Enteroendocrine Hormone Secretion and Metabolic Control: Importance of the Region of the Gut Stimulation
Abstract
:1. Introduction
2. Nutrient Transport, Digestion, and Absorption
3. Secretion and Actions of Gastrointestinal Hormones
3.1. Glucagon-Like Peptide-1 (GLP-1)
3.2. Glucose-Dependent Insulinotropic Polypeptide (GIP)
3.3. Cholecystokinin (CCK)
3.4. Peptide YY (PYY)
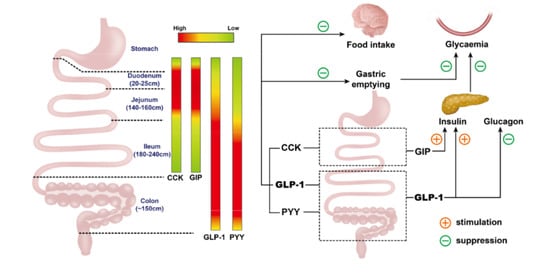
4. Regional Differences in Nutrient Absorption and Gastrointestinal Hormone Secretion, and Associated Impact on Postprandial Glycemia and Appetite
4.1. Nutrient Absorption
4.2. Gastrointestinal Hormone Secretion
4.3. Regulation of Postprandial Glycaemia and Appetite
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steinert, R.E.; Feinle-Bisset, C.; Asarian, L.; Horowitz, M.; Beglinger, C.; Geary, N. Ghrelin, CCK, GLP-1, and PYY(3-36): Secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol. Rev. 2017, 97, 411–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gribble, F.M.; Reimann, F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat. Rev. Endocrinol. 2019, 15, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.K.; Deane, A.M.; Jones, K.L.; Rayner, C.K.; Horowitz, M. Gastric emptying and glycaemia in health and diabetes mellitus. Nat. Rev. Endocrinol. 2015, 11, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Hussain, M.M. Intestinal lipid absorption. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1183–E1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, R.H.; Kim, Y.S. Digestion and absorption of dietary protein. Annu. Rev. Med. 1990, 41, 133–139. [Google Scholar] [CrossRef]
- Gray, G.M. Carbohydrate digestion and absorption. Role of the small intestine. N. Engl. J. Med. 1975, 292, 1225–1230. [Google Scholar] [CrossRef]
- Song, P.; Onishi, A.; Koepsell, H.; Vallon, V. Sodium glucose cotransporter SGLT1 as a therapeutic target in diabetes mellitus. Expert Opin. Ther. Targets 2016, 20, 1109–1125. [Google Scholar] [CrossRef] [Green Version]
- Wright, E.M.; Hirayama, B.A.; Loo, D.F. Active sugar transport in health and disease. J. Intern. Med. 2007, 261, 32–43. [Google Scholar] [CrossRef]
- Balen, D.; Ljubojevic, M.; Breljak, D.; Brzica, H.; Zlender, V.; Koepsell, H.; Sabolic, I. Revised immunolocalization of the Na+-D-glucose cotransporter SGLT1 in rat organs with an improved antibody. Am. J. Physiol. Cell Physiol. 2008, 295, C475–C489. [Google Scholar] [CrossRef] [Green Version]
- Thazhath, S.S.; Wu, T.; Young, R.L.; Horowitz, M.; Rayner, C.K. Glucose absorption in small intestinal diseases. Expert Rev. Gastroenterol. Hepatol. 2014, 8, 301–312. [Google Scholar] [CrossRef]
- Gouyon, F.; Caillaud, L.; Carriere, V.; Klein, C.; Dalet, V.; Citadelle, D.; Kellett, G.L.; Thorens, B.; Leturque, A.; Brot-Laroche, E. Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: A study in GLUT2-null mice. J. Physiol. 2003, 552, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Davidson, N.O.; Hausman, A.M.; Ifkovits, C.A.; Buse, J.B.; Gould, G.W.; Burant, C.F.; Bell, G.I. Human intestinal glucose transporter expression and localization of GLUT5. Am. J. Physiol. 1992, 262, C795–C800. [Google Scholar] [CrossRef] [PubMed]
- Abumrad, N.A.; Davidson, N.O. Role of the gut in lipid homeostasis. Physiol. Rev. 2012, 92, 1061–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paalvast, Y.; de Boer, J.F.; Groen, A.K. Developments in intestinal cholesterol transport and triglyceride absorption. Curr. Opin. Lipidol. 2017, 28, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Carter, D.; Howlett, H.C.; Wiernsperger, N.F.; Bailey, C.J. Differential effects of metformin on bile salt absorption from the jejunum and ileum. Diabetes Obes. Metab. 2003, 5, 120–125. [Google Scholar] [CrossRef]
- Broer, S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef]
- Broer, S.; Fairweather, S.J. Amino acid transport across the mammalian intestine. Compr. Physiol. 2018, 9, 343–373. [Google Scholar] [CrossRef]
