tavares, L.; Barros, H.L.B.; Vaghetti, J.C.P.; Noreña, C.P.Z. Microencapsulation of Garlic Extract by Complex Coacervation Using Whey Protein Isolate/Chitosan and Gum Arabic/Chitosan as Wall Materials: Influence of Anionic Biopolymers on the Physicochemical and Structural Properties of Microparticles. Food Bioprocess. Technol. 2019, 12, 2093–2106. [Google Scholar] [CrossRef]
- Li, Y.; Liang, M.; Dou, X.; Feng, C.; Pang, J.; Cheng, X.; Liu, H.; Liu, T.; Wang, Y.; Chen, X. Development of alginate hydrogel/gum Arabic/gelatin based composite capsules and their application as oral delivery carriers for antioxidant. Int. J. Biol. Macromol. 2019, 132, 1090–1097. [Google Scholar] [CrossRef]
- Rajabi, H.; Jafari, S.M.; Rajabzadeh, G.; Sarfarazi, M.; Sedaghati, S. Chitosan-gum Arabic complex nanocarriers for encapsulation of saffron bioactive components. Colloids Surf. APhysicochem. Eng. Asp. 2019, 578, 123644. [Google Scholar] [CrossRef]
- Chakraborty, S. Carrageenan for encapsulation and immobilization of flavor, fragrance, probiotics, and enzymes: A review. J. Carbohydr. Chem. 2017, 36, 1–19. [Google Scholar] [CrossRef]
- Belscak-Cvitanovic, A.; Komes, D.; Karlovic, S.; Djakovic, S.; Spoljaric, I.; Mrsic, G.; Jezek, D. Improving the controlled delivery formulations of caffeine in alginate hydrogel beads combined with pectin, carrageenan, chitosan and psyllium. Food Chem. 2015, 167, 378–386. [Google Scholar] [CrossRef]
- Dima, C.; Cotârlet, M.; Alexe, P.; Dima, S. Microencapsulation of essential oil of pimento [Pimentadioica (L) Merr.] by chitosan/k-carrageenan complex coacervation method. Innov. Food Sci. Emerg. Technol. 2014, 22, 203–211. [Google Scholar] [CrossRef]
- Mehyar, G.F.; Al-Isamil, K.M.; Al-Ghizzawi, H.A.M.; Holley, R.A. Stability of Cardamom (Elettaria Cardamomum) Essential Oil in Microcapsules Made of Whey Protein Isolate, Guar Gum, and Carrageenan. J. Food Sci. 2014, 79, C1939–C1949. [Google Scholar] [CrossRef]
- Gómez-Aldapa, C.A.; Castro-Rosas, J.; Rangel-Vargas, E.; Navarro-Cortez, R.O.; Cabrera-Canales, Z.E.; Díaz-Batalla, L.; Martínez-Bustos, F.; Guzmán-Ortiz, F.A.; Falfan-Cortes, R.N. A modified Achira (Canna indica L.) starch as a wall material for the encapsulation of Hibiscus sabdariffa extract using spray drying. Food Res. Int. 2019, 119, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Das, A.B.; Goud, V.; Das, C. Microencapsulation of anthocyanin extract from purple rice bran using modified rice starch and its effect on rice dough rheology. Int. J. Biol. Macromol. 2019, 124, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Rehan, M.; Ahmed-Farid, O.A.; Ibrahim, S.R.; Hassan, A.A.; Abdelrazek, A.M.; Khafaga, N.I.; Khattab, T.A. Green and sustainable encapsulation of Guava leaf extracts (Psidium guajava L.) into alginate/starch microcapsules for multifunctional finish over cotton gauze. ACS Sustain. Chem. Eng. 2019, 7, 18612–18623. [Google Scholar] [CrossRef]
- Mehran, M.; Masoum, S.; Memarzadeh, M. Improvement of thermal stability and antioxidant activity of anthocyanins of Echium amoenum petal using maltodextrin/modified starch combination as wall material. Int. J. Biol. Macromol. 2020, 148, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Urzúa, C.; González, E.; Dueik, V.; Bouchon, P.; Giménez, B.; Robert, P. Olive leaves extract encapsulated by spray-drying in vacuum fried starch–gluten doughs. Food Bioprod. Process. 2017, 106, 171–180. [Google Scholar] [CrossRef]
- Akbari-Alavijeh, S.; Shaddel, R.; Jafari, S.M. Encapsulation of food bioactives and nutraceuticals by various chitosan-based nanocarriers. Food Hydrocoll. 2020, 105, 105774. [Google Scholar] [CrossRef]
- Desai, K.G. Chitosan Nanoparticles Prepared by Ionotropic Gelation: An Overview of Recent Advances. Crit. Rev. Ther. Drug Carr. Syst. 2016, 33, 107–158. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Tran, T.; Zhang, J.; Kong, L. Manufacturing Techniques and Surface Engineering of Polymer Based Nanoparticles for Targeted Drug Delivery to Cancer. Nanomaterials 2016, 6, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, K.G.H.; Park, H.J. Preparation and characterization of drug-loaded chitosan–tripolyphosphate microspheres by spray drying. Drug Dev. Res. 2005, 64, 114–128. [Google Scholar] [CrossRef]
- Fogaça de Oliveira, B.; Santana, M.H.; Ré, M. Spray-dried chitosan microspheres cross-linked with D, L-glyceraldehyde as a potential drug delivery system: Preparation and characterization. Braz. J. Chem. Eng. 2005, 22, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Gzyra-Jagieła, K.; Pęczek, B.; Wiśniewska-Wrona, M.; Gutowska, N. Physicochemical Properties of Chitosan and its Degradation Products. Chitin Chitosan Prop. Appl. 2019, 61–80. [Google Scholar] [CrossRef]
- Kumari, S.; Annamareddy, S.H.K.; Abanti, S.; Rath, P.K. Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells. Int. J. Biol. Macromol. 2017, 104, 1697–1705. [Google Scholar] [CrossRef]
- Oh, D.-W.; Kang, J.-H.; Lee, H.-J.; Han, S.-D.; Kang, M.-H.; Kwon, Y.-H.; Jun, J.-H.; Kim, D.-W.; Rhee, Y.-S.; Kim, J.-Y. Formulation and in vitro/in vivo evaluation of chitosan-based film forming gel containing ketoprofen. Drug Deliv. 2017, 24, 1056–1066. [Google Scholar] [CrossRef] [Green Version]
- Frank, L.; Onzi, G.; Morawski, A.; Pohlmann, A.; Guterres, S.; Contri, R. Chitosan as a coating material for nanoparticles intended for biomedical applications. React. Funct. Polym. 2019, 147, 104459. [Google Scholar] [CrossRef]
- De Souza Soares, L.; Perim, R.B.; de Alvarenga, E.S.; de Moura Guimarães, L.; de Carvalho Teixeira, A.V.N.; dos Reis Coimbra, J.S.; de Oliveira, E.B. Insights on physicochemical aspects of chitosan dispersion in aqueous solutions of acetic, glycolic, propionic or lactic acid. Int. J. Biol. Macromol. 2019, 128, 140–148. [Google Scholar] [CrossRef]
- Hong, S.; Choi, H.; Jo, S.; Kim, M.J.; Lee, S.; Ahn, S.; Lee, J. Modification of chitosan using hydrogen peroxide and ascorbic acid and its physicochemical properties including water solubility, oil entrapment and in vitro lipase activity. Int. J. Food Sci. Technol. 2019, 54, 2300–2308. [Google Scholar] [CrossRef]
- Panda, P.K.; Yang, J.-M.; Chang, Y.-H.; Su, W.-W. Modification of different molecular weights of chitosan by p-Coumaric acid: Preparation, characterization and effect of molecular weight on its water solubility and antioxidant property. Int. J. Biol. Macromol. 2019, 136, 661–667. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, H.; Huang, J.; Zhao, J.; Yan, B.; Ma, S.; Zhang, H.; Fan, D. The physicochemical properties of chitosan prepared by microwave heating. Food Sci. Nutr. 2020, 8, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the Development of Chitosan-Based Biomaterials for Tissue Engineering and Regenerative Medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Zhang, M.; Chen, S.; Wang, X.; Tian, Z.; Chen, Y.; Xu, P.; Zhang, L.; Zhang, L.; Zhang, L. Peptide-modified chitosan hydrogels accelerate skin wound healing by promoting fibroblast proliferation, migration, and secretion. Cell Transpl. 2017, 26, 1331–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasile, C. Polymeric Nanocomposites and Nanocoatings for Food Packaging: A Review. Materials 2018, 11, 1834. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, M.A.; Syeda, J.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Xu, X.; Feng, J.; Liu, M.; Hu, K. Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int. J. Pharm. 2019, 560, 282–293. [Google Scholar] [CrossRef]
- Pontillo, A.R.N.; Detsi, A. Nanoparticles for ocular drug delivery: Modified and non-modified chitosan as a promising biocompatible carrier. Nanomedicine 2019, 14, 1889–1909. [Google Scholar] [CrossRef]
- Zheng, L.-Y.; Zhu, J.-F. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Divya, K.; Vijayan, S.; George, T.K.; Jisha, M. Antimicrobial properties of chitosan nanoparticles: Mode of action and factors affecting activity. Fibers Polym. 2017, 18, 221–230. [Google Scholar] [CrossRef]
- Yen, M.-T.; Yang, J.-H.; Mau, J.-L. Antioxidant properties of chitosan from crab shells. Carbohydr. Polym. 2008, 74, 840–844. [Google Scholar] [CrossRef]
- Krausz, A.E.; Adler, B.L.; Cabral, V.; Navati, M.; Doerner, J.; Charafeddine, R.A.; Chandra, D.; Liang, H.; Gunther, L.; Clendaniel, A. Curcumin-encapsulated nanoparticles as innovative antimicrobial and wound healing agent. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 195–206. [Google Scholar] [CrossRef] [Green Version]
- Kavaz, D.; Idris, M.; Onyebuchi, C. Physiochemical characterization, antioxidative, anticancer cells proliferation and food pathogens antibacterial activity of chitosan nanoparticles loaded with Cyperus articulatus rhizome essential oils. Int. J. Biol. Macromol. 2019, 123, 837–845. [Google Scholar] [CrossRef]
- Taher, F.; Ibrahim, S.A.; El-Aziz, A.A.; El-Nour, M.F.A.; El-Sheikh, M.A.; El-Husseiny, N.; Mohamed, M.M. Anti-proliferative effect of chitosan nanoparticles (extracted from crayfish Procambarusclarkii, Crustacea: Cambaridae) against MDA-MB-231 and SK-BR-3 human breast cancer cell lines. Int. J. Biol. Macromol. 2019, 126, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.D.; Patel, H.M.; Surana, S.J.; Vanjari, Y.H.; Belgamwar, V.S.; Pardeshi, C.V. N, N, N-Trimethyl chitosan: An advanced polymer with myriad of opportunities in nanomedicine. Carbohydr. Polym. 2017, 157, 875–902. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Han, Q.; Zhang, F.; Meng, X.; Liu, B. Preparation, characterization and antibacterial properties of 6-deoxy-6-arginine modified chitosan. Carbohydr. Polym. 2020, 230, 115635. [Google Scholar] [CrossRef]
- Sinani, G.; Sessevmez, M.; Gök, M.K.; Özgümüş, S.; Alpar, H.O.; Cevher, E. Modified chitosan-based nanoadjuvants enhance immunogenicity of protein antigens after mucosal vaccination. Int. J. Pharm. 2019, 569, 118592. [Google Scholar] [CrossRef]
- Garcia-Valdez, O.; Champagne-Hartley, R.; Saldivar-Guerra, E.; Champagne, P.; Cunningham, M. Modification of chitosan with polystyrene and poly (n-butyl acrylate) via nitroxide-mediated polymerization and grafting from approach in homogeneous media. Polym. Chem. 2015, 6, 2827–2836. [Google Scholar] [CrossRef]
- Ziegler-Borowska, M.; Chełminiak, D.; Kaczmarek, H. Thermal stability of magnetic nanoparticles coated by blends of modified chitosan and poly (quaternary ammonium) salt. J. Therm. Anal. Calorim. 2015, 119, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Saekhor, K.; Udomsinprasert, W.; Honsawek, S.; Tachaboonyakiat, W. Preparation of an injectable modified chitosan-based hydrogel approaching for bone tissue engineering. Int. J. Biol. Macromol. 2019, 123, 167–173. [Google Scholar] [CrossRef]
