Protein Expression Knockdown in Cancer Cells Induced by a Gemini Cationic Lipid Nanovector with Histidine-Based Polar Heads
Abstract
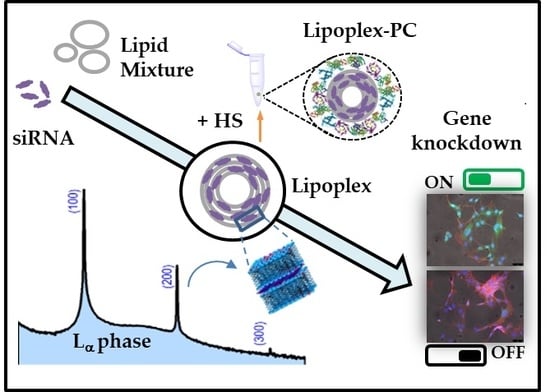
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Lipoplexes
2.3. Electrochemical study. Ζ Potential and Agarose Gel Electrophoresis
2.4. Structure Study. SAXS and Cryo-TEM
2.5. In Vitro Evaluation
2.5.1. Cell Culturing
2.5.2. Cytotoxicity, Epifluorescence Microscopy and Flow Cytometry
2.6. Protein Ccorona Studies
3. Results and Discussion
3.1. Electrochemical Study. Ζ Potential and Agarose Gel Electrophoresis
3.2. Structural Study. Cryo-TEM and SAXS
3.3. In Vitro Studies
3.4. Protein Corona Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dunbar, C.E.; High, K.A.; Joung, J.K.; Kohn, D.B.; Ozawa, K.; Sadelain, M. Gene therapy comes of age. Science 2018, 359, eaan4672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szybalski, W. The 50th anniversary of gene therapy: Beginnings and present realities. Gene 2013, 525, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Mangala, L.S.; Rodriguez-Aguayo, C.; Kong, X.C.; Lopez-Berestein, G.; Sood, A.K. RNA interference-based therapy and its delivery systems. Cancer Metastasis Rev. 2018, 37, 107–124. [Google Scholar] [CrossRef]
- Kim, D.H.; Rossi, J.J. Strategies for silencing human disease using RNA interference. Nat. Rev. Genet. 2007, 8, 173–184. [Google Scholar] [CrossRef]
- Liu, F.; Wang, C.F.; Gao, Y.T.; Li, X.; Tian, F.; Zhang, Y.T.; Fu, M.Y.; Li, P.F.; Wang, Y.L.; Wang, F. Current transport systems and clinical applications for small interfering RNA (siRNA) drugs. Mol. Diagn. Ther. 2018, 22, 551–569. [Google Scholar] [CrossRef]
- Nayak, S.; Herzog, R.W. Progress and prospects: Immune responses to viral vectors. Gene Ther. 2010, 17, 295–304. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.E.; Ehrhardt, A.; Kay, M.A. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003, 4, 346–358. [Google Scholar] [CrossRef]
- Wang, X.Y.; Tai, Z.G.; Tian, J.; Zhang, W.; Yao, C.; Zhang, L.J.; Gao, Y.; Zhu, Q.G.; Gao, J.; Gao, S. Reducible chimeric polypeptide consisting of octa-D-arginine and tetra-L-histidine peptides as an efficient gene delivery vector. Int. J. Nanomed. 2015, 10, 4669–4690. [Google Scholar] [CrossRef] [Green Version]
- Lehto, T.; Kurrikoff, K.; Langel, U. Cell-penetrating peptides for the delivery of nucleic acids. Expert Opin. Drug Deliv. 2012, 9, 823–836. [Google Scholar] [CrossRef]
- Liu, Z.H.; Li, M.Y.; Cui, D.F.; Fei, J. Macro-branched cell-penetrating peptide design for gene delivery. J. Control. Release 2005, 102, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-w.; Lee, J.-j.; Choi, J.S.; Kim, H.-S. Electrostatically assembled dendrimer complex with a high-affinity protein binder for targeted gene delivery. Int. J. Pharm. 2018, 544, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Y.T.; Wang, H.; Shao, N.M.; Chen, Y.Y.; Cheng, Y.Y. Synergistic effect of amino acids modified on dendrimer surface in gene delivery. Biomaterials 2014, 35, 9187–9198. [Google Scholar] [CrossRef] [PubMed]