- Daniel, H.; Zietek, T. Taste and move: Glucose and peptide transporters in the gastrointestinal tract. Exp. Physiol. 2015, 100, 1441–1450. [Google Scholar] [CrossRef]
- Gill, S.; Chater, P.I.; Wilcox, M.D.; Pearson, J.P.; Brownlee, I.A. The impact of dietary fibres on the physiological processes of the large intestine. Bioact. Carbohydr. Diet. Fibre 2018, 16, 62–74. [Google Scholar] [CrossRef]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The influence of diet on the gut microbiota. Pharm. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Kreuch, D.; Keating, D.J.; Wu, T.; Horowitz, M.; Rayner, C.K.; Young, R.L. Gut mechanisms linking intestinal sweet sensing to glycemic control. Front. Endocrinol. (Lausanne) 2018, 9, 741. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Bound, M.J.; Standfield, S.D.; Bellon, M.; Young, R.L.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Artificial sweeteners have no effect on gastric emptying, glucagon-like peptide-1, or glycemia after oral glucose in healthy humans. Diabetes Care 2013, 36, e202–e203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.J.; Kokrashvili, Z.; Theodorakis, M.J.; Carlson, O.D.; Kim, B.J.; Zhou, J.; Kim, H.H.; Xu, X.; Chan, S.L.; Juhaszova, M.; et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl. Acad. Sci. USA 2007, 104, 15069–15074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mace, O.J.; Schindler, M.; Patel, S. The regulation of K- and L-cell activity by GLUT2 and the calcium-sensing receptor CasR in rat small intestine. J. Physiol. 2012, 590, 2917–2936. [Google Scholar] [CrossRef]
- Gorboulev, V.; Schurmann, A.; Vallon, V.; Kipp, H.; Jaschke, A.; Klessen, D.; Friedrich, A.; Scherneck, S.; Rieg, T.; Cunard, R.; et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012, 61, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Zhao, B.R.; Bound, M.J.; Checklin, H.L.; Bellon, M.; Little, T.J.; Young, R.L.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effects of different sweet preloads on incretin hormone secretion, gastric emptying, and postprandial glycemia in healthy humans. Am. J. Clin. Nutr. 2012, 95, 78–83. [Google Scholar] [CrossRef]
- Beglinger, S.; Drewe, J.; Schirra, J.; Goke, B.; D’Amato, M.; Beglinger, C. Role of fat hydrolysis in regulating glucagon-like Peptide-1 secretion. J. Clin. Endocrinol. Metab. 2010, 95, 879–886. [Google Scholar] [CrossRef]
- Kuo, P.; Stevens, J.E.; Russo, A.; Maddox, A.; Wishart, J.M.; Jones, K.L.; Greville, H.; Hetzel, D.; Chapman, I.; Horowitz, M.; et al. Gastric emptying, incretin hormone secretion, and postprandial glycemia in cystic fibrosis--effects of pancreatic enzyme supplementation. J. Clin. Endocrinol. Metab. 2011, 96, E851–E855. [Google Scholar] [CrossRef] [Green Version]
- Perano, S.J.; Couper, J.J.; Horowitz, M.; Martin, A.J.; Kritas, S.; Sullivan, T.; Rayner, C.K. Pancreatic enzyme supplementation improves the incretin hormone response and attenuates postprandial glycemia in adolescents with cystic fibrosis: A randomized crossover trial. J. Clin. Endocrinol. Metab. 2014, 99, 2486–2493. [Google Scholar] [CrossRef] [Green Version]
- Edfalk, S.; Steneberg, P.; Edlund, H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 2008, 57, 2280–2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauffer, L.M.; Iakoubov, R.; Brubaker, P.L. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes 2009, 58, 1058–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sankoda, A.; Harada, N.; Iwasaki, K.; Yamane, S.; Murata, Y.; Shibue, K.; Thewjitcharoen, Y.; Suzuki, K.; Harada, T.; Kanemaru, Y.; et al. Long-chain free fatty acid receptor GPR120 mediates oil-induced GIP secretion through CCK in male mice. Endocrinology 2017, 158, 1172–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poreba, M.A.; Dong, C.X.; Li, S.K.; Stahl, A.; Miner, J.H.; Brubaker, P.L. Role of fatty acid transport protein 4 in oleic acid-induced glucagon-like peptide-1 secretion from murine intestinal L cells. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E899–E907. [Google Scholar] [CrossRef] [Green Version]