- Naskar, S.; Sharma, S.; Kuotsu, K. Chitosan-based nanoparticles: An overview of biomedical applications and its preparation. J. Drug Deliv. Sci. Technol. 2019, 49, 66–81. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical applications of chitosan and its derivative nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [Green Version]
- Kamel, N.A.; El-messieh, S.L.A.; Saleh, N.M. Chitosan/banana peel powder nanocomposites for wound dressing application: Preparation and characterization. Mat. Sci. Eng. C 2017, 72, 543–550. [Google Scholar] [CrossRef] [PubMed]
- El-Naby, F.S.A.; Naiel, M.A.; Al-Sagheer, A.A.; Negm, S.S. Dietary chitosan nanoparticles enhance the growth, production performance, and immunity in Oreochromis niloticus. Aquaculture 2019, 501, 82–89. [Google Scholar] [CrossRef]
- Li, R.; He, J.; Xie, H.; Wang, W.; Bose, S.K.; Sun, Y.; Hu, J.; Yin, H. Effects of chitosan nanoparticles on seed germination and seedling growth of wheat (Triticum aestivum L.). Int. J. Biol. Macromol. 2019, 126, 91–100. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Puligundla, P.; Gao, X.; Zhou, Y.; Wan, X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A review. Food Hydrocoll. 2017, 69, 286–292. [Google Scholar] [CrossRef]
- Othman, A.I.; El-Sherbiny, I.M.; ElMissiry, M.A.; Ali, D.A.; AbdElhakim, E. Polyphenon-E encapsulated into chitosan nanoparticles inhibited proliferation and growth of Ehrlich solid tumor in mice. Egypt. J. Basic Appl. Sci. 2018, 5, 110–120. [Google Scholar] [CrossRef]
- Almutairi, F.M.; El Rabey, H.A.; Tayel, A.A.; Alalawy, A.I.; Al-Duais, M.A.; Sakran, M.I.; Zidan, N.S. Augmented anticancer activity of curcumin loaded fungal chitosan nanoparticles. Int. J. Biol. Macromol. 2019, 155, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, F.; Karimi, E.; Oskoueian, E. Synthesis and Characterization of Chitosan-Encapsulated Genistein, its Antiproliferative and Antiangiogenic Activities. J. Microencapsul. 2020, 37, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Omwenga, E.; Hensel, A.; Shitandi, A.; Goycoolea, F. Chitosan nanoencapsulation of flavonoids enhances their quorum sensing and biofilm formation inhibitory activities against an E. coli Top 10 biosensor. Colloids Surf. B Biointerfaces 2018, 164, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Ilk, S.; Sağlam, N.; Özgen, M.; Korkusuz, F. Chitosan nanoparticles enhances the anti-quorum sensing activity of kaempferol. Int. J. Biol. Macromol. 2017, 94, 653–662. [Google Scholar] [CrossRef]
- Mekawey, A.A.; El-Metwally, M.M. Impact of nanoencapsulated natural bioactive phenolic metabolites on chitosan nanoparticles as aflatoxins inhibitor. J. Basic Microbiol. 2019, 59, 599–608. [Google Scholar] [CrossRef]
- Panwar, R.; Pemmaraju, S.C.; Sharma, A.K.; Pruthi, V. Efficacy of ferulic acid encapsulated chitosan nanoparticles against Candida albicans biofilm. Microbial Pathogen. 2016, 95, 21–31. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Rashed, M.M.; Lin, L. The antibacterial activity of clove oil/chitosan nanoparticles embedded gelatin nanofibers against Escherichia coli O157: H7 biofilms on cucumber. Int. J. Food Microbiol. 2018, 266, 69–78. [Google Scholar] [CrossRef]
- Hasheminejad, N.; Khodaiyan, F.; Safari, M. Improving the antifungal activity of clove essential oil encapsulated by chitosan nanoparticles. Food Chem. 2019, 275, 113–122. [Google Scholar] [CrossRef]
- Hasheminejad, N.; Khodaiyan, F. The effect of clove essential oil loaded chitosan nanoparticles on the shelf life and quality of pomegranate arils. Food Chem. 2020, 309, 125520. [Google Scholar] [CrossRef]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan nanoparticles loaded with clove essential oil: Characterization, antioxidant and antibacterial activities. Carbohydr. Polym. 2020, 236, 116075. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Iqbal, H.M. Development and characterization of essential oils incorporated chitosan-based cues with antibacterial and antifungal potentialities. J. Radiat. Res. Appl. Sci. 2020, 13, 174–179. [Google Scholar] [CrossRef] [Green Version]
- Barzegar, M.; Ghaderi Ghahfarokhi, M.; Sahari, M.; Azizi, M. Enhancement of thermal stability and antioxidant activity of thyme essential oil by encapsulation in chitosan nanoparticles. J. Agricult. Sci. Technol. 2016, 18, 1781–1792. [Google Scholar]
- Perdones, Á.; Chiralt, A.; Vargas, M. Properties of film-forming dispersions and films based on chitosan containing basil or thyme essential oil. Food Hydrocoll. 2016, 57, 271–279. [Google Scholar] [CrossRef]
- Sangsuwan, J.; Pongsapakworawat, T.; Bangmo, P.; Sutthasupa, S. Effect of chitosan beads incorporated with lavender or red thyme essential oils in inhibiting Botrytis cinerea and their application in strawberry packaging system. LWT 2016, 74, 14–20. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.; Correa-Pacheco, Z.; Bautista-Baños, S.; Gómez, Y.G. Release study and inhibitory activity of thyme essential oil-loaded chitosan nanoparticles and nanocapsules against foodborne bacteria. Int. J. Biol. Macromol. 2017, 103, 409–414. [Google Scholar] [CrossRef]
- Nouri, A. Chitosan nano-encapsulation improves the effects of mint, thyme, and cinnamon essential oils in broiler chickens. Br. Poult. Sci. 2019, 60, 530–538. [Google Scholar] [CrossRef]
- Ashrafi, B.; Rashidipour, M.; Marzban, A.; Soroush, S.; Azadpour, M.; Delfani, S.; Ramak, P. Mentha piperita essential oils loaded in a chitosan nanogel with inhibitory effect on biofilm formation against S. mutans on the dental surface. Carbohydr. Polym. 2019, 212, 142–149. [Google Scholar] [CrossRef]
- Jamil, B.; Abbasi, R.; Abbasi, S.; Imran, M.; Khan, S.U.; Ihsan, A.; Javed, S.; Bokhari, H. Encapsulation of cardamom essential oil in chitosan nano-composites: In-vitro efficacy on antibiotic-resistant bacterial pathogens and cytotoxicity studies. Front. Microbiol. 2016, 7, 1580. [Google Scholar] [CrossRef]
- Haider, J.; Majeed, H.; Williams, P.A.; Safdar, W.; Zhong, F. Formation of chitosan nanoparticles to encapsulate krill oil (Euphausia superba) for application as a dietary supplement. Food Hydrocoll. 2017, 63, 27–34. [Google Scholar] [CrossRef]
- Sotelo-Boyás, M.; Correa-Pacheco, Z.; Bautista-Baños, S.; Corona-Rangel, M. Physicochemical characterization of chitosan nanoparticles and nanocapsules incorporated with lime essential oil and their antibacterial activity against food-borne pathogens. LWT 2017, 77, 15–20. [Google Scholar] [CrossRef]
- Velmurugan, P.; Ganeshan, V.; Nishter, N.F.; Jonnalagadda, R.R. Encapsulation of orange and lavender essential oils in chitosan nanospherical particles and its application in leather for aroma enrichment. Surf. Interfaces 2017, 9, 124–132. [Google Scholar] [CrossRef]
- Ahmadi, Z.; Saber, M.; Bagheri, M.; Mahdavinia, G.R. Achillea millefolium essential oil and chitosan nanocapsules with enhanced activity against Tetranychusurticae. J. Pest Sci. 2018, 91, 837–848. [Google Scholar] [CrossRef]
- Kalagatur, N.K.; Ghosh, N.; Oriparambil, S.; Sundararaj, N.; Mudili, V. Antifungal activity of chitosan nanoparticles encapsulated with Cymbopogon martinii essential oil on plant pathogenic fungi Fusarium graminearum. Fronti. Pharmacol. 2018, 9, 610. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wu, C.; Wu, T.; Wang, L.; Chen, S.; Ding, T.; Hu, Y. Preparation and characterization of citrus essential oils loaded in chitosan microcapsules by using different emulsifiers. J. Food Eng. 2018, 217, 108–114. [Google Scholar] [CrossRef]
- Rajkumar, V.; Gunasekaran, C.; Dharmaraj, J.; Chinnaraj, P.; Paul, C.A.; Kanithachristy, I. Structural characterization of chitosan nanoparticle loaded with Piper nigrum essential oil for biological efficacy against the stored grain pest control. Pestic. Biochem. Physiol. 2020, 166, 104566. [Google Scholar] [CrossRef]
- Shetta, A.A.A. Encapsulation of Essential Oils in Chitosan Nanoparticle Formulations and Investigation on Their Antioxidant and Antibacterial Properties. Master’s Thesis, The American University in Cairo, Cairo, Egypt, 9 November 2017. [Google Scholar]
- Yulianti, L.; Bramono, K.; Mardliyati, E.; Freisleben, H.-J. Effects of Centellaasiatica ethanolic extract encapsulated in chitosan nanoparticles on proliferation activity of skin fibroblasts and keratinocytes, type I and III collagen synthesis and aquaporin 3 expression in vitro. J. Pharm. Biomed. Sci. 2016, 6. [Google Scholar] [CrossRef]
- Fachriyah, E. Cinnamomum casia extract encapsulated Nanochitosan as Antihypercholesterol. IOP Conf. Ser. Mat. Sci. Eng. 2017, 172, 012035. [Google Scholar]
- Cabral, B.R.P.; de Oliveira, P.M.; Gelfuso, G.M.; Quintão, T.D.S.C.; Chaker, J.A.; de Oliveira Karnikowski, M.G.; Gris, E.F. Improving stability of antioxidant compounds from Pliniacauliflora (jabuticaba) fruit peel extract by encapsulation in chitosan microparticles. J. Food Eng. 2018, 238, 195–201. [Google Scholar] [CrossRef]
- Jeyakumari, A.; Zynudheen, A.; Parvathy, U.; Binsi, P. Impact of chitosan and oregano extract on the physicochemical properties of microencapsulated fish oil stored at different temperature. Int. J. Food Prop. 2018, 21, 943–956. [Google Scholar] [CrossRef] [Green Version]
- El-Aziz, A.; Mohamed, A.R.; Al-Othman, M.R.; Mahmoud, M.A.; Shehata, S.M.; Abdelazim, N.S. Chitosan nanoparticles as a carrier for Mentha longifolia extract: Synthesis, characterization and antifungal activity. Curr. Sci. 2018, 114, 2116. [Google Scholar]
- Mahmoudi, R.; Ardakani, M.T.; Verdom, B.H.; Bagheri, A.; Mohammad-Beigi, H.; Aliakbari, F.; Salehpour, Z.; Alipour, M.; Afrouz, S.; Bardania, H. Chitosan nanoparticles containing Physalis alkekengi-L extract: Preparation, optimization and their antioxidant activity. Bull. Mat. Sci. 2019, 42, 131. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, S.M.M.; Ferreira-Nunes, R.; Amore, C.R.; Martins, D.H.; Pic-Taylor, A.; Fonseca-Bazzo, Y.M.; Silveira, D.; Gelfuso, G.M.; Magalhães, P.O. Emulsion incorporating Eugenia dysenterica aqueous extract entrapped in chitosan microparticles as a novel topical treatment of cutaneous infections. J. Drug Deliv. Sci. Technol. 2020, 55, 101372. [Google Scholar] [CrossRef]