- Leng, Q.X.; Chou, S.T.; Scaria, P.V.; Woodle, M.C.; Mixson, A.J. Increased tumor distribution and expression of histidine-rich plasmid polyplexes. J. Gene Med. 2014, 16, 317–328. [Google Scholar] [CrossRef] [Green Version]
- Chou, S.T.; Hom, K.; Zhang, D.; Leng, Q.; Tricoli, L.J.; Hustedt, J.M.; Lee, A.; Shapiro, M.J.; Seog, J.; Kahn, J.D.; et al. Enhanced silencing and stabilization of siRNA polyplexes by histidine-mediated hydrogen bonds. Biomaterials 2014, 35, 846–855. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.T.; Yi, W.J.; Su, R.C.; Liu, Q.; Zhao, Z.G. Reducible amino acid based cationic lipids as highly efficient and serum-tolerant gene vectors. ChemPlusChem 2016, 81, 125–134. [Google Scholar] [CrossRef]
- Obata, Y.; Suzuki, D.; Takeoka, S. Evaluation of cationic assemblies constructed with amino acid based lipids for plasmid DNA delivery. Bioconjugate Chem. 2008, 19, 1055–1063. [Google Scholar] [CrossRef]
- Martínez-Negro, M.; Sánchez-Arribas, N.; Guerrero-Martínez, A.; Moyá, M.L.; de Ilarduya, C.T.; Mendicuti, F.; Aicart, E.; Junquera, E. A non-viral plasmid DNA delivery system consisting on a lysine-derived cationic lipid mixed with a fusogenic lipid. Pharmaceutics 2019, 11, 632. [Google Scholar] [CrossRef] [Green Version]
- Damen, M.; Cristobal-Lecina, E.; Sanmarti, G.C.; van Dongen, S.F.M.; Garcia Rodriguez, C.L.; Dolbnya, I.P.; Nolte, R.J.M.; Feiters, M.C. Structure-delivery relationships of lysine-based gemini surfactants and their lipoplexes. Soft Matter 2014, 10, 5702–5714. [Google Scholar] [CrossRef]
- Su, R.-C.; Liu, Q.; Yi, W.-J.; Zheng, L.-T.; Zhao, Z.-G. Lipoic acid functionalized amino acids cationic lipids as gene vectors. Bioorg. Med. Chem. Lett. 2016, 26, 4692–4697. [Google Scholar] [CrossRef]
- Midoux, P.; Pichon, C.; Yaouanc, J.J.; Jaffres, P.A. Chemical vectors for gene delivery: A current review on polymers, peptides and lipids containing histidine or imidazole as nucleic acids carriers. Br. J. Pharmacol. 2009, 157, 166–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.V.; Pichon, C.; Refregiers, M.; Guerin, B.; Midoux, P.; Chaudhuri, A. Single histidine residue in head-group region is sufficient to impart remarkable gene transfection properties to cationic lipids: Evidence for histidine-mediated membrane fusion at acidic pH. Gene Ther. 2003, 10, 1206–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kichler, A.; Leborgne, C.; Marz, J.; Danos, O.; Bechinger, B. Histidine-rich amphipathic peptide antibiotics promote efficient delivery of DNA into mammalian cells. Proc. Natl. Acad. Sci. USA 2003, 100, 1564–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mevel, M.; Breuzard, G.; Yaouanc, J.J.; Clement, J.C.; Lehn, P.; Pichon, C.; Jaffres, P.A.; Midoux, P. Synthesis and transfection activity of new cationic phosphoramidate lipids: High efficiency of an imidazolium derivative. ChemBioChem 2008, 9, 1462–1471. [Google Scholar] [CrossRef]