- Sundaresan, S.; Shahid, R.; Riehl, T.E.; Chandra, R.; Nassir, F.; Stenson, W.F.; Liddle, R.A.; Abumrad, N.A. CD36-dependent signaling mediates fatty acid-induced gut release of secretin and cholecystokinin. FASEB J. 2013, 27, 1191–1202. [Google Scholar] [CrossRef] [Green Version]
- Pais, R.; Gribble, F.M.; Reimann, F. Signalling pathways involved in the detection of peptones by murine small intestinal enteroendocrine L-cells. Peptides 2016, 77, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Rose, A.J.; Sijmonsma, T.P.; Broer, A.; Pfenninger, A.; Herzig, S.; Schmoll, D.; Broer, S. Mice lacking neutral amino acid transporter B(0)AT1 (Slc6a19) have elevated levels of FGF21 and GLP-1 and improved glycaemic control. Mol. Metab. 2015, 4, 406–417. [Google Scholar] [CrossRef]
- Clemmensen, C.; Jorgensen, C.V.; Smajilovic, S.; Brauner-Osborne, H. Robust GLP-1 secretion by basic L-amino acids does not require the GPRC6A receptor. Diabetes Obes. Metab. 2017, 19, 599–603. [Google Scholar] [CrossRef]
- Trabelsi, M.S.; Daoudi, M.; Prawitt, J.; Ducastel, S.; Touche, V.; Sayin, S.I.; Perino, A.; Brighton, C.A.; Sebti, Y.; Kluza, J.; et al. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat. Commun. 2015, 6, 7629. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Kuhre, R.E.; Wewer Albrechtsen, N.J.; Larsen, O.; Jepsen, S.L.; Balk-Moller, E.; Andersen, D.B.; Deacon, C.F.; Schoonjans, K.; Reimann, F.; Gribble, F.M.; et al. Bile acids are important direct and indirect regulators of the secretion of appetite- and metabolism-regulating hormones from the gut and pancreas. Mol. Metab. 2018, 11, 84–95. [Google Scholar] [CrossRef]
- Tough, I.R.; Schwartz, T.W.; Cox, H.M. Synthetic G protein-coupled bile acid receptor agonists and bile acids act via basolateral receptors in ileal and colonic mucosa. Neurogastroenterol. Motil. 2020, e13943. [Google Scholar] [CrossRef]
- Xie, C.; Wang, X.; Young, R.L.; Horowitz, M.; Rayner, C.K.; Wu, T. Role of Intestinal Bitter Sensing in Enteroendocrine Hormone Secretion and Metabolic Control. Front. Endocrinol. (Lausanne) 2018, 9, 576. [Google Scholar] [CrossRef]
- Wu, T.; Rayner, C.K.; Watson, L.E.; Jones, K.L.; Horowitz, M.; Little, T.J. Comparative effects of intraduodenal fat and glucose on the gut-incretin axis in healthy males. Peptides 2017, 95, 124–127. [Google Scholar] [CrossRef]
- Ryan, A.T.; Luscombe-Marsh, N.D.; Saies, A.A.; Little, T.J.; Standfield, S.; Horowitz, M.; Feinle-Bisset, C. Effects of intraduodenal lipid and protein on gut motility and hormone release, glycemia, appetite, and energy intake in lean men. Am. J. Clin. Nutr. 2013, 98, 300–311. [Google Scholar] [CrossRef] [Green Version]
- Jensen, C.Z.; Bojsen-Moller, K.N.; Svane, M.S.; Holst, L.M.; Hermansen, K.; Hartmann, B.; Wewer Albrechtsen, N.J.; Kuhre, R.E.; Kristiansen, V.B.; Rehfeld, J.F.; et al. Responses of gut and pancreatic hormones, bile acids, and fibroblast growth factor-21 differ to glucose, protein, and fat ingestion after gastric bypass surgery. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G661–G672. [Google Scholar] [CrossRef]
- Katsuma, S.; Hirasawa, A.; Tsujimoto, G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem. Biophys. Res. Commun. 2005, 329, 386–390. [Google Scholar] [CrossRef]
- Andersen, A.; Lund, A.; Knop, F.K.; Vilsboll, T. Glucagon-like peptide 1 in health and disease. Nat. Rev. Endocrinol. 2018, 14, 390–403. [Google Scholar] [CrossRef]
- Holst, J.J.; Albrechtsen, N.J.W.; Rosenkilde, M.M.; Deacon, C.F. Physiology of the incretin hormones, GIP and GLP-1-regulation of release and posttranslational modifications. Compr. Physiol. 2019, 9, 1339–1381. [Google Scholar] [CrossRef]
- Wu, T.; Rayner, C.K.; Horowitz, M. Incretins. Handb. Exp. Pharm. 2016, 233, 137–171. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. 1), 5–21. [Google Scholar] [CrossRef]