- Ahmed, G.H.G.; González, A.F.; García, M.E.D. Nano-encapsulation of grape and apple pomace phenolic extract in chitosan and soy protein via nanoemulsification. Food Hydrocoll. 2020, 108, 105806. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, D.; Burton-Freeman, B.M.; Edirisinghe, I. Chemical changes of bioactive phytochemicals during thermal processing. Mat. Sci. 2016. [Google Scholar] [CrossRef]
- Cseke, L.J.; Kirakosyan, A.; Kaufman, P.B.; Warber, S.; Duke, J.A.; Brielmann, H.L. Natural Products from Plants; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Martinez, K.; Mackert, J.; McIntosh, M. Nutrition and Functional Foods for Healthy Aging; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- De Oliveira, J.L.; Campos, E.N.V.R.; Pereira, A.E.; Nunes, L.E.; Da Silva, C.C.; Pasquoto, T.; Lima, R.; Smaniotto, G.; Polanczyk, R.A.; Fraceto, L.F. Geraniol encapsulated in chitosan/gum arabic nanoparticles: A promising system for pest management in sustainable agriculture. J. Agricult. Food Chem. 2018, 66, 5325–5334. [Google Scholar] [CrossRef]
- Gopalakrishnan, L.; Ramana, L.N.; Sethuraman, S.; Krishnan, U.M. Ellagic acid encapsulated chitosan nanoparticles as anti-hemorrhagic agent. Carbohydr. Polym. 2014, 111, 215–221. [Google Scholar] [CrossRef]
- Akolade, J.O.; Oloyede, H.O.B.; Onyenekwe, P.C. Encapsulation in chitosan-based polyelectrolyte complexes enhances antidiabetic activity of curcumin. J. Funct. Foods 2017, 35, 584–594. [Google Scholar] [CrossRef]
- Khan, N.; Bharali, D.J.; Adhami, V.M.; Siddiqui, I.A.; Cui, H.; Shabana, S.M.; Mousa, S.A.; Mukhtar, H. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis 2014, 35, 415–423. [Google Scholar] [CrossRef]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the plasma exposure of (−)-epigallocatechin gallate in mice through an enhancement in intestinal stability. Eur. J. Pharm. Sci. 2011, 44, 422–426. [Google Scholar] [CrossRef]
- Nallamuthu, I.; Devi, A.; Khanum, F. Chlorogenic acid loaded chitosan nanoparticles with sustained release property, retained antioxidant activity and enhanced bioavailability. Asian J. Pharm. Sci. 2015, 10, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yang, Y.; Tang, K.; Hu, X.; Zou, G. Physicochemical characterization and antioxidant activity of quercetin-loaded chitosan nanoparticles. J. Appl. Polym. Sci. 2008, 107, 891–897. [Google Scholar] [CrossRef]
- Yadav, A.; Lomash, V.; Samim, M.; Flora, S.J. Curcumin encapsulated in chitosan nanoparticles: A novel strategy for the treatment of arsenic toxicity. Chem. Biol. Interact. 2012, 199, 49–61. [Google Scholar] [CrossRef]
- Panwar, R.; Sharma, A.K.; Kaloti, M.; Dutt, D.; Pruthi, V. Characterization and anticancer potential of ferulic acid-loaded chitosan nanoparticles against ME-180 human cervical cancer cell lines. Appl. Nanosci. 2016, 6, 803–813. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.P.; Birundha, K.; Kaveri, K.; Devi, K.R. Antioxidant studies of chitosan nanoparticles containing naringenin and their cytotoxicity effects in lung cancer cells. Int. J. Biol. Macromol. 2015, 78, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.B.; Ferreira, D.; Pintado, M.; Sarmento, B. Chitosan-based nanoparticles for rosmarinic acid ocular delivery—In vitro tests. Int. J. Biol. Macromol. 2016, 84, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Leonida, M.D.; Belbekhouche, S.; Benzecry, A.; Peddineni, M.; Suria, A.; Carbonnier, B. Antibacterial hop extracts encapsulated in nanochitosan matrices. Int. J. Biol. Macromol. 2018, 120, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, P.; Arbabi, E.; Atyabi, F.; Dinarvand, R. Ferulic acid exhibits antiepileptogenic effect and prevents oxidative stress and cognitive impairment in the kindling model of epilepsy. Life Sci. 2017, 179, 9–14. [Google Scholar] [CrossRef]
- Panwar, R.; Raghuwanshi, N.; Srivastava, A.K.; Sharma, A.K.; Pruthi, V. In-vivo sustained release of nanoencapsulated ferulic acid and its impact in induced diabetes. Mat. Sci. Eng. C 2018, 92, 381–392. [Google Scholar] [CrossRef]
- Ratul Kumar, D.; Naresh, K.; Utpal, B. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomed: Nanotech. Biol. Med. 2010, 6, 153–160. [Google Scholar]
- Gupta, V.; Aseh, A.; Ríos, C.N.; Aggarwal, B.B.; Mathur, A.B. Fabrication and characterization of silk fibroin-derived curcumin nanoparticles for cancer therapy. Int. J. Nanomed. 2009, 4, 115. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Yu, D.; Ying, Z.; Pan, C.; Wang, N.; Huang, F.; Ling, J.; Ouyang, X.-K. Fabrication of Ion-Crosslinking Aminochitosan Nanoparticles for Encapsulation and Slow Release of Curcumin. Pharmaceutics 2019, 11, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuah, L.H.; Roberts, C.J.; Billa, N.; Abdullah, S.; Rosli, R. Cellular uptake and anticancer effects of mucoadhesive curcumin-containing chitosan nanoparticles. Colloids Surf. B Biointerfaces 2014, 116, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.S.; Rashed, H.M.; Fayez, H.; Farouk, F.; Shamma, R.N. Nanoparticle-Mediated Dual Targeting: An Approach for Enhanced Baicalin Delivery to the Liver. Pharmaceutics 2020, 12, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zu, Y.; Zhang, Y.; Wang, W.; Zhao, X.; Han, X.; Wang, K.; Ge, Y. Preparation and in vitro/in vivo evaluation of resveratrol-loaded carboxymethyl chitosan nanoparticles. Drug Deliv. 2016, 23, 971–981. [Google Scholar] [CrossRef]
- Rassu, G.; Porcu, E.P.; Fancello, S.; Obinu, A.; Senes, N.; Galleri, G.; Migheli, R.; Gavini, E.; Giunchedi, P. Intranasal Delivery of Genistein-Loaded Nanoparticles as a Potential Preventive System against Neurodegenerative Disorders. Pharmaceutics 2019, 11, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woranuch, S.; Yoksan, R. Eugenol-loaded chitosan nanoparticles: I. Thermal stability improvement of eugenol through encapsulation. Carbohydr. Polym. 2013, 96, 578–585. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, S.-Q.; Peng, H.; Yu, L.; He, B.; Zhao, Q. In vivo anti-apoptosis activity of novel berberine-loaded chitosan nanoparticles effectively ameliorates osteoarthritis. Int. Immunopharmacol. 2015, 28, 34–43. [Google Scholar] [CrossRef]
- Mirhadi, E.; Rezaee, M.; Malaekeh-Nikouei, B. Nano strategies for berberine delivery, a natural alkaloid of Berberis. Biomed. Pharmacother. 2018, 104, 465–473. [Google Scholar] [CrossRef]
- Durán-Lobato, M.; Martín-Banderas, L.; Gonçalves, L.M.; Fernández-Arévalo, M.; Almeida, A.J. Comparative study of chitosan-and PEG-coated lipid and PLGA nanoparticles as oral delivery systems for cannabinoids. J. Nanopart. Res. 2015, 17, 61. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Zorzi, G.K.; Carvalho, E.L.S.; von Poser, G.L.; Teixeira, H.F. On the use of nanotechnology-based strategies for association of complex matrices from plant extracts. Rev. Bras. Farmacogn. 2015, 25, 426–436. [Google Scholar] [CrossRef]
- Safer, A.-M.A.; Hanafy, N.A.; Bharali, D.J.; Cui, H.; Mousa, S.A. Effect of green tea extract encapsulated into chitosan nanoparticles on hepatic fibrosis collagen fibers assessed by atomic force microscopy in rat hepatic fibrosis model. J. Nanosci. Nanotechnol. 2015, 15, 6452–6459. [Google Scholar] [CrossRef] [PubMed]
- Bharali, D.J.; Siddiqui, I.A.; Adhami, V.M.; Chamcheu, J.C.; Aldahmash, A.M.; Mukhtar, H.; Mousa, S.A. Nanoparticle delivery of natural products in the prevention and treatment of cancers: Current status and future prospects. Cancers 2011, 3, 4024–4045. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Kesharwani, S.; Sharma, N.; Gupta, M.K. Formulation and Evaluation of Herbal Extract of Allivum sativum (Garlic) Loaded Chitosan Nanoparticles. J. Drug Deliv. Ther. 2019, 9, 715–718. [Google Scholar]
- Rejinold, N.S.; Muthunarayanan, M.; Muthuchelian, K.; Chennazhi, K.; Nair, S.V.; Jayakumar, R. Saponin-loaded chitosan nanoparticles and their cytotoxicity to cancer cell lines in vitro. Carbohydr. Polym. 2011, 84, 407–416. [Google Scholar] [CrossRef]
- Alfaro-Viquez, E.; Esquivel-Alvarado, D.; Madrigal-Carballo, S.; Krueger, C.G.; Reed, J.D. Cranberry proanthocyanidin-chitosan hybrid nanoparticles as a potential inhibitor of extra-intestinal pathogenic Escherichia coli invasion of gut epithelial cells. Int. J. Biol. Macromol. 2018, 111, 415–420. [Google Scholar] [CrossRef]
- Beconcini, D.; Fabiano, A.; Zambito, Y.; Berni, R.; Santoni, T.; Piras, A.M.; Di Stefano, R. Chitosan-based nanoparticles containing cherry extract from Prunus avium L. to improve the resistance of endothelial cells to oxidative stress. Nutrients 2018, 10, 1598. [Google Scholar] [CrossRef] [Green Version]
- Fabiano, A.; Piras, A.M.; Uccello-Barretta, G.; Balzano, F.; Cesari, A.; Testai, L.; Citi, V.; Zambito, Y. Impact of mucoadhesive polymeric nanoparticulate systems on oral bioavailability of a macromolecular model drug. Eur. J. Pharm. Biopharm. 2018, 130, 281–289. [Google Scholar] [CrossRef]
- Barrera-Necha, L.L.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Hernández-López, M.; Jiménez, J.E.M.; Mejía, A.F.M. Synthesis and characterization of chitosan nanoparticles loaded botanical extracts with antifungal activity on Colletotrichum gloeosporioides and Alternaria species. Adv. Microbiol. 2018, 8, 286. [Google Scholar]
- He, B.; Ge, J.; Yue, P.; Yue, X.; Fu, R.; Liang, J.; Gao, X. Loading of anthocyanins on chitosan nanoparticles influences anthocyanin degradation in gastrointestinal fluids and stability in a beverage. Food Chem. 2017, 221, 1671–1677. [Google Scholar] [CrossRef]
- Kailaku, S.I.; Mulyawanti, I.; Alamsyah, A.N. Formulation of nanoencapsulated catechin with chitosan as encapsulation material. Procedia Chem. 2014, 9, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Swarnalatha, Y.; Gunna, G.K.; Jacob, C.M. Synthesis of alkaloid loaded chitosan nanoparticles for enhancing the anticancer activity in A549 lung cancer cell line. Pharm. Lett. 2015, 7, 378. [Google Scholar]
- Inoue, E.; Shimizu, Y.; Masui, R.; Hayakawa, T.; Tsubonoya, T.; Hori, S.; Sudoh, K. Effects of saffron and its constituents, crocin-1, crocin-2, and crocetin on α-synuclein fibrils. J. Nat. Med. 2018, 72, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Shahid-ul-Islam; Rather, L.J.; Mohammad, F. Phytochemistry, biological activities and potential of annatto in natural colorant production for industrial applications-A review. J. Adv. Res. 2016, 7, 499–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, T.; Percival, S.S.; Cheng, Q.; Li, Z.; Rowe, C.A.; Gu, L. Preparation, characterization, and induction of cell apoptosis of cocoa procyanidins–gelatin–chitosan nanoparticles. Eur. J. Pharm. Biopharm. 2012, 82, 36–42. [Google Scholar] [CrossRef]