- Karmali, P.P.; Majeti, B.K.; Sreedhar, B.; Chaudhuri, A. In vitro gene transfer efficacies and serum compatibility profiles of novel mono-, di-, and tri-histidinylated cationic transfection lipids: A structure-activity investigation. Bioconjugate Chem. 2006, 17, 159–171. [Google Scholar] [CrossRef]
- Martínez-Negro, M.; Blanco-Fernández, L.; Tentori, P.M.; Pérez, L.; Pinazo, A.; de Ilarduya, C.T.; Aicart, E.; Junquera, E. A gemini cationic lipid with histidine residues as a novel lipid-based gene nanocarrier: A biophysical and biochemical study. Nanomaterials 2018, 8, 1061. [Google Scholar] [CrossRef] [Green Version]
- Junquera, E.; Aicart, E. Recent progress in gene therapy to deliver nucleic acids with multivalent cationic vectors. Adv. Colloid Interface Sci. 2016, 233, 161–175. [Google Scholar] [CrossRef]
- Junquera, E.; Aicart, E. Cationic lipids as transfecting agents of DNA in gene therapy. Curr. Top. Med. Chem. 2014, 14, 649–663. [Google Scholar] [CrossRef]
- Adami, R.C.; Seth, S.; Harvie, P.; Johns, R.; Fam, R.; Fosnaugh, K.; Zhu, T.Y.; Farber, K.; McCutcheon, M.; Goodman, T.T.; et al. An amino acid-based amphoteric liposomal delivery system for systemic administration of siRNA. Mol. Ther. 2011, 19, 1141–1151. [Google Scholar] [CrossRef]
- Suh, M.S.; Shim, G.; Lee, H.Y.; Han, S.E.; Yu, Y.H.; Choi, Y.; Kim, K.; Kwon, I.C.; Weon, K.Y.; Kim, Y.B.; et al. Anionic amino acid-derived cationic lipid for siRNA delivery. J. Control. Release 2009, 140, 268–276. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Meng, Y.; Zhao, Y.N.; Zhu, J.; Xu, H.; Zhang, E.X.; Shi, L.; Du, L.Y.; Liu, G.L.; Zhang, C.M.; et al. Toxicological exploration of peptide-based cationic liposomes in siRNA delivery. Colloids Surf. B 2019, 179, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Ibaraki, H.; Kanazawa, T.; Kurano, T.; Oogi, C.; Takashima, Y.; Seta, Y. Anti-RelA siRNA-encapsulated flexible liposome with tight junction-opening peptide as a non-invasive topical therapeutic for atopic dermatitis. Biol. Pharm. Bull. 2019, 42, 1216–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobbs, W.; Heinrich, B.; Bourgogne, C.; Donnio, B.; Terazzi, E.; Bonnet, M.E.; Stock, F.; Erbacher, P.; Bolcato-Bellemin, A.L.; Douce, L. Mesomorphic imidazolium salts: New vectors for efficient siRNA transfection. J. Am. Chem. Soc. 2009, 131, 13338–13346. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Kamel, A.O.; Wettig, S.D. Interactions between DNA and gemini surfactant: Impact on gene therapy: Part I. Nanomedicine 2016, 11, 289–306. [Google Scholar] [CrossRef]
- Ahmed, T.; Kamel, A.O.; Wettig, S.D. Interactions between DNA and gemini surfactant: Impact on gene therapy: Part II. Nanomedicine 2016, 11, 403–420. [Google Scholar] [CrossRef]
- Martínez-Negro, M.; Barrán-Berdón, A.L.; Aicart-Ramos, C.; Moyá, M.L.; de Ilarduya, C.T.; Aicart, E.; Junquera, E. Transfection of plasmid DNA by nanocarriers containing a gemini cationic lipid with an aromatic spacer or its monomeric counterpart. Colloids Surf. B 2018, 161, 519–527. [Google Scholar] [CrossRef]