- Hare, K.J.; Vilsboll, T.; Asmar, M.; Deacon, C.F.; Knop, F.K.; Holst, J.J. The glucagonostatic and insulinotropic effects of glucagon-like peptide 1 contribute equally to its glucose-lowering action. Diabetes 2010, 59, 1765–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maida, A.; Hansotia, T.; Longuet, C.; Seino, Y.; Drucker, D.J. Differential importance of glucose-dependent insulinotropic polypeptide vs glucagon-like peptide 1 receptor signaling for beta cell survival in mice. Gastroenterology 2009, 137, 2146–2157. [Google Scholar] [CrossRef]
- Seghieri, M.; Rebelos, E.; Gastaldelli, A.; Astiarraga, B.D.; Casolaro, A.; Barsotti, E.; Pocai, A.; Nauck, M.; Muscelli, E.; Ferrannini, E. Direct effect of GLP-1 infusion on endogenous glucose production in humans. Diabetologia 2013, 56, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Nauck, M.A.; Niedereichholz, U.; Ettler, R.; Holst, J.J.; Orskov, C.; Ritzel, R.; Schmiegel, W.H. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am. J. Physiol. 1997, 273, E981–E988. [Google Scholar] [CrossRef]
- Little, T.J.; Pilichiewicz, A.N.; Russo, A.; Phillips, L.; Jones, K.L.; Nauck, M.A.; Wishart, J.; Horowitz, M.; Feinle-Bisset, C. Effects of intravenous glucagon-like peptide-1 on gastric emptying and intragastric distribution in healthy subjects: Relationships with postprandial glycemic and insulinemic responses. J. Clin. Endocrinol. Metab. 2006, 91, 1916–1923. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.L.; Rigda, R.S.; Buttfield, M.D.M.; Hatzinikolas, S.; Pham, H.T.; Marathe, C.S.; Wu, T.; Lange, K.; Trahair, L.G.; Rayner, C.K.; et al. Effects of lixisenatide on postprandial blood pressure, gastric emptying and glycaemia in healthy people and people with type 2 diabetes. Diabetes Obes. Metab. 2019, 21, 1158–1167. [Google Scholar] [CrossRef]
- Deane, A.M.; Nguyen, N.Q.; Stevens, J.E.; Fraser, R.J.; Holloway, R.H.; Besanko, L.K.; Burgstad, C.; Jones, K.L.; Chapman, M.J.; Rayner, C.K.; et al. Endogenous glucagon-like peptide-1 slows gastric emptying in healthy subjects, attenuating postprandial glycemia. J. Clin. Endocrinol. Metab. 2010, 95, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, M.; Pfeiffer, C.; Steinstrasser, A.; Becker, R.H.; Rutten, H.; Ruus, P.; Horowitz, M. Effects of lixisenatide once daily on gastric emptying in type 2 diabetes--relationship to postprandial glycemia. Regul. Pept. 2013, 185, 1–8. [Google Scholar] [CrossRef]
- Stevens, J.E.; Buttfield, M.; Wu, T.; Hatzinikolas, S.; Pham, H.; Lange, K.; Rayner, C.K.; Horowitz, M.; Jones, K.L. Effects of sitagliptin on gastric emptying of, and the glycaemic and blood pressure responses to, a carbohydrate meal in type 2 diabetes. Diabetes Obes. Metab. 2020, 22, 51–58. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Okerson, T.; Viswanathan, P.; Guan, X.; Holcombe, J.H.; MacConell, L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: A randomized, cross-over study. Curr. Med. Res. Opin. 2008, 24, 2943–2952. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.; Longuet, C.; Baker, C.L.; Qin, B.; Federico, L.M.; Drucker, D.J.; Adeli, K. The glucagon-like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice. Diabetologia 2010, 53, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Shen, H.; Liu, M.; Yang, Q.; Zheng, S.; Sabo, M.; D’Alessio, D.A.; Tso, P. GLP-1 reduces intestinal lymph flow, triglyceride absorption, and apolipoprotein production in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G943–G949. [Google Scholar] [CrossRef]
- Xiao, C.; Bandsma, R.H.; Dash, S.; Szeto, L.; Lewis, G.F. Exenatide, a glucagon-like peptide-1 receptor agonist, acutely inhibits intestinal lipoprotein production in healthy humans. Arter. Thromb. Vasc. Biol. 2012, 32, 1513–1519. [Google Scholar] [CrossRef] [Green Version]
- Xiao, C.; Dash, S.; Morgantini, C.; Patterson, B.W.; Lewis, G.F. Sitagliptin, a DPP-4 inhibitor, acutely inhibits intestinal lipoprotein particle secretion in healthy humans. Diabetes 2014, 63, 2394–2401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hjerpsted, J.B.; Flint, A.; Brooks, A.; Axelsen, M.B.; Kvist, T.; Blundell, J. Semaglutide improves postprandial glucose and lipid metabolism, and delays first-hour gastric emptying in subjects with obesity. Diabetes Obes. Metab. 2018, 20, 610–619. [Google Scholar] [CrossRef] [Green Version]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef]