- Ang, L.F.; Darwis, Y.; Yee, L.; Yam, M.F. Microencapsulation Curcuminoids for Effective Delivery in Pharmaceutical Application. Pharmaceutics 2019, 11, 451. [Google Scholar] [CrossRef] [Green Version]
- Piazzini, V.; Vasarri, M.; Degl’Innocenti, D.; Guastini, A.; Barletta, E.; Salvatici, M.C.; Bergonzi, M.C. Comparison of Chitosan Nanoparticles and Soluplus Micelles to Optimize the Bioactivity of Posidonia oceanica Extract on Human Neuroblastoma Cell Migration. Pharmaceutics 2019, 11, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimirad, R.; Behnamian, M.; Dezhsetan, S. Bitter orange oil incorporated into chitosan nanoparticles: Preparation, characterization and their potential application on antioxidant and antimicrobial characteristics of white button mushroom. Food Hydrocoll. 2020, 100, 105387. [Google Scholar] [CrossRef]
- Hasani, S.; Ojagh, S.M.; Ghorbani, M. Nanoencapsulation of lemon essential oil in Chitosan-Hicap system. Part 1: Study on its physical and structural characteristics. Int. J. Biol. Macromol. 2018, 115, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Chitosan nanoparticles loaded with Cinnamomum zeylanicum essential oil enhance the shelf life of cucumber during cold storage. Postharvest Biol. Technol. 2015, 110, 203–213. [Google Scholar] [CrossRef]
- Djenane, D.; Aboudaou, M.; Ferhat, M.A.; Ouelhadj, A.; Ariño, A. Effect of the aromatisation with summer savory (Saturejahortensis L.) essential oil on the oxidative and microbial stabilities of liquid whole eggs during storage. J. Essent. Oil Res. 2019, 31, 444–455. [Google Scholar] [CrossRef]
- Skubij, N.; Dzida, K. Essential oil composition of summer savory (Saturejahortensis L.) cv. Saturn depending on nitrogen nutrition and plant development phases in raw material cultivated for industrial use. Ind. Crops Prod. 2019, 135, 260–270. [Google Scholar] [CrossRef]
- Ferreira, T.P.; Haddi, K.; Corrêa, R.F.; Zapata, V.L.; Piau, T.B.; Souza, L.F.; Santos, S.-M.G.; Oliveira, E.E.; Jumbo, L.O.; Ribeiro, B.M. Prolonged mosquitocidal activity of Siparunaguianensis essential oil encapsulated in chitosan nanoparticles. PLoSNegl. Trop. Dis. 2019, 13, e0007624. [Google Scholar]
- Thakur, V.K. Handbook of Polymers for Pharmaceutical Technologies, Structure and Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2015; Volume 1. [Google Scholar]
- Matshetshe, K.I.; Parani, S.; Manki, S.M.; Oluwafemi, O.S. Preparation, characterization and in vitro release study of β-cyclodextrin/chitosan nanoparticles loaded Cinnamomumzeylanicum essential oil. Int. J. Biol. Macromol. 2018, 118, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Gibis, M.; Rahn, N.; Weiss, J. Physical and oxidative stability of uncoated and chitosan-coated liposomes containing grape seed extract. Pharmaceutics 2013, 5, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Piazzini, V.; Cinci, L.; D’Ambrosio, M.; Luceri, C.; Bilia, A.R.; Bergonzi, M.C. Solid Lipid Nanoparticles and Chitosan-coated Solid Lipid Nanoparticles as Promising Tool for Silybin Delivery: Formulation, Characterization, and In vitro Evaluation. Curr. Drug Deliv. 2019, 16, 142–152. [Google Scholar] [CrossRef]
- Rabelo, R.S.; Oliveira, I.F.; da Silva, V.M.; Prata, A.S.; Hubinger, M.D. Chitosan coated nanostructured lipid carriers (NLCs) for loading Vitamin D: A physical stability study. Int. J. Biol. Macromol. 2018, 119, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Lin, Y.-S.; Wu, S.-J.; Mi, F.-L. Mutlifunctional nanoparticles prepared from arginine-modified chitosan and thiolated fucoidan for oral delivery of hydrophobic and hydrophilic drugs. Carbohydr. Polym. 2018, 193, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Nalini, T.; Basha, S.K.; Sadiq, A.M.M.; Kumari, V.S.; Kaviyarasu, K. Development and characterization of alginate/chitosan nanoparticulate system for hydrophobic drug encapsulation. J. Drug Deliv. Sci. Technol. 2019, 52, 65–72. [Google Scholar] [CrossRef]
- Fathy Abd-Ellatef, G.-E.; Gazzano, E.; Chirio, D.; Hamed, A.R.; Belisario, D.C.; Zuddas, C.; Peira, E.; Rolando, B.; Kopecka, J.; Assem Said Marie, M. Curcumin-Loaded Solid Lipid Nanoparticles Bypass P-Glycoprotein Mediated Doxorubicin Resistance in Triple Negative Breast Cancer Cells. Pharmaceutics 2020, 12, 96. [Google Scholar] [CrossRef] [Green Version]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Controll. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Elmowafy, M.; Alruwaili, N.K.; Shalaby, K.; Alharbi, K.S.; Altowayan, W.M.; Ahmed, N.; Zafar, A.; Elkomy, M. Long-Acting Paliperidone Parenteral Formulations Based on Polycaprolactone Nanoparticles; the Influence of Stabilizer and Chitosan on In vitro Release, Protein Adsorption, and Cytotoxicity. Pharmaceutics 2020, 12, 160. [Google Scholar] [CrossRef] [Green Version]
- Trotta, V.; Pavan, B.; Ferraro, L.; Beggiato, S.; Traini, D.; Des Reis, L.G.; Scalia, S.; Dalpiaz, A. Brain targeting of resveratrol by nasal administration of chitosan-coated lipid microparticles. Eur. J. Pharm. Biopharm. 2018, 127, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Bruinsmann, F.A.; Pigana, S.; Aguirre, T.; DadaltSouto, G.; Garrastazu Pereira, G.; Bianchera, A.; TiozzoFasiolo, L.; Colombo, G.; Marques, M.; Raffin Pohlmann, A. Chitosan-coated nanoparticles: Effect of chitosan molecular weight on nasal transmucosal delivery. Pharmaceutics 2019, 11, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buttini, F.; Colombo, P.; Rossi, A.; Sonvico, F.; Colombo, G. Particles and powders: Tools of innovation for non-invasive drug administration. J. Controll. Release 2012, 161, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Sezer, A.D.; Cevher, E. Topical drug delivery using chitosan nano-and microparticles. Expert Opin. Drug Deliv. 2012, 9, 1129–1146. [Google Scholar] [CrossRef]
- Rodrigues, S.F.; Fiel, L.A.; Shimada, A.L.; Pereira, N.R.; Guterres, S.S.; Pohlmann, A.R.; Farsky, S.H. Lipid-core nanocapsules act as a drug shuttle through the blood brain barrier and reduce glioblastoma after intravenous or oral administration. J. Biomed. Nanotechnol. 2016, 12, 986–1000. [Google Scholar] [CrossRef]
- Bahadur, S.; Pathak, K. Physicochemical and physiological considerations for efficient nose-to-brain targeting. Expert Opin. Drug Deliv. 2012, 9, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Arafa, M.G.; Girgis, G.N.; El-Dahan, M.S. Chitosan-Coated PLGA Nanoparticles for Enhanced Ocular Anti-Inflammatory Efficacy of Atorvastatin Calcium. Int. J. Nanomed. 2020, 15, 1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Adhikari, U.; Rijal, N.P.; Bhattarai, S.R.; Sankar, J.; Bhattarai, N. pH-responsive PLGA nanoparticle for controlled payload delivery of diclofenac sodium. J. Funct. Biomat. 2016, 7, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, S.; Casettari, L.; Illum, L. Transmucosal Absorption Enhancers in the Drug Delivery Field. Pharmaceutics 2019, 11, 339. [Google Scholar] [CrossRef] [Green Version]
- Üstündağ-Okur, N.; Gökçe, E.H.; Bozbıyık, D.I.; Eğrilmez, S.; Özer, Ö.; Ertan, G. Preparation and in vitro–in vivo evaluation of ofloxacin loaded ophthalmic nano structured lipid carriers modified with chitosan oligosaccharide lactate for the treatment of bacterial keratitis. Eur. J. Pharm. Sci. 2014, 63, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-C.; Hon, M.-H. The effect of the molecular weight of chitosan nanoparticles and its application on drug delivery. Microchem. J. 2009, 92, 87–91. [Google Scholar] [CrossRef]
- Quagliariello, V.; Masarone, M.; Armenia, E.; Giudice, A.; Barbarisi, M.; Caraglia, M.; Barbarisi, A.; Persico, M. Chitosan-coated liposomes loaded with butyric acid demonstrate anticancer and anti-inflammatory activity in human hepatoma HepG2 cells. Oncol. Rep. 2019, 41, 1476–1486. [Google Scholar] [CrossRef] [Green Version]
- Goy, R.; de Britto, D.; Assis, O.B. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Markl, D.; Wahl, P.R.; Menezes, J.C.; Koller, D.M.; Kavsek, B.; Francois, K.; Roblegg, E.; Khinast, J.G. Supervisory control system for monitoring a pharmaceutical hot melt extrusion process. AAPS PharmSciTech 2013, 14, 1034–1044. [Google Scholar] [CrossRef] [Green Version]
- Al-Nemrawi, N.K.; Alshraiedeh, N.A.H.; Zayed, A.L.; Altaani, B.M. Low molecular weight chitosan-coated PLGA nanoparticles for pulmonary delivery of tobramycin for cystic fibrosis. Pharmaceuticals 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senthilkumar, P.; Yaswant, G.; Kavitha, S.; Chandramohan, E.; Kowsalya, G.; Vijay, R.; Sudhagar, B.; Kumar, D.R.S. Preparation and characterization of hybrid chitosan-silver nanoparticles (Chi-Ag NPs); A potential antibacterial agent. Int. J. Biol. Macromol. 2019, 141, 290–297. [Google Scholar] [CrossRef]
- Li, J.; Hwang, I.-C.; Chen, X.; Park, H.J. Effects of chitosan coating on curcumin loaded nano-emulsion: Study on stability and in vitro digestibility. Food Hydrocoll. 2016, 60, 138–147. [Google Scholar] [CrossRef]
- Braber, N.L.V.; Paredes, A.J.; Rossi, Y.E.; Porporatto, C.; Allemandi, D.A.; Borsarelli, C.D.; Correa, S.G.; Montenegro, M.A. Controlled release and antioxidant activity of chitosan or its glucosamine water-soluble derivative microcapsules loaded with quercetin. Int. J. Biol. Macromol. 2018, 112, 399–404. [Google Scholar] [CrossRef]
- Singh, P.K.; Sah, P.; Meher, J.G.; Joshi, S.; Pawar, V.K.; Raval, K.; Singh, Y.; Sharma, K.; Kumar, A.; Dube, A. Macrophage-targeted chitosan anchored PLGA nanoparticles bearing doxorubicin and amphotericin B against visceral leishmaniasis. RSC Adv. 2016, 6, 71705–71718. [Google Scholar] [CrossRef]
- Lima, I.A.D.; Khalil, N.M.; Tominaga, T.T.; Lechanteur, A.; Sarmento, B.; Mainardes, R.M. Mucoadhesive chitosan-coated PLGA nanoparticles for oral delivery of ferulic acid. Artif. Cells Nanomed. Biotechnol. 2018, 46, 993–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.; Khanna, K.; Bhatnagar, A.; Ahmad, F.J.; Ali, A. Chitosan coated PLGA nanoparticles amplify the ocular hypotensive effect of forskolin: Statistical design, characterization and in vivo studies. Int. J. Biol. Macromol. 2018, 116, 648–663. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, F.; Hanieh, P.N.; Chan, L.K.N.; Angeloni, L.; Passeri, D.; Rossi, M.; Wang, J.T.-W.; Imbriano, A.; Carafa, M.; Marianecci, C. Chitosan glutamate-coated niosomes: A proposal for nose-to-brain delivery. Pharmaceutics 2018, 10, 38. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, P.; Kong, L. Chitosan-modified PLGA nanoparticles with versatile surface for improved drug delivery. AAPS Pharm. Sci. Tech. 2013, 14, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Bang, S.; Hwang, I.; Yu, Y.; Kwon, H.; Kim, D.; Park, H.J. Influence of chitosan coating on the liposomal surface on physicochemical properties and the release profile of nanocarrier systems. J. Microencapsul. 2011, 28, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Khare, V.; Saxena, A.K.; Gupta, P.N. Toxicology Considerations in Nanomedicine. In Nanotechnology Applications for Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2015; pp. 239–261. [Google Scholar]