- Zhou, T.; Llizo, A.; Li, P.; Wang, C.X.; Guo, Y.Y.; Ao, M.Q.; Bai, L.L.; Wang, C.; Yang, Y.L.; Xu, G.Y. High transfection efficiency of homogeneous DNA nanoparticles induced by imidazolium gemini surfactant as nonviral vector. J. Phys. Chem. C 2013, 117, 26573–26581. [Google Scholar] [CrossRef]
- Martínez-Negro, M.; Kumar, K.; Barrán-Berdón, A.L.; Datta, S.; Kondaiah, P.; Junquera, E.; Bhattacharya, S.; Aicart, E. Efficient cellular knockdown mediated by siRNA nanovectors of gemini cationic lipids having delocalizable headgroups and oligo-oxyethylene spacers. ACS Appl. Mat. Interfaces 2016, 8, 22113–22126. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Manzanares, D.; Cena, V. Endocytosis: The nanoparticle and submicron nanocompounds gateway into the cell. Pharmaceutics 2020, 12, 371. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, C.V.; Wachter, W.; Iglesias-Salto, G.; Engelskirchen, S.; Ahualli, S. Monoolein: A magic lipid? Phys. Chem. Chem. Phys. 2011, 13, 3004–3021. [Google Scholar] [CrossRef] [PubMed]
- Leal, C.; Bouxsein, N.F.; Ewert, K.K.; Safinya, C.R. Highly efficient gene silencing activity of siRNA embedded in a nanostructured gyroid cubic lipid matrix. J. Am. Chem. Soc. 2010, 132, 16841–16847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Arribas, N.; Martínez-Negro, M.; Villar-Álvarez, E.; Pérez, L.; Aicart, E.; Taboada, P.; Guerrero-Martínez, A.; Junquera, E. Biocompatible nanovector of siRNA consisting of arginine-based cationic lipid for gene knockdown in cancer cells. ACS Appl. Mat. Interfaces 2020. [Google Scholar] [CrossRef]
- Walczyk, D.; Bombelli, F.B.; Monopoli, M.P.; Lynch, I.; Dawson, K.A. What the cell “sees” in bionanoscience. J. Am. Chem. Soc. 2010, 132, 5761–5768. [Google Scholar] [CrossRef] [PubMed]
- Cedervall, T.; Lynch, I.; Lindman, S.; Berggard, T.; Thulin, E.; Nilsson, H.; Dawson, K.A.; Linse, S. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 2007, 104, 2050–2055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caracciolo, G.; Palchetti, S.; Colapicchioni, V.; Digiacomo, L.; Pozzi, D.; Capriotti, A.L.; La Barbera, G.; Laganà, A. Stealth effect of biomolecular corona on nanoparticle uptake by immune cells. Langmuir 2015, 31, 10764–10773. [Google Scholar] [CrossRef] [PubMed]
- Mirshafiee, V.; Mahmoudi, M.; Lou, K.; Cheng, J.; Kraft, M.L. Protein corona significantly reduces active targeting yield. Chem. Commun. 2013, 49, 2557–2559. [Google Scholar] [CrossRef]
- Corbo, C.; Molinaro, R.; Parodi, A.; Furman, N.E.T.; Salvatore, F.; Tasciotti, E. The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. Nanomedicine 2016, 11, 81–100. [Google Scholar] [CrossRef] [Green Version]
- Kelly, P.M.; Aberg, C.; Polo, E.; O’Connell, A.; Cookman, J.; Fallon, J.; Krpetic, Z.; Dawson, K.A. Mapping protein binding sites on the biomolecular corona of nanoparticles. Nat. Nanotechnol. 2015, 10, 472–479. [Google Scholar] [CrossRef]