- Plamboeck, A.; Veedfald, S.; Deacon, C.F.; Hartmann, B.; Wettergren, A.; Svendsen, L.B.; Meisner, S.; Hovendal, C.; Vilsboll, T.; Knop, F.K.; et al. The effect of exogenous GLP-1 on food intake is lost in male truncally vagotomized subjects with pyloroplasty. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G1117–G1127. [Google Scholar] [CrossRef]
- Krieger, J.P.; Arnold, M.; Pettersen, K.G.; Lossel, P.; Langhans, W.; Lee, S.J. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes 2016, 65, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Turton, M.D.; O’Shea, D.; Gunn, I.; Beak, S.A.; Edwards, C.M.; Meeran, K.; Choi, S.J.; Taylor, G.M.; Heath, M.M.; Lambert, P.D.; et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 1996, 379, 69–72. [Google Scholar] [CrossRef]
- ten Kulve, J.S.; Veltman, D.J.; van Bloemendaal, L.; Barkhof, F.; Deacon, C.F.; Holst, J.J.; Konrad, R.J.; Sloan, J.H.; Drent, M.L.; Diamant, M.; et al. Endogenous GLP-1 mediates postprandial reductions in activation in central reward and satiety areas in patients with type 2 diabetes. Diabetologia 2015, 58, 2688–2698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pratley, R.; Amod, A.; Hoff, S.T.; Kadowaki, T.; Lingvay, I.; Nauck, M.; Pedersen, K.B.; Saugstrup, T.; Meier, J.J. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): A randomised, double-blind, phase 3a trial. Lancet 2019, 394, 39–50. [Google Scholar] [CrossRef]
- Xu, F.; Lin, B.; Zheng, X.; Chen, Z.; Cao, H.; Xu, H.; Liang, H.; Weng, J. GLP-1 receptor agonist promotes brown remodelling in mouse white adipose tissue through SIRT1. Diabetologia 2016, 59, 1059–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, L.; Hogan, A.E.; Duquette, D.; Lester, C.; Banks, A.; LeClair, K.; Cohen, D.E.; Ghosh, A.; Lu, B.; Corrigan, M.; et al. iNKT cells induce FGF21 for thermogenesis and are required for maximal weight loss in GLP1 therapy. Cell Metab. 2016, 24, 510–519. [Google Scholar] [CrossRef] [Green Version]
- Beiroa, D.; Imbernon, M.; Gallego, R.; Senra, A.; Herranz, D.; Villarroya, F.; Serrano, M.; Ferno, J.; Salvador, J.; Escalada, J.; et al. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 2014, 63, 3346–3358. [Google Scholar] [CrossRef] [Green Version]
- Tomas, E.; Stanojevic, V.; McManus, K.; Khatri, A.; Everill, P.; Bachovchin, W.W.; Habener, J.F. GLP-1(32-36)amide pentapeptide increases basal energy expenditure and inhibits weight gain in obese mice. Diabetes 2015, 64, 2409–2419. [Google Scholar] [CrossRef] [Green Version]
- Goldsmith, F.; Keenan, M.J.; Raggio, A.M.; Ye, X.; Hao, Z.; Durham, H.; Geaghan, J.; Jia, W.P.; Martin, R.J.; Ye, J.P. Induction of energy expenditure by sitagliptin is dependent on GLP-1 receptor. PLoS ONE 2015, 10, e0126177. [Google Scholar] [CrossRef] [Green Version]
- Fukuda-Tsuru, S.; Kakimoto, T.; Utsumi, H.; Kiuchi, S.; Ishii, S. The novel dipeptidyl peptidase-4 inhibitor teneligliptin prevents high-fat diet-induced obesity accompanied with increased energy expenditure in mice. Eur. J. Pharm. 2014, 723, 207–215. [Google Scholar] [CrossRef]
- Maciel, M.G.; Beserra, B.T.S.; Oliveira, F.C.B.; Ribeiro, C.M.; Coelho, M.S.; Neves, F.A.R.; Amato, A.A. The effect of glucagon-like peptide 1 and glucagon-like peptide 1 receptor agonists on energy expenditure: A systematic review and meta-analysis. Diabetes Res. Clin. Pr. 2018, 142, 222–235. [Google Scholar] [CrossRef]
- Heruc, G.A.; Horowitz, M.; Deacon, C.F.; Feinle-Bisset, C.; Rayner, C.K.; Luscombe-Marsh, N.; Little, T.J. Effects of dipeptidyl peptidase IV inhibition on glycemic, gut hormone, triglyceride, energy expenditure, and energy intake responses to fat in healthy males. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E830–E837. [Google Scholar] [CrossRef]