- Almalik, A.; Alradwan, I.; Kalam, M.A.; Alshamsan, A. Effect of cryoprotection on particle size stability and preservation of chitosan nanoparticles with and without hyaluronate or alginate coating. Saudi Pharm. J. 2017, 25, 861–867. [Google Scholar] [CrossRef]
- Almalik, A.; Benabdelkamel, H.; Masood, A.; Alanazi, I.O.; Alradwan, I.; Majrashi, M.A.; Alfadda, A.A.; Alghamdi, W.M.; Alrabiah, H.; Tirelli, N. Hyaluronic acid coated chitosan nanoparticles reduced the immunogenicity of the formed protein corona. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Zaki, O.; Sarah, S.; Ibrahim, M.N.; Katas, H. Particle size affects concentration-dependent cytotoxicity of chitosan nanoparticles towards mouse hematopoietic stem cells. J. Nanotechnol. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.; Ben Messaoud, G.; Michaux, F.; Tamayol, A.; Kahn, C.J.F.; Belhaj, N.; Linder, M.; Arab-Tehrany, E. Chitosan-coated liposomes encapsulating curcumin: Study of lipid–polysaccharide interactions and nanovesicle behavior. RSC Adv. 2016, 6, 45290–45304. [Google Scholar] [CrossRef]
- Holubnycha, V.; Kalinkevich, O.; Ivashchenko, O.; Pogorielov, M. Antibacterial activity of in situ prepared chitosan/silver nanoparticles solution against methicillin-resistant strains of Staphylococcus aureus. Nanoscale Res. Lett. 2018, 13, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nate, Z.; Moloto, M.J.; Mubiayi, P.K.; Sibiya, P.N. Green synthesis of chitosan capped silver nanoparticles and their antimicrobial activity. MRS Adv. 2018, 3, 2505–2517. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J. Chitosan-based zinc oxide nanoparticle for enhanced anticancer effect in cervical cancer: A physicochemical and biological perspective. Saudi Pharm. J. 2018, 26, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, L.D.; Lyu, G.M.; Shen, B.Y.; Han, Y.F.; Shi, J.L.; Teng, J.L.; Feng, L.; Si, S.Y.; Wu, J.H.; et al. Chitosan-coated cerium oxide nanocubes accelerate cutaneous wound healing by curtailing persistent inflammation. Inorg. Chem. Front. 2018, 5, 386–393. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Bondarenko, O.; Sihtmäe, M.M.; Kuzmičiova, J.; Ragelienė, L.; Kahru, A.; Daugelavičius, R. Bacterial plasma membrane is the main cellular target of silver nanoparticles in Escherichia coli and Pseudomonas aeruginosa. bioRxiv 2018. [Google Scholar] [CrossRef]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Li, L.; Zhang, Y.; Chen, K.; Wang, H.; Gong, R. Carboxymethyl-β-cyclodextrin grafted chitosan nanoparticles as oral delivery carrier of protein drugs. React. Funct. Polym. 2017, 117, 10–15. [Google Scholar] [CrossRef]
- Brasselet, C.; Pierre, G.; Dubessay, P.; Dols-Lafargue, M.; Coulon, J.; Maupeu, J.; Vallet-Courbin, A.; De Baynast, H.; Doco, T.; Michaud, P. Modification of chitosan for the generation of functional derivatives. Appl. Sci. 2019, 9, 1321. [Google Scholar] [CrossRef] [Green Version]
- Upadhyaya, L.; Singh, J.; Agarwal, V.; Tewari, R.P. Biomedical applications of carboxymethyl chitosans. Carbohydr. Polym. 2013, 91, 452–466. [Google Scholar] [CrossRef]
- Campos, E.V.; Proença, P.L.; Oliveira, J.L.; Melville, C.C.; Della Vechia, J.F.; De Andrade, D.J.; Fraceto, L.F. Chitosan nanoparticles functionalized with β-cyclodextrin: A promising carrier for botanical pesticides. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.V.; Proença, P.L.; Oliveira, J.L.; Pereira, A.E.; de Morais Ribeiro, L.N.; Fernandes, F.O.; Gonçalves, K.C.; Polanczyk, R.A.; Pasquoto-Stigliani, T.; Lima, R. Carvacrol and linalool co-loaded in β-cyclodextrin-grafted chitosan nanoparticles as sustainable biopesticide aiming pest control. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yaneva, Z.; Ivanova, D.; Nikolova, N.; Tzanova, M. The 21st century revival of chitosan in service to bio-organic chemistry. Biotechnol. Biotechnol. Equip. 2020, 34, 221–237. [Google Scholar] [CrossRef]
- Kosaraju, S.L.; Weerakkody, R.; Augustin, M.A. Chitosan−glucose conjugates: Influence of extent of Maillard reaction on antioxidant properties. J. Agricult. Food Chem. 2010, 58, 12449–12455. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Hussain, Z.; Katas, H.; Amin, M.C.I.M.; Kumolosasi, E.; Buang, F.; Sahudin, S. Self-assembled polymeric nanoparticles for percutaneous co-delivery of hydrocortisone/hydroxytyrosol: An ex vivo and in vivo study using an NC/Nga mouse model. Int. J. Pharm. 2013, 444, 109–119. [Google Scholar] [CrossRef]
- Siddique, M.I.; Katas, H.; Amin, M.C.I.M.; Ng, S.-F.; Zulfakar, M.H.; Buang, F.; Jamil, A. Minimization of local and systemic adverse effects of topical glucocorticoids by nanoencapsulation: In vivo safety of hydrocortisone–hydroxytyrosol loaded chitosan nanoparticles. J. Pharm. Sci. 2015, 104, 4276–4286. [Google Scholar] [CrossRef]
- Song, W.; Su, X.; Gregory, D.A.; Li, W.; Cai, Z.; Zhao, X. Magnetic alginate/chitosan nanoparticles for targeted delivery of curcumin into human breast cancer cells. Nanomaterials 2018, 8, 907. [Google Scholar] [CrossRef] [Green Version]
- Manconi, M.; Manca, M.L.; Valenti, D.; Escribano, E.; Hillaireau, H.; Fadda, A.M.; Fattal, E. Chitosan and hyaluronan coated liposomes for pulmonary administration of curcumin. Int. J. Pharm. 2017, 525, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Marongiu, F.; Castangia, I.; Manca, M.L.; Caddeo, C.; Tuberoso, C.I.G.; D’Hallewin, G.; Bacchetta, G.; Fadda, A.M. Polymer-associated liposomes for the oral delivery of grape pomace extract. Colloids Surf. B Biointerfaces 2016, 146, 910–917. [Google Scholar] [CrossRef]
- Miele, D.; Catenacci, L.; Sorrenti, M.; Rossi, S.; Sandri, G.; Malavasi, L.; Dacarro, G.; Ferrari, F.; Bonferoni, M.C. Chitosan Oleate Coated Poly Lactic-Glycolic Acid (PLGA) Nanoparticles versus Chitosan Oleate Self-Assembled Polymeric Micelles, Loaded with Resveratrol. Mar. Drugs 2019, 17, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leena, R.; Vairamani, M.; Selvamurugan, N. Alginate/Gelatin scaffolds incorporated with Silibinin-loaded Chitosan nanoparticles for bone formation in vitro. Colloids Surf. B Biointerfaces 2017, 158, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Caro, N.; Abugoch, L.; Gamboa, A.; Díaz-Dosque, M.; Tapia, C. Chitosan thymol nanoparticles improve the antimicrobial effect and the water vapour barrier of chitosan-quinoa protein films. J. Food Eng. 2019, 240, 191–198. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Cui, H. Moringa oil/chitosan nanoparticles embedded gelatin nanofibers for food packaging against Listeria monocytogenes and Staphylococcus aureus on cheese. Food Packag. Shelf Life 2019, 19, 86–93. [Google Scholar] [CrossRef]
- Gómez Chabala, L.F.; Cuartas, C.E.E.; López, M.E.L. Release behavior and antibacterial activity of chitosan/alginate blends with aloe vera and silver nanoparticles. Mar. Drugs 2017, 15, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straccia, M.C.; d’Ayala, G.G.; Romano, I.; Oliva, A.; Laurienzo, P. Alginate hydrogels coated with chitosan for wound dressing. Mar. Drugs 2015, 13, 2890–2908. [Google Scholar] [CrossRef] [Green Version]







| Source Name | Extract | Biological Study | Main Constituents | Chitosan (CS) Characteristics | Preparation Method | Outcome | Ref. |
|---|---|---|---|---|---|---|---|
| Centella asiatica | C. asiatica ethanolic extract | Microculture tetrazolium assay for analysis of the proliferation of normal human dermal fibroblasts (NHDF) and normal human epidermal keratinocytes (NHEK), test on type I and III collagen synthesis using ELISA, immunocytochemistry in combination with ImageJ software for the evaluation of Aquaporin 3 expression | Asiatic acid, madecassic acid, asiaticoside and madecassoside | CS with a deacetylation degree >70% | Ionic gelation | Anti-aging activity by inducing skin cell (fibroblasts and keratinocytes) proliferation and AQP3 expression | [122] |
| Physalis alkekengi | Hydro-alcoholic extract of seeds of P. alkekengi | Non-biological but antioxidant assays: DPPH, FRAP | Physalins, carotenoids, alkaloids, polyphenols, flavonoids | Low MW CS | Ionic gelation using TPP | Improved antioxidant capacity | [126] |
| Theobroma cacao | Golden apple and red grape | DPPH assay | Nanoemulsification-solvent displacement method and Tween as the emulsifier | Enhanced antioxidant activity | [129] | ||
| Cocoa bean procyanidins (CPs) extract | Cell apoptosis with annexin V staining and cytotoxicity assay in the THP-1 cell line | Procyanidin oligomers (from monomer to decamers) and polymers, with polymers being the predominant component | CS (low MW, 75–85% deacetylated) | Preparation of CPs-gelatin-CS nanoparticles | Improved stability and good apoptotic effects at lower concentrations in human acute monocytic leukemia THP-1 cells | [173] | |
| Camellia sinensis | Green Tea Extract (GTE) distilled water extract | Uptake study in HepG2 cells, test on carbon tetrachloride (CCl4)-induced hepatic fibrosis in rats | epicatechin gallate(ECG), epigallocatechin (EGC), epicatechin(EC) and caffeine | Water-soluble, low MW CS obtained from mushroom | Ionic gelation using TPP | Effective in removing all the extracellular collagen caused byCCl4 in the hepatic fibrosis rat liver | [159] |
| Allivum sativum | Garlic aqueous extract | In vitro drug release | Ionic gelation | High stability and in vitro release for future use in many diseases such as cancer | [162] | ||
| Sapindus emarginatus | Sapindus extract with distilled ethanol | Specific cytotoxic assay (MTT) against prostate/oral cancer cells/normal cells | Saponin | Average molecular weight (MW) 20 kDa, degree ofN-deacetylation (75–80%) | Ionic gelation using TPP | Potential therapeutic agent for cancer, inducing dose-dependent cancer cell death with lower toxicity on normal cells | [163] |