- Martínez-Negro, M.; Caracciolo, G.; Palchetti, S.; Pozzi, D.; Capriotti, A.L.; Cavaliere, C.; Lagana, A.; Ortiz Mellet, C.; Benito, J.M.; García Fernandez, J.M.; et al. Biophysics and protein corona analysis of Janus cyclodextrin-DNA nanocomplexes. Efficient cellular transfection on cancer cells. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1737–1749. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Caracciolo, G.; Caruso, G.; Foglia, P.; Pozzi, D.; Samperi, R.; Lagana, A. Differential analysis of "protein corona" profile adsorbed onto different nonviral gene delivery systems. Anal. Biochem. 2011, 419, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Corbo, C.; Molinaro, R.; Taraballi, F.; Furman, N.E.T.; Sherman, M.B.; Parodi, A.; Salvatore, F.; Tasciotti, E. Effects of the protein corona on liposome-liposome and liposome-cell interactions. Int. J. Nanomed. 2016, 11, 3049–3063. [Google Scholar] [CrossRef] [Green Version]
- Barrán-Berdón, A.L.; Muñoz-Úbeda, M.; Aicart-Ramos, C.; Pérez, L.; Infante, M.R.; Castro-Hartmann, P.; Martín-Molina, A.; Aicart, E.; Junquera, E. Ribbon-type and cluster-type lipoplexes constituted by a chiral lysine based cationic gemini lipid and plasmid DNA. Soft Matter 2012, 8, 7368–7380. [Google Scholar] [CrossRef]
- Lasic, D.D.; Templeton, N.S. Liposomes in gene therapy. Adv. Drug Deliv. Rev. 1996, 20, 221–266. [Google Scholar] [CrossRef]
- Rodriguez-Pulido, A.; Aicart, E.; Llorca, O.; Junquera, E. Compaction process of calf thymus DNA by mixed cationic-zwitterionic liposomes: A physicochemical study. J. Phys. Chem. B 2008, 112, 2187–2197. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Muñoz-Úbeda, M.; Datta, S.; Barrán-Berdón, A.L.; Aicart-Ramos, C.; Castro-Hartmann, P.; Kondaiah, P.; Junquera, E.; Bhattacharya, S.; Aicart, E. Effects of a delocalizable cation on the headgroup of gemini lipids on the lipoplex-type nano-aggregates directly formed from plasmid DNA. Biomacromolecules 2013, 14, 3951–3963. [Google Scholar] [CrossRef]
- Muñoz-Úbeda, M.; Misra, S.K.; Barrán-Berdón, A.L.; Datta, S.; Aicart-Ramos, C.; Castro-Hartmann, P.; Kondaiah, P.; Junquera, E.; Bhattacharya, S.; Aicart, E. How does the spacer length of cationic gemini lipids influence the lipoplex formation with plasmid DNA? Physicochemical and biochemical characterizations and their relevance in gene therapy. Biomacromolecules 2012, 13, 3926–3937. [Google Scholar] [CrossRef]
- Muñoz-Úbeda, M.; Misra, S.K.; Barrán-Berdón, A.L.; Aicart-Ramos, C.; Sierra, M.B.; Biswas, J.; Kondaiah, P.; Junquera, E.; Bhattacharya, S.; Aicart, E. Why is less cationic lipid required to prepare lipoplexes from plasmid DNA than linear DNA in gene therapy? J. Am. Chem. Soc. 2011, 133, 18014–18017. [Google Scholar] [CrossRef]
- Bednar, J.; Woodcock, C.L. Chromatin; Academic Press Inc.: San Diego, CA, USA, 1999; Volume 304, pp. 191–213. [Google Scholar]