- Xie, C.; Wang, X.; Jones, K.L.; Horowitz, M.; Sun, Z.; Little, T.J.; Rayner, C.K.; Wu, T. Role of endogenous glucagon-like peptide-1 enhanced by vildagliptin in the glycaemic and energy expenditure responses to intraduodenal fat infusion in type 2 diabetes. Diabetes Obes. Metab. 2020, 22, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, K.; Christensen, L.L.; Holst, J.J.; Orskov, C. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul. Pept. 2003, 114, 189–196. [Google Scholar] [CrossRef]
- Svendsen, B.; Pedersen, J.; Albrechtsen, N.J.; Hartmann, B.; Torang, S.; Rehfeld, J.F.; Poulsen, S.S.; Holst, J.J. An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology 2015, 156, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Rayner, C.K.; Jones, K.; Horowitz, M. Dietary effects on incretin hormone secretion. Vitam Horm 2010, 84, 81–110. [Google Scholar] [CrossRef] [PubMed]
- Holst, J.J.; Gromada, J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E199–E206. [Google Scholar] [CrossRef] [PubMed]
- Mentis, N.; Vardarli, I.; Köthe, L.D.; Holst, J.J.; Deacon, C.F.; Theodorakis, M.; Meier, J.J.; Nauck, M.A. GIP does not potentiate the antidiabetic effects of GLP-1 in hyperglycemic patients with type 2 diabetes. Diabetes 2011, 60, 1270–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, M.; Calanna, S.; Sparre-Ulrich, A.H.; Kristensen, P.L.; Rosenkilde, M.M.; Faber, J.; Purrello, F.; van Hall, G.; Holst, J.J.; Vilsboll, T.; et al. Glucose-dependent insulinotropic polypeptide augments glucagon responses to hypoglycemia in type 1 diabetes. Diabetes 2015, 64, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, N.C.; Lund, A.; Gasbjerg, L.S.; Meessen, E.C.E.; Andersen, M.M.; Bergmann, S.; Hartmann, B.; Holst, J.J.; Jessen, L.; Christensen, M.B.; et al. Effects of combined GIP and GLP-1 infusion on energy intake, appetite and energy expenditure in overweight/obese individuals: A randomised, crossover study. Diabetologia 2019, 62, 665–675. [Google Scholar] [CrossRef] [Green Version]
- Meier, J.J.; Goetze, O.; Anstipp, J.; Hagemann, D.; Holst, J.J.; Schmidt, W.E.; Gallwitz, B.; Nauck, M.A. Gastric inhibitory polypeptide does not inhibit gastric emptying in humans. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E621–E625. [Google Scholar] [CrossRef]
- Miyawaki, K.; Yamada, Y.; Ban, N.; Ihara, Y.; Tsukiyama, K.; Zhou, H.; Fujimoto, S.; Oku, A.; Tsuda, K.; Toyokuni, S.; et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat. Med. 2002, 8, 738–742. [Google Scholar] [CrossRef]
- Naitoh, R.; Miyawaki, K.; Harada, N.; Mizunoya, W.; Toyoda, K.; Fushiki, T.; Yamada, Y.; Seino, Y.; Inagaki, N. Inhibition of GIP signaling modulates adiponectin levels under high-fat diet in mice. Biochem. Biophys. Res. Commun. 2008, 376, 21–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Yamada, Y.; Tsukiyama, K.; Miyawaki, K.; Hosokawa, M.; Nagashima, K.; Toyoda, K.; Naitoh, R.; Mizunoya, W.; Fushiki, T.; et al. Gastric inhibitory polypeptide modulates adiposity and fat oxidation under diminished insulin action. Biochem. Biophys. Res. Commun. 2005, 335, 937–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansotia, T.; Maida, A.; Flock, G.; Yamada, Y.; Tsukiyama, K.; Seino, Y.; Drucker, D.J. Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J. Clin. Investig. 2007, 117, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Boylan, M.O.; Glazebrook, P.A.; Tatalovic, M.; Wolfe, M.M. Gastric inhibitory polypeptide immunoneutralization attenuates development of obesity in mice. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E1008–E1018. [Google Scholar] [CrossRef] [Green Version]
- Asmar, M.; Asmar, A.; Simonsen, L.; Gasbjerg, L.S.; Sparre-Ulrich, A.H.; Rosenkilde, M.M.; Hartmann, B.; Dela, F.; Holst, J.J.; Bulow, J. The gluco- and liporegulatory and vasodilatory effects of glucose-dependent insulinotropic polypeptide (GIP) are abolished by an antagonist of the human GIP receptor. Diabetes 2017, 66, 2363–2371. [Google Scholar] [CrossRef] [Green Version]