| Vacciniumma crocarpon | Cranberry proanthocyanidins (PAC) | Determinationof the effect on the (extra-intestinal pathogenic Escherichia coli) ExPEC invasion of gut epithelial cells in vitro | Flavonolglycosides, anthocyanins, proanthocyanidins, and hydroxycinnamic acids, but use only of proanthocyanidin enriched fraction (PAC) | CS from shrimp shells (deacetylation degree of 92%, MW185 kDa | Ionic gelation | Increased stability and molecular adhesion of PAC to ExPEC | [164] |
| Prunus avium L. | Crognola cherry fruits extract | In vitro test on HUVECs (Human umbilical vein endothelialcells)stressed with H2O2 | Polyphenols | S-protected thiolated derivative | Protection of the endothelial cells from oxidative stress related to vascular dysfunction implied in a number of cardiovascular pathologies. | [165] | |
| Vaccinium corymbosum | Blueberry fruit ethanol extract | In vitro antifungal evaluation (sporulation and germination were measured) on Alternaria alternata from Ficuscarica and Rosmarinus officinalis | Flavonoids, phenolic acids, tannins, and anthocyanins | Medium MWCS (deacetylation degree 75–85%) | Weak antifungal activity against A. alternata from fig and rosemary | [167] | |
| Byrsonima crassifolia | Nanche leaves methanol extract | In vitro antifungal evaluation (sporulation and germination were measured) on Colletotrichum gloeosporioides isolated from Carica papaya L. and Annona muricata L. | Fatty acids, diterpenes, phenolic compounds and monoterpenes | Medium MW CS (deacetylation degree 75–85%) | Improved control of C. gloeosporioides isolated from papaya and soursop leading to synergistic effect | [167] | |
| Uncaria gambier Roxb. | Catechin (gambier) extract | No-biological assays, DPPH assay | Higher levels of catechin (42%): catechin acid and catechu tannat acid and small quantity of quercetin | CS (deacetylation degree: 85%) | Good particle surface topography, internal structure of the particles and emulsion stability, good antioxidant activity | [169] | |
| Sphaeranthus amaranthoides | Alkaloid extract | Alkaloids, tannins, saponins, flavonoids, alkaloids, proteins and steroids | CS-alginate nanoparticles | Good apoptotic inducer in vitro, inhibition of the cell growth via induction of apoptosis in A549 cell line. | [170] | ||
| Crocus sativus | Saffron and ultrafine saffron aqueous extract | In vitro cytotoxicity study measuring the viability of HUVE cells incorporation in sunscreen emulsions (emulsion stability and SPF determination assays) | Crocin-1, crocin-2, crocetin, safranal | CS with high MW (MW: 350,000 g/moL, deacetylation degree >75%, and viscosity 800–2000 cps) | Ionic gelation using TPP | Formed nanoparticles with spherical and irregular shape, and size varied from ~150 to ~500 nm, crystalline dispersion, for sunscreen emulsions: good stability, viscosity, low cytotoxicity. | [171] |
| Bixaorellana | Annatto and ultrafine annatto (UF) | In vitrocytotoxicity study measuring the viability of HUVE cells incorporation in sunscreen emulsions (emulsion stability and SPF determination assays) | Carotenoids, apocarotenoids, sterols, aliphatic compounds, monoterpenes and sesquiterpenes, triterpenoids | CS with high MW (MW: 350,000 g/moL, deacetylation degree >75%, and viscosity 800–2000 cps) | Ionotropic gelation method using TPP | Formed nanoparticles with spherical and irregular shape, and size varied from ~150 to ~500 nm, amorphous dispersion in the case of annatto and UF annatto, for sunscreen emulsions: good stability, viscosity, low cytotoxicity. | [172] |
| Rhizome of turmeric | Curcumin (~77%), demethoxycurcumin (~17%) and bisdemethoxycurcumin (~3%) | Complex coacervation, using Tween 80 as the emulsifier and formaldehyde as the cross-linking agent | [174] | ||||
| Posidonia oceanica (L.) Delile. | Hydroalcoholic extract | Ionic gelation method with TPP | Improvement of the aqueous solubility of the extract | [175] |
| Source/Plant Name | Essential Oil | Biological Study | Main Constituents | Chitosan Characteristics | Preparation Method | Outcome | Ref. |
| Coriandrum sativum | C. sativum essential oil (CSEO) | 14 different food borne mold swere used for fungitoxic spectrum determination, determination of AFB1 inhibitory efficacy, ABTS•+ assay, TPC determination, phytotoxicity assay | Linalool (65.18%), geranyl acetate (12.06%) and α-pinene (4.76%) | MW = 193,400 | Ionic gelation | Efficient broad spectrum antifungal, antiaflatoxigenic and antioxidant agent, inhibitor of methylglyoxal (aflatoxin inducer), inhibitor of AFB1 (aflatoxin B1) secretion | [2] |
| Camellia sinensis | GreenTea oil (GTO) | Agar dilution and colony counting methods against Gram-positive (S.aureus) and Gram-negative bacteria (Escherichia coli), DPPH assay | Monoterpenes, terpene alcohol, sesquiterpene and phenolic compounds such as flavanones and flavanols | Medium MW CS (84.8% degree of dealkylation) | Emulsification/ionic gelation | High antibacterial activities against S. aureus and E. coli | [3] |
| Mentha piperita | Peppermint oil | Agar dilution and colony counting methods against Gram-positive (Staphylococcus aureus and Gram-negative bacteria (Escherichia coli) | Oxygenated terpenoids: menthone and menthol | Medium MW CS (84.8% degree of dealkylation) | Emulsification/ ionic gelation | Weak antibacterial activity against S. aureus | [3] |
| Rosmarinus officinalis | Rosemary essential oil | DPPH assay, TPC determination with Folin-Ciocalteu assay | 1,8 cineole, camphor, α-terpineol, α-pinene, camphene | Low MW CS | Homogenization | Increased thermal stability | [6] |
| Eugenia caryophyllata | Clove essential oil (CEO) | Pour-plate technique for antifungal assays against Aspergillus niger isolated from spoiled pomegranate | Eugenol, phenylpropanoid, eugenyl acetate, monoterpeneester and β-caryophyllene, a sesquiterpene | Medium MW and 75–85% degree of deacetylation | Emulsion-ionic gelation using TPP | Promising natural fungicide with improved efficacy against Aspergillus niger | [103] |
| Hydrodistillation of air-dried clove buds | Clove essential oil (CEO) | Oil-in-water emulsification followed by TPP induced ionic gelation | Antioxidant activity and potent antimicrobial activity against L. monocytogenes and S. aureus | [104] | |||
| Thymus (plant) | Thyme essential oil (TEO) | Six bacterial strains: S. aureus, L. monocytogenes, B. cereus, Salmonella typhi, Shigella dysenteriae and E. coli tested using agar plate technique | Thymol and carvacrol | Medium MWCS (deacetylation degree75–85%) | Two different procedures for nanoparticles (CSNPs and nanocapsules (CSNCs) preparation | TEO-CSNPs had the highest inhibitory activity against Staphylococcus aureus and TEO-CSNCs against Bacillus cereus | [110] |
| Mentha piperita | l-Menthol 45.05% L-menthalone 17.53% Menthofuran 8.58%, cis-Carane 8.22%, neo-Menthol 4.33%, 1,8-Cineole 4.26% etc. | Ionic gelation | Loaded nanoparticles effectively inhibited the biofilm formation and were found to specifically inhibit some glygosyltransferase genes | [112] | |||
| Citrus species | Lime essential oil | Four strains of bacteria: Staphylococcus aureus, Listeria monocytogenes-Shigella dysenteriae, and Escherichia coli, were used astest microorganisms in agar plate | Limonene and otherterpenes | Medium MW CS (deacetylation degree75–85%) | Nanoparticles preparation: nanoprecipitation, oil-in-water emulsion followed by ionic gelation and nano- encapsulation preparation: oxidative degradation of medium MW CS using the solvent displacement technique | Synergistic effect in the antibacterial activity against testedpathogens, greater for the nanoparticles compared to the nanocapsules for S. aureus, L. monocytogenes, S. dysenteriae, and E. coli with the highest antibacterial activity being against S. dysenteriae | [115] |
| Cymbopogon martinii | C. martinii essential oil (CMEO) | Antifungal activity determined by the microwell dilution method on mycotoxigenic, F. graminearum, determination of intracellular ROS, lipid peroxidation and ergosterol | Geraniol, geranial, geranyl propionate, geranyl acetone, geranyl acetate, a-phellandrene, and linalool | High purity CS: 99%degree of deacetylation, and MW of 100 kDa | Enhanced antifungal and antimycotoxin activity against F. graminearum | [118] | |
| Citrus aurantium | Bitter orange essential oil | Inoculated potato dextrose agar (PDA) media for yeast and mold determination and inoculated plate count agar (PCA) for aerobic mesophilic and psychrophilic bacteria determination, determination of glutathione reductase (GR) and peroxidase (POD) activity | Monoterpenes, limone, pinene, synephrine alkaloids, limonoids, phytosterols, flavonoids including hesperidin, naringin and nobiletin | MediumMW CS, 190–310 KDa, viscosity: 200–800 cP, degree of deacetylation: 75–85% | Ionic gelation using TPP | Improved microbial safety and antioxidant enzymes activity (glutathione reductase (GR) and ascorbate peroxidase (APX)) of white button mushroom (Agaricusbisporus) | [176] |
| Citrus limon L. | Lemon essential oil | 22 compounds of which limone in the largest proportion, C-pinene, J-terpinene, p-cymene | Low MW with 75–85% DD and modified starch (Hi-cap) | Freeze-drying | Highest encapsulation efficiency and zeta potential with prolonged release value and improved stability | [177] | |
| Cinnamomum zeylanicum | C. zeylanicum essential oil | Antifungal assays performed with the pour-plate method | Cinnamaldehyde, benzaldehyde, (E)-cinnamyl acetate, limonene and eugenol | Medium MW with DD 75–85% | Ionic gelation | Reduction in severity and incidence of infectedcucumbers by Phytophthora drechsleri and enhancement of cucumber shelf life | [178] |
| Satureja hortensis L. | Summer savory essential oil | DPPH assay and antibacterial assay against E.coli, L. monocytogenes, S. aureus | Carvacrol, γ-terpinene and p-cymene | CS from crab shells, 85% deacylated | Emulsion and ionic gelation using TPP | Strong antibacterial activity against Staphylococcus aureus, Listeria monocytogenes and Escherichia coli and antioxidant activity | [179,180] |
| Siparuna guianensis | Siparuna guianensis essential oil | Bioassay for determination of toxic activity against Aedes aegypti larvae | Monoterpene β-, myrcene, sesquiterpene epicurzerenone, Germacrene D, γ-elemene, non-terpene acyclic ketone 2-undecanone | Viscosity-average MW CS with deacetylation degree 76.5% | Potential larvicide control against mosquito Aedes aegypti (vector ofinfectious diseases such as yellow fever, dengue, zika, and chikungunya) | [181] |