- Llorca, O.; McCormack, E.; Hynes, G.; Grantham, J.; Cordell, J.; Carrascosa, J.L.; Willison, K.R.; Fernández, J.J.; Valpuesta, J.M. Eukaryotic type II chaperonin CCT interacts with actin through specific subunits. Nature 1999, 402, 693–696. [Google Scholar] [CrossRef]
- Dubochet, J.; Adrian, M.; Chang, J.J.; Homo, J.C.; Lepault, J.; McDowall, A.W.; Schultz, P. Cryo-electron microscopy of vitrified specimens. Q. Rev. Biophys. 1988, 21, 129–228. [Google Scholar] [CrossRef] [Green Version]
- Seoane, M.; Iglesias, P.; Gonzalez, T.; Dominguez, F.; Fraga, M.; Aliste, C.; Forteza, J.; Costoya, J.A. Retinoblastoma loss modulates DNA damage response favoring tumor progression. PLoS ONE 2008, 3, e3632. [Google Scholar] [CrossRef] [PubMed]
- Kantner, K.; Rejman, J.; Kraft, K.V.L.; Soliman, M.G.; Zyuzin, M.V.; Escudero, A.; del Pino, P.; Parak, W.J. Laterally and temporally controlled intracellular staining by light-triggered release of encapsulated fluorescent markers. Chem. Eur. J. 2018, 24, 2098–2102. [Google Scholar] [CrossRef] [PubMed]
- Barrán-Berdón, A.L.; Misra, S.K.; Datta, S.; Muñoz-Úbeda, M.; Kondaiah, P.; Junquera, E.; Bhattacharya, S.; Aicart, E. Cationic gemini lipids containing polyoxyethylene spacers as improved transfecting agents of plasmid DNA in cancer cells. J. Mater. Chem. B 2014, 2, 4640–4652. [Google Scholar] [CrossRef] [PubMed]
- Tassler, S.; Woelk, C.; Janich, C.; Dobner, B.; Brezesinski, G. Lysine-based amino-functionalized lipids for gene transfection: The protonation state in monolayers at the air-liquid interface. Phys. Chem. Chem. Phys. 2017, 19, 20271–20280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, K.; Barrán-Berdón, A.L.; Datta, S.; Muñoz-Úbeda, M.; Aicart-Ramos, C.; Kondaiah, P.; Junquera, E.; Bhattacharya, S.; Aicart, E. A delocalizable cationic headgroup together with an oligo-oxyethylene spacer in gemini cationic lipids improves their biological activity as vectors of plasmid DNA. J. Mater. Chem. B 2015, 3, 1495–1506. [Google Scholar] [CrossRef]
- Badwaik, V.D.; Aicart, E.; Mondjinou, Y.A.; Johnson, M.A.; Bowman, V.D.; Thompson, D.H. Structure-property relationship for in vitro siRNA delivery performance of cationic 2-hydroxypropyl-beta-cyclodextrin: PEG-PPG-PEG polyrotaxane vectors. Biomaterials 2016, 84, 86–98. [Google Scholar] [CrossRef] [Green Version]
- Caracciolo, G.; Pozzi, D.; Amici, A.; Amenitsch, H. Universality of DNA adsorption behavior on the cationic membranes of nanolipoplexes. J. Phys. Chem. B 2010, 114, 2028–2032. [Google Scholar] [CrossRef]
- Tanford, C. The Hydrophobic Effect: Formation of Micelles and Biological Membranes; Wiley & Sons: New York, NY, USA, 1980. [Google Scholar] [CrossRef]
- Tanford, C. Micelle shape and size. J. Phys. Chem. 1972, 76, 3020–3024. [Google Scholar] [CrossRef]
- Tanford, C. Theory of micelle formation in aqueous solutions. J. Phys. Chem. 1974, 78, 2469–2479. [Google Scholar] [CrossRef]
- Schroeder, A.; Levins, C.G.; Cortez, C.; Langer, R.; Anderson, D.G. Lipid-based nanotherapeutics for siRNA delivery. J. Intern. Med. 2009, 267, 9–21. [Google Scholar] [CrossRef]