- Frias, J.P.; Nauck, M.A.; Van, J.; Kutner, M.E.; Cui, X.; Benson, C.; Urva, S.; Gimeno, R.E.; Milicevic, Z.; Robins, D.; et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: A randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet 2018, 392, 2180–2193. [Google Scholar] [CrossRef]
- Frias, J.P.; Bastyr, E.J., 3rd; Vignati, L.; Tschop, M.H.; Schmitt, C.; Owen, K.; Christensen, R.H.; DiMarchi, R.D. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes. Cell Metab. 2017, 26, 343–352. [Google Scholar] [CrossRef]
- Bergmann, N.C.; Gasbjerg, L.S.; Heimburger, S.M.; Krogh, L.S.L.; Dela, F.; Hartmann, B.; Holst, J.J.; Jessen, L.; Christensen, M.B.; Vilsboll, T.; et al. No acute effects of exogenous glucose-dependent insulinotropic polypeptide on energy intake, appetite, or energy expenditure when added to treatment with a long-acting glucagon-like peptide 1 receptor agonist in men with type 2 diabetes. Diabetes Care 2020, 43, 588–596. [Google Scholar] [CrossRef]
- Sykaras, A.G.; Demenis, C.; Cheng, L.; Pisitkun, T.; McLaughlin, J.T.; Fenton, R.A.; Smith, C.P. Duodenal CCK cells from male mice express multiple hormones including ghrelin. Endocrinology 2014, 155, 3339–3351. [Google Scholar] [CrossRef] [Green Version]
- Little, T.J.; Doran, S.; Meyer, J.H.; Smout, A.J.; O’Donovan, D.G.; Wu, K.L.; Jones, K.L.; Wishart, J.; Rayner, C.K.; Horowitz, M.; et al. The release of GLP-1 and ghrelin, but not GIP and CCK, by glucose is dependent upon the length of small intestine exposed. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E647–E655. [Google Scholar] [CrossRef]
- Liddle, R.A.; Rushakoff, R.J.; Morita, E.T.; Beccaria, L.; Carter, J.D.; Goldfine, I.D. Physiological role for cholecystokinin in reducing postprandial hyperglycemia in humans. J. Clin. Investig. 1988, 81, 1675–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahren, B.; Holst, J.J.; Efendic, S. Antidiabetogenic action of cholecystokinin-8 in type 2 diabetes. J. Clin. Endocrinol. Metab. 2000, 85, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Harding, P.E.; Maddox, A.F.; Wishart, J.M.; Akkermans, L.M.; Chatterton, B.E.; Shearman, D.J. Gastric and oesophageal emptying in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 1989, 32, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reidelberger, R.D.; Hernandez, J.; Fritzsch, B.; Hulce, M. Abdominal vagal mediation of the satiety effects of CCK in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R1005–R1012. [Google Scholar] [CrossRef] [PubMed]
- MacIntosh, C.G.; Morley, J.E.; Wishart, J.; Morris, H.; Jansen, J.B.; Horowitz, M.; Chapman, I.M. Effect of exogenous cholecystokinin (CCK)-8 on food intake and plasma CCK, leptin, and insulin concentrations in older and young adults: Evidence for increased CCK activity as a cause of the anorexia of aging. J. Clin. Endocrinol. Metab. 2001, 86, 5830–5837. [Google Scholar] [CrossRef] [PubMed]
- Brennan, I.M.; Feltrin, K.L.; Horowitz, M.; Smout, A.J.; Meyer, J.H.; Wishart, J.; Feinle-Bisset, C. Evaluation of interactions between CCK and GLP-1 in their effects on appetite, energy intake, and antropyloroduodenal motility in healthy men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1477–R1485. [Google Scholar] [CrossRef] [Green Version]
- Brennan, I.M.; Little, T.J.; Feltrin, K.L.; Smout, A.J.; Wishart, J.M.; Horowitz, M.; Feinle-Bisset, C. Dose-dependent effects of cholecystokinin-8 on antropyloroduodenal motility, gastrointestinal hormones, appetite, and energy intake in healthy men. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1487–E1494. [Google Scholar] [CrossRef] [Green Version]
- Beglinger, C.; Degen, L.; Matzinger, D.; D’Amato, M.; Drewe, J. Loxiglumide, a CCK-A receptor antagonist, stimulates calorie intake and hunger feelings in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R1149–R1154. [Google Scholar] [CrossRef]
- de Krom, M.; van der Schouw, Y.T.; Hendriks, J.; Ophoff, R.A.; van Gils, C.H.; Stolk, R.P.; Grobbee, D.E.; Adan, R. Common genetic variations in CCK, leptin, and leptin receptor genes are associated with specific human eating patterns. Diabetes 2007, 56, 276–280. [Google Scholar] [CrossRef] [Green Version]