| Compound Name | Category | Plant Source | Biological Activity of the Phytochemical | Biological Study | Chitosan Characteristics | Preparation Method | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|
| Baicalein and Quercetin (separately tested) | Flavone/ Flavonoids/ polyphenols | Onions, many fruits, or in herbs | AQS (anti-quorum sensing) and antibiofilm activities of pure and nanoencapsulated compounds against the bioengineered E. coli Top10 biosensor | Stability test, in vitro release assay in the M9 bacterial growth medium, bacterial assays with E. coli Top10 biosensor QS assay, antibiofilm assay, cell viability assay, Mammalian cell (MDCK-C7) line cytotoxicity test using MTT assay | MW∼115,000 g/mol and DD∼42% | Preparation method of nanocapsules | Anti-quorum sensing activity against E. coli Top 10 and inhibition of biofilm formation | [98] |
| Kaempferol | Flavonol/flavonoids/polyphenols | Anti-inflammatory, anticancer and antioxidant activities | Modulation of QS (quorum sensing) mediated by AI (autoinducers) in model bioassay test systems, QS inhibition against C. violaceum CV026 with disc diffusion assay and quantitative determination of violacein inhibition, DPPH assay, FRAP, in vitro release and stability studies | 75–85% DD, low MW | Anionic gelation method using TPP | QS (based anti-biofilm) inhibitory against C.violaceum CV026, for effective antimicrobial chemotherapy, good stability | [99] | |
| Ferulic acid | Hydroxy- cinnamic acid/ polyphenols | Antibiofilm potential against C. albicans | Biocompatibility on hek-293 cell lines by MTT assay, Fesem and fluorescent microscopy, c. Albicans biofilm formation test with XTT assay and scanning electron microscopy | Medium MW (190e310 kDa) with 75–85% DD | Ionic gelation | Effective, safe and powerful antifungal (antibiofilm activity against C. albicans) agent | [101] | |
| Ferulic acid | Hydroxy- Cinnamic acid/ polyphenols | Various cereals, plants and fruits | Antioxidant and anticancer activities, antimicrobial, anti-inflammatory, cholesterol-lowering activities, thrombosis and atherosclerosis prevention, photoprotectiveactivity [132] against diabetes and neurological disorders, antimicrobial, and hepatoprotective activities, and protective effects against the UV, reduction in triglycerides and cholesterol [136] | In vitro antiproliferative potential against ME-180 human cervical cancer cell lines, cytocompatibility evaluation on HEK-293 cells (MTTassay and FESEM analysis) | Low MW (85% DD) | Ionic gelation | Potential therapeutic agent against cancer cells (ME-180 cell lines) proliferation due to apoptotic induction, enhanced cytocompatibility and solubility | [141,146] |
| Ferulic acid | Hydroxy- cinnamic acid/ polyphenols | Anti-diabetic effect due to its antioxidant capacity | In vitrorelease profile, in vivo pharmacokinetic study (in Wistar albino rats), anti-diabetic studies: Oral Glucose Tolerance Test (OGTT) on wistar albino rats, biochemical studies:blood glucose levels, lipid profile and plasma insulin estimation in rat by ELISA kit, histo- pathological study on pancreas of scarified rats | medium MW (190-310 kDa) | Ionic gelation using TPP | Extended plasma retention time, maximum plasma concentration and/or bioavailability and attenuation of the diabetes-associated symptoms | [146] | |
| Geraniol | Monoterpene alcohol | Coming from flowers and tissues of many herbs and essential oils (ninde, rose, palmarosa, citronella EO) | Antimicrobial, antioxidant, anti-inflammatory, and antitumor, repellent activity | Photostability and release assays in vitro at different temperatures, biological effects were investigated in whitefly (Bemisiatabaci). | CS/gum arabic nanoparticles, CS MW: 27 kDa; degree of deacetylation: 75−85% | Emulsification followed by ionic gelation | Good colloidal properties, improved stability from UV radiation, decreased degradation rates, significant attraction activity against whitefly with potential use in pest management | [133] |
| Ellagic acid (EA) | Hydroxy- Benzoic acid/ polyphenols | (generally) pomegranates, raspberries, strawberries, pecans, blackberries, several vegetables | Antioxidant, anti-proliferative, wound healing properties, coagulation promotion of blood.EA-CS-NPs activities: inhibition of the proliferation of glioblastoma, proliferation of melanoma cells and colorectal cancer cells, against oral cancer cell lines and able to promote apoptosis and DNA fragmentation | Blood clotting time analysis (WBCT) by the Lee-White method and the clot retraction, time (CRT) on rat blood, blood retraction time analysis | CS 85% deacetylated, 140 kDa | Ionic gelation using TPP | Synergism for anti- hemorrhagic activity, efficient promoting blood coagulation factor | [134] |
| Curcumin | Hydroxy- cinnamic acids/ polyphenols | Curcuma longa | Excellent antioxidantand antidiabetic properties | In vitro amylase inhibitory activity assay, in vivo antidiabetic assay intissues of rats | DD 78%, MW: 94 kDa; and viscosity = 3 m2/s | Ionic gelation using TPP and CS-alginate complex | More effective the CS-alginate- curcumin complex than CS-curcumin, significant reductions in hyperglycemia | [135] |
| Curcumin | Hydroxy- cinnamic acids/ polyphenols | Curcumalonga | Anti-cancer and anti-inflammatory properties, anti-bacterial, anti-parasitic and anti-malaria, antioxidant, metal chelating effects in metal toxicity | Evaluation of therapeutic efficacy in arsenic-induced toxic Wistar rats for 4 weeks with many assays | MW 400 kDa | Antioxidant and metal-chelating properties, stable detoxifyingagent for arsenic poisoning, neuroprotective efficacy | [140] | |
| Curcumin | Hydroxy- cinnamic acids/ polyphenols | Curcuma longa | Antioxidant, anti-inflammatory, anticarcinogenic/ antitumor, and antimicrobial properties | In vitro cytotoxicity assay on HeLa cells (human cervical cancer cell line),in vitrorelease studies | CS: MW ~50 kDa, 90% deacetylated | Tripolymeric composite of alginate (ALG), CS and pluronic. Preparation method: ionotropic pre-gelation followed by polycationic cross-linking | Suitable size distribution, drug encapsulation efficiency, and drug release kinetics in delivery of hydrophobic drugs | [147] |
| Curcumin | Hydroxy- cinnamic acids /polyphenols | Curcuma longa | Anticancer properties against breast cancer | Assay for intracellular uptake of curcumin and cell viability with MTT assay on breast cancer cell lines MCF-7 (Her2-) and MDA-MB-453(Her2+) and in vitro curcumin release | Silk fibroin (SF) and CS polymers, SFCS nanoparticles | Devised capillary-microdot technique | Weaker efficacy of SFCS nanoparticles againstbreast cancer cells (and potential for in vivo breast tumor treatment) than SF-curcumin nanoparticles (showed the highest curcumin entrapment, release, intracellular uptake and highest biological activity) | [148] |
| Curcumin | Hydroxy- cinnamic acids/ polyphenols | Blood lipid-lowering, anticoagulant, antioxidant, anticancer activities, various clinical applications | (1) Cytotoxicity and uptake by tumor cells | Deacetylation degree of CS: 95%, viscosity: 100–200 mPas | Ionic cross linking of folate- modified aminated CS (FA-AmCS-TPP) using TPP | (1) Slow and controlled release of curcumin at pH 7.4 (2) Suitable NPs to carry fat-soluble drugs (3) Possible good tumor-targeting effect Potent injectable agents | [149] | |
| Curcumin | Hydroxy- cinnamic acids/ polyphenols | Curcuma longa | Antioxidant, anti-inflammatory, antibacterial, anticancer activities particularly against colorectal cancer | (1) Ex vivo mucoadhesion study (2) In vitro effect of mucoadhesive interaction between the nanoparticles and colorectal cancer cells. | Low MW CS (75–85% deacetylated) | Ionic gelation using TPP | Better anticancer activity of NPs against colorectal cancer, improved cellular uptake compared to free curcumin | [150] |
| (-)- Epigallo- catechin- 3-gallate (EGCG) | Flavan-3-ols/ flavonoids/ polyphenols | Camelia sinensis | Excellent potential in treating/preventing many cancers including prostate cancer | In vivo antitumor efficacy assay on 22Rν1 tumor xenografts in athymic nude mice, PSA (prostate specific antigen) estimation by ELISA, immunohistochemical analysis, inhibition of cell proliferation markers and inhibition of angiogenesis markers assays in mice | water-soluble CS | Preparation in aqueous conditions using TPP | Inhibition of the growth of prostate cancer cells and secreted prostate-specific antigen levels | [136] |
| (-)-epigallo- Catechin gallate (EGCG) | Flavan-3-ols/ flavonoids/ polyphenols | Green tea | Generally antioxidant, anti-viral, anti-inflammatory, cardioprotective, neuro-protective and anti-cancer effect | Determination of the stability in the GIT (stomach and jejunum) and plasma exposure in mice | Enhanced oral delivery, plasma exposure, and therapeutic applicationin many diseases | [137] | ||
| Chlorogenic acid (CGA) | Hydroxy- cinnamic acid/ polyphenols | Generally apples, pears, berries, plum, vegetableslike sweet potato, lettuce, spinach, coffee beans, tea etc. | Anti-obese, anti-inflammatory, neuroprotective, anti-diabetic, antioxidant, anti-cancerous, radio protective, neuroprotectiveproperties, and also for treating Alzheimer’s disease, inhibit oxidation of LDL and thereforeprotect against cardiovascular diseases | In vitroantioxidant assay with ABTS, in vivo pharmacokinetics on wistar male rats | Low MW DD 86.6% | Ionic gelation using TPP | Controlled release profile, preserved antioxidant activity, increased bioavailability | [138] |
| Lupulone And xanthohumol | Beta-bitter acid and chalcones/ polyphenols respectively | Extract dried hopflowers of Humulus lupulus L. | Antimicrobial and antioxidant tests against a Gram-positive (Staphylococcus aureus) and Gram-negative bacterium (Pseudomonas aeruginosa) | First type of used CS: heterogeneous and of high molar weight, obtained by chemical deacetylation/ partial depolymerization of chitin, Second used CS: obtained through an enzymatic process | Ionotropic gelation method using TPP | Activity against several Gram-positive (S. Aureus), Gram-negative (P. Aeruginosa) and Candida strains, good stability | [141] | |
| Naringenin | Flavanone/ flavonoids/ polyphenols | Anti-inflammatory agent, significant antitumor effects with low toxicity | Antioxidant assays (nitrate scavenging, DPPH, hydroxyl radical scavenging assay), cell cytotoxicity in lung cancer cells by MTT | Ionic gelation using TPP | Significant antioxidant and anticancer (against A549 lung cancer cells) activity | [142] | ||