- Motta, S.; Rondelli, V.; Cantu, L.; Del Favero, E.; Aureli, M.; Pozzi, D.; Caracciolo, G.; Brocca, P. What the cell surface does not see: The gene vector under the protein corona. Colloids Surf. B 2016, 141, 170–178. [Google Scholar] [CrossRef]
- Jiang, Q.; Yue, D.; Nie, Y.; Xu, X.H.; He, Y.Y.; Zhang, S.Y.; Wagner, E.; Gu, Z.W. Specially-made lipid-based assemblies for improving transmembrane gene delivery: Comparison of basic amino acid residue rich periphery. Mol. Pharm. 2016, 13, 1809–1821. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Xu, X.H.; Li, H.P.; Jian, Y.T.; Wang, G.; He, B.; Gu, Z.W. Components simulation of viral envelope via amino acid modified chitosans for efficient nucleic acid delivery: In vitro and in vivo study. Adv. Funct. Mater. 2013, 23, 2691–2699. [Google Scholar] [CrossRef]
- Ju, J.; Huan, M.L.; Wan, N.; Qiu, H.; Zhou, S.Y.; Zhang, B.L. Novel cholesterol-based cationic lipids as transfecting agents of DNA for efficient gene delivery. Int. J. Mol. Sci. 2015, 16, 5666–5681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Q.; Huang, J.; Feng, Q.; Chen, X.; Liu, X.; Li, X.; Zhang, T.; Xiao, S.; Li, H.; Zhong, Z.; et al. Size- and cell type-dependent cellular uptake, cytotoxicity and in vivo distribution of gold nanoparticles. Int. J. Nanomed. 2019, 14, 6957–6970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labouta, H.I.; Sarsons, C.; Kennard, J.; Gomez-Garcia, M.J.; Villar, K.; Lee, H.; Cramb, D.T.; Rinker, K.D. Understanding and improving assays for cytotoxicity of nanoparticles: What really matters? RSC Adv. 2018, 8, 23027–23039. [Google Scholar] [CrossRef] [Green Version]
- Poncelet, P.; Besson-Faure, I.; Lavabre-Bertrand, T. Clinical Applications of Quantitative Immunophenotyping. In Immunophenotyping; Stewart, C., Nicholson, J., Eds.; John Wiley & Sons: Toronto, ON, Canada, 2000. [Google Scholar]
- Walkey, C.D.; Olsen, J.B.; Guo, H.B.; Emili, A.; Chan, W.C.W. Nanoparticle Size and Surface Chemistry Determine Serum Protein Adsorption and Macrophage Uptake. J. Am. Chem. Soc. 2012, 134, 2139–2147. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Grenier, P.; Mahmoudi, M.; Lima, E.M.; Appel, E.A.; Dormont, F.; Lim, J.M.; Karnik, R.; Langer, R.; Farokhzad, O.C. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat. Commun. 2017, 8, 8. [Google Scholar] [CrossRef]
- Kreuter, J.; Shamenkov, D.; Petrov, V.; Ramge, P.; Cychutek, K.; Koch-Brandt, C.; Alyautdin, R. Apolipoprotein-mediated transport of nanoparticle-bound drugs across the blood-brain barrier. J. Drug Target. 2002, 10, 317–325. [Google Scholar] [CrossRef]
- Saha, K.; Rahimi, M.; Yazdani, M.; Kim, S.T.; Moyano, D.F.; Hou, S.; Das, R.; Mout, R.; Rezaee, F.; Mahmoudi, M.; et al. Regulation of macrophage recognition through the interplay of nanoparticle surface functionality and protein corona. ACS Nano 2016, 10, 4421–4430. [Google Scholar] [CrossRef] [Green Version]
- Zanganeh, S.; Spitler, R.; Erfanzadeh, M.; Alkilany, A.M.; Mahmoudi, M. Protein corona: Opportunities and challenges. Int. J. Biochem. Cell Biol. 2016, 75, 143–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| α = 0.2 | C3 (C16His)2/MOG-siRNA | |||
|---|---|---|---|---|
| qhkl or dhkl | ρeff = 4 | ρeff = 4 (+ PC) | ρeff = 10 | ρeff = 10 (+ PC) |
| q100 | - | 0.6 | - | 0.6 |
| 1.0 | 1.0 | 1.0 | 1.0 | |
| d100 | - | 10.6 | - | 10.8 |
| 6.0 | 6.2 | 6.0 | 6.2 | |
| q200 | - | 1.1 | - | 1.2 |
| 2.1 | 2.0 | 2.1 | 2.0 | |
| d200 | - | 10.9 | - | 10.8 |
| 6.1 | 6.2 | 6.0 | 6.2 | |
| q300 | - | 1.7 | - | 1.7 |
| 3.1 | - | 3.1 | - | |
| d300 | - | 10.9 | - | 10.8 |
| 6.1 | - | 6.1 | - | |
| Protein Number | Description | % |
|---|---|---|
| 1 | Apolipoprotein A-I | 12.85 |
| 2 | Apolipoprotein A-II | 7.39 |
| 3 | Serum albumin | 5.38 |
| 4 | Ig kappa constant | 3.73 |
| 5 | Ig lambda constant 2 | 3.13 |
| 6 | Alpha-1-antitrypsin SV = 3 | 2.97 |
| 7 | Alpha-1-antitrypsin SV = 1 | 2.94 |
| 8 | Apolipoprotein A-IV | 2.90 |
| 9 | Complement C3 | 2.89 |
| 10 | Apolipoprotein E | 1.99 |
| 11 | Apolipoprotein C-III | 1.81 |
| 12 | Complement C4-B | 1.72 |
| 13 | Complement C4-A | 1.67 |
| 14 | Ig heavy constant mu | 1.62 |
| 15 | Trypsin | 1.54 |
| 16 | Apolipoprotein C-I | 1.35 |
| 17 | Retinol-binding protein 4 | 1.34 |
| 18 | APOC4-APOC2 readthrough (NMD candidate) | 1.31 |
| 19 | Serotransferrin | 1.25 |
| 20 | Apolipoprotein B-100 | 1.22 |
| 21 | SAA2-SAA4 readthrough | 1.19 |
| 22 | Haptoglobin | 1.17 |
| 23 | Vitronectin | 1.10 |
| 24 | Isoform 2 of Clusterin | 1.09 |
| 25 | Ig lambda-like polypeptide 5 | 1.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Arribas, N.; Martínez-Negro, M.; Villar, E.M.; Pérez, L.; Osío Barcina, J.; Aicart, E.; Taboada, P.; Guerrero-Martínez, A.; Junquera, E. Protein Expression Knockdown in Cancer Cells Induced by a Gemini Cationic Lipid Nanovector with Histidine-Based Polar Heads. Pharmaceutics 2020, 12, 791. https://doi.org/10.3390/pharmaceutics12090791
Sánchez-Arribas N, Martínez-Negro M, Villar EM, Pérez L, Osío Barcina J, Aicart E, Taboada P, Guerrero-Martínez A, Junquera E. Protein Expression Knockdown in Cancer Cells Induced by a Gemini Cationic Lipid Nanovector with Histidine-Based Polar Heads. Pharmaceutics. 2020; 12(9):791. https://doi.org/10.3390/pharmaceutics12090791
Chicago/Turabian StyleSánchez-Arribas, Natalia, María Martínez-Negro, Eva M. Villar, Lourdes Pérez, José Osío Barcina, Emilio Aicart, Pablo Taboada, Andrés Guerrero-Martínez, and Elena Junquera. 2020. "Protein Expression Knockdown in Cancer Cells Induced by a Gemini Cationic Lipid Nanovector with Histidine-Based Polar Heads" Pharmaceutics 12, no. 9: 791. https://doi.org/10.3390/pharmaceutics12090791
APA StyleSánchez-Arribas, N., Martínez-Negro, M., Villar, E. M., Pérez, L., Osío Barcina, J., Aicart, E., Taboada, P., Guerrero-Martínez, A., & Junquera, E. (2020). Protein Expression Knockdown in Cancer Cells Induced by a Gemini Cationic Lipid Nanovector with Histidine-Based Polar Heads. Pharmaceutics, 12(9), 791. https://doi.org/10.3390/pharmaceutics12090791