| Rosmarinic acid | Hydroxycinnamic acids/polyphenols | Salvia officinalis (sage) and Saturejamontana extracts and many more | Treatment of vasoproliferative retinopathies, anti-angiogenic activity to retinal neovascularization a mouse model of retinopathy with no retinal toxicity, antioxidant | Mucoadhesion proprieties evaluation by mucin interaction method, cell viabilityon ARPE-19 and HCE-T cell lines and cytotoxicity (using chorioallantoic membrane), permeability studies in cells, transepithelial electrical resistance | low MW (≈50 kDa) with DD 86%, | Ionic gelation using TPP | Efficient drug delivery systems for ocular application in oxidative eye conditions | [143] |
| Baicalin | Flavonoid | Glucoronide of baicalein (Chinese herbal medicine) | Among others, anti-inflammatory, antihypertensive, antifungal, antioxidant, neuroprotective | In vivo biodistribution | CS oligosaccharide lactate (CL, Mn = 5000, deacetylation degree > 90%) | Ionic gelation using TPP | Ιncrease local bioavailability of therapeutic agents in the liver | [151] |
| Resveratrol | Stilbene/polyphenol | Mainly derived from Polygonum cuspidatum, grapes and peanuts | Antitumor, antioxidative, anti-bacterial, anti-inflammatory effects, providesprotection against cardiovascular and hepatic diseasesand participates in immune regulation | In vitro DPPH assay, in vitro release assay, in vivo bioavailability studies in Sprague- Dawley (SD) rats | Carboxymethyl CS MW = 14.2 × 104 Da | Emulsion cross-linking method | Increased absorption, prolonged duration of action and increased relative bioavailability | [152] |
| Genistein | Isoflavonoid phytoestrogen | Antioxidant and neuroprotective activity | Ex vivo permeation studies on nasal mucosa, in vitro cytotoxicity studies | Chitoclear® 1360, MW 35 kDa, 96% deacetylated | Ionic gelation with sodium hexametaphosphate as cross-linker | Enhanced genistein penetration through the nasal mucosa | [153] | |
| Eugenol | Phenol | Antimicrobial and antioxidant properties | DPPH method | DD 0.95 and MW of ∼760 kDa | Ionic gelation of an oil-in-water emulsion using TPP | Potential antioxidants for various thermal processing applications | [154] | |
| Berberine (chloride) | Isoquinoline alkaloid | From Rhizoma Coptidis (Huanglian in Chinese) | Osteoarthritis (OA) treatment | Histological analysis, TUNEL staining assay, quantitative real-time polymerase chain reaction, Western blot, and immunohistochemical methods on male Sprague- Dawley rats analyses of caspase-3, Bcl-2 and Bax expressions, in vitro release assay | Mw = 9.0 × 105, DD 90% | Ionic cross-linking method using sodium TPP | Effective anti- 439 apoptosis activity in the rat OA model, potential therapeutic agent for OA | [155] |
| Berberine | Isoquinoline alkaloid | Berberis vulgaris, Berberis aristata, Berberis petiolaris, Berberisaquifolium, Berberis asiatica, Berberis thunbergii, Coptisteeta, Coptischinensis, Hydrastis canadensis, Phellodendronamurense and Caulis mahoniae | Anti-viral, anti-microbial, anti-diarrhea, anti-inflammatoryand anti-tumor, anti-diabetic, glycolysis stimulator and mitochondrial functioninhibitor, improved lipid and glucose metabolism, for heart failure, cardiac arrhythmia and hypertension | (1) Nano-hydroxyapatite/CS (n-HA/CS) (2) Fucose-CS/heparin nanoparticles (3) CS and fucoidan-taurine (FD-Tau) conjugates (4) CSNPs | (1) Treating bone defects (2) Activity against Helicobacter pylori (3) Inhibition of the redistribution of tight junction (TJ) ZO-1 protein and improved intestinal epithelial TJ disruption (4) Protective against osteoarthritis | [156] | ||
| 1-naphthalenyl [4 -(pentyloxy)-1-naphthalenyl]methanone (CB13) | Cannabinoid derivative | Analgesic in chronic pain with less penetration into brain | In vitro drug release, human blood compatibility test, uptake assay on THP1 cells and MTT assay on human colon adenocarcinoma cells, Caco-2 cells(ECACC) | low MW 67,000 g/mol, 75–85% deacetylated, | Polymeric poly (lactic-co-glycolic) acid (PLGA) and lipid nanoparticles (LNPs) surfaces have been modified with CS | Adequate blood compatibility and absence of cytotoxicity, good oral carriers for CB13 | [157] |
| Encapsulated Product | Coating | Modification | Chitosan Characteristics | Outcome | Ref. |
|---|---|---|---|---|---|
| Curcumin | Curcumin-loaded SLNs with chitosan coating | Down-regulation of P-glycoprotein expression and rescue doxorubicin efficacy against resistant triple negative breast cancer (TNBC) tumors | [189] | ||
| Paliperidone | Paliperidone-loaded PCL nanoparticles with chitosan coating | MW: 100–300 kDa | Minimization of stabilizer induced cytotoxicity, cytokine secretion, and oxidative stress response | [190] | |
| Resveratrol | Resveratrol-loaded Lipid microparticles with chitosan coating | Medium MW; 190–310 kDa; Viscosity 200–800 cP, 1 wt. % in 1% acetic acid, 25 °C, DD 75–85% | Enhancement of the targeting of resveratrol to the brain via nasal administration | [192] | |
| Butyric acid | Chitosan-coated liposomes loaded with butyric acid | Increased cytotoxic activity and important anti-inflammatory effects by inhibiting production of cytokines with a central role in liver cell survival. | [204] | ||
| Silver | Chitosan–silver nanoparticles | Low MW; DD 75–85% | Chitosan hybrid silver nanoparticles with antimicrobial potency | [208] | |
| Curcumin | Chitosan-coated nanoemulsion | High MW: 190,000–310,000; DD: 85% Medium MW: 30,000; DD: 89.2% Low MW: 3000; DD: 90% | Preparation of a promising delivery system to promote the applications of curcumin in functional food and beverage system | [209] | |
| Quercetin | Chitosan and GACh as coating material for the microencapsulation of Quercetin | Modified chitosan with glucosamine by Maillard reaction (GACh) | Μedium MW (583 kDa) DD 78% | Enhancement of the bioavailability and antioxidant properties of Quercetin | [210] |
| Amphotericin B and doxorubicin | Chitosan-coated PLGA nanoparticles | Lower cytotoxicity against toward macrophages and active against leishmania in vitro | [211] | ||
| Ferulic acid | Chitosan-coated PLGA nanoparticles | Medium MW; DD 75–85% | Promising carriers for oral delivery of Ferulic acid | [212] | |
| Forskolin | Chitosan-coated PLGA nanoparticles | MW: around 110 kDa; DD 96%; viscosity 15cp | Excellent vehicle for forskolin in ocular delivery | [213] | |
| Bovine serum proteins | HA coating of CSNPs and alginate coating of CSNPs | DD > 60% mol, from white mushrooms | Different surface chemistry HA–CSNPs less immunogenic HA–CSNPs adsorb anti-inflammatory proteins Alginate–CSNPs adsorb proinflammatory | [218] | |
| - | HA coating of CSNPs and Alginate coating of CSNPs | DD > 60% mol, from white mushrooms | Optimum cryoprotectant and its concentration for the stability of nanoparticles during freeze-drying process | [219] | |
| - | - | Medium MW and high MW | The size and zeta potential of the particles affect the cytotoxicity | [220] | |
| Curcumin | Nanoliposomes prepared from salmon purified phospholipid coated with chitosan | From shrimp shells, practical grade DD up to 75% | Increase in the dispersion stability Improvement of mechanical stability | [221] | |
| Forskolin | Chitosan–PLGA nanoparticles prepared with emulsion-sonication process | MW: around 110 kDa; DD 96%; viscosity 15cp | Prolonged retention increased effectiveness in reducing the intraocular pressure Ocular tolerance confirmed ex-vivo and in vivo | [213] | |
| Bovine serum albumin | Carboxymethyl-β-CD grafted on chitosan | MW: 4.6 × 104 DD approximately 90–95% | Prolongation of release profiles in simulated intestinal fluid and simulated colonic fluid | [229] | |
| Carvacrol or linalool | Chitosan glycol functionalized with β-CD ICs | Chitosan glycol | Chitosan glycol (≥60% titration), LMW Chitosan | - Repellency and acaricidal effect oviposition activity against Tetranychusurticae | [232] |
| Carvacrol and linalool | Chitosan glycol functionalized with β-CD ICs chitosan nanoparticles crosslinked with gum arabic | Chitosan glycol | Chitosan glycol (≥60% titration; degree of polymerization ≥400) | Decreased toxicity 1. Insecticidal activity against Helicover paarmigera and Tetranychusurticae | [233] |
| Curcumin | Consecutive coatings of Fe3O4 magnetic nanoparticles with PLGA and CS layers | Enhanced anticancer effect - 3–6 fold increase in the uptake of curcumin from cancer cells Lower uptake of curcumin from the non-cancer model cells HDF | [239] | ||
| Resveratrol | Chitosan: Oleic acid micelles coated with PLGA | Chitosan oleate | Low MW; DD 80% | 1. Strong cytotoxic activity against both the colonic adenocarcinoma and the human cervical cancer cell lines | [242] |
| Silibinin | Chitosan nanoparticles incorporated in Alginate/Gelatin scaffolds | Low MW; DD 75–85% | Prolongation of release profile Increase in bioavailability 2. osteo-conductive and osteo-inductive properties | [243] | |
| Thymol | Chitosan nanoparticles immobilised in edible chitosan-quinoa protein films | Medium WC; DD 88.5% Low WC; viscosity reduction (ηsp/c) = 203 (mL/g), viscosity average molar mass MW = 269 kDa DD 78.3% | Food packaging extended the shelf-life of the food tested | [244] | |
| Moringa oil | Chitosan nanoparticles immobilised in gelatin nanofibers | 85% deacylated | Decreased the hydrophilicity of the system and the permeability of water vapor antimicrobial activity against Listeria monocytogenes and Staphylococcus aureus | [245] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix. Pharmaceutics 2020, 12, 669. https://doi.org/10.3390/pharmaceutics12070669
Detsi A, Kavetsou E, Kostopoulou I, Pitterou I, Pontillo ARN, Tzani A, Christodoulou P, Siliachli A, Zoumpoulakis P. Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix. Pharmaceutics. 2020; 12(7):669. https://doi.org/10.3390/pharmaceutics12070669
Chicago/Turabian StyleDetsi, Anastasia, Eleni Kavetsou, Ioanna Kostopoulou, Ioanna Pitterou, Antonella Rozaria Nefeli Pontillo, Andromachi Tzani, Paris Christodoulou, Aristeia Siliachli, and Panagiotis Zoumpoulakis. 2020. "Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix" Pharmaceutics 12, no. 7: 669. https://doi.org/10.3390/pharmaceutics12070669
APA StyleDetsi, A., Kavetsou, E., Kostopoulou, I., Pitterou, I., Pontillo, A. R. N., Tzani, A., Christodoulou, P., Siliachli, A., & Zoumpoulakis, P. (2020). Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix. Pharmaceutics, 12(7), 669. https://doi.org/10.3390/pharmaceutics12070669