- Lieverse, R.J.; Jansen, J.B.; Masclee, A.A.; Lamers, C.B. Satiety effects of a physiological dose of cholecystokinin in humans. Gut 1995, 36, 176–179. [Google Scholar] [CrossRef] [Green Version]
- Habib, A.M.; Richards, P.; Rogers, G.J.; Reimann, F.; Gribble, F.M. Co-localisation and secretion of glucagon-like peptide 1 and peptide YY from primary cultured human L cells. Diabetologia 2013, 56, 1413–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Essah, P.A.; Levy, J.R.; Sistrun, S.N.; Kelly, S.M.; Nestler, J.E. Effect of macronutrient composition on postprandial peptide YY levels. J. Clin. Endocrinol. Metab. 2007, 92, 4052–4055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brennan, I.M.; Luscombe-Marsh, N.D.; Seimon, R.V.; Otto, B.; Horowitz, M.; Wishart, J.M.; Feinle-Bisset, C. Effects of fat, protein, and carbohydrate and protein load on appetite, plasma cholecystokinin, peptide YY, and ghrelin, and energy intake in lean and obese men. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G129–G140. [Google Scholar] [CrossRef] [PubMed]
- Helou, N.; Obeid, O.; Azar, S.T.; Hwalla, N. Variation of postprandial PYY 3-36 response following ingestion of differing macronutrient meals in obese females. Ann. Nutr. Metab. 2008, 52, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Batterham, R.L.; Heffron, H.; Kapoor, S.; Chivers, J.E.; Chandarana, K.; Herzog, H.; Le Roux, C.W.; Thomas, E.L.; Bell, J.D.; Withers, D.J. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006, 4, 223–233. [Google Scholar] [CrossRef] [Green Version]
- Broberger, C.; Landry, M.; Wong, H.; Walsh, J.N.; Hokfelt, T. Subtypes Y1 and Y2 of the neuropeptide Y receptor are respectively expressed in pro-opiomelanocortin- and neuropeptide-Y-containing neurons of the rat hypothalamic arcuate nucleus. Neuroendocrinology 1997, 66, 393–408. [Google Scholar] [CrossRef]
- Batterham, R.L.; Cowley, M.A.; Small, C.J.; Herzog, H.; Cohen, M.A.; Dakin, C.L.; Wren, A.M.; Brynes, A.E.; Low, M.J.; Ghatei, M.A.; et al. Gut hormone PYY3-36 physiologically inhibits food intake. Nature 2002, 418, 650–654. [Google Scholar] [CrossRef]
- Abbott, C.R.; Small, C.J.; Kennedy, A.R.; Neary, N.M.; Sajedi, A.; Ghatei, M.A.; Bloom, S.R. Blockade of the neuropeptide Y Y2 receptor with the specific antagonist BIIE0246 attenuates the effect of endogenous and exogenous peptide YY(3-36) on food intake. Brain Res. 2005, 1043, 139–144. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, L.; Enriori, P.J.; Koska, J.; Franks, P.W.; Brookshire, T.; Cowley, M.A.; Salbe, A.D.; Delparigi, A.; Tataranni, P.A. Physiological evidence for the involvement of peptide YY in the regulation of energy homeostasis in humans. Obesity (Silver Spring) 2006, 14, 1562–1570. [Google Scholar] [CrossRef]
- Stoeckel, L.E.; Weller, R.E.; Giddings, M.; Cox, J.E. Peptide YY levels are associated with appetite suppression in response to long-chain fatty acids. Physiol. Behav. 2008, 93, 289–295. [Google Scholar] [CrossRef]
- Degen, L.; Oesch, S.; Casanova, M.; Graf, S.; Ketterer, S.; Drewe, J.; Beglinger, C. Effect of peptide YY3-36 on food intake in humans. Gastroenterology 2005, 129, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- le Roux, C.W.; Borg, C.M.; Murphy, K.G.; Vincent, R.P.; Ghatei, M.A.; Bloom, S.R. Supraphysiological doses of intravenous PYY3-36 cause nausea, but no additional reduction in food intake. Ann. Clin. Biochem. 2008, 45, 93–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangwala, S.M.; D’Aquino, K.; Zhang, Y.M.; Bader, L.; Edwards, W.; Zheng, S.; Eckardt, A.; Lacombe, A.; Pick, R.; Moreno, V.; et al. A long-acting PYY3-36 analog mediates robust anorectic efficacy with minimal emesis in nonhuman primates. Cell Metab. 2019, 29, 837–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savage, A.P.; Adrian, T.E.; Carolan, G.; Chatterjee, V.K.; Bloom, S.R. Effects of peptide YY (PYY) on mouth to caecum intestinal transit time and on the rate of gastric emptying in healthy volunteers. Gut 1987, 28, 166–170. [Google Scholar] [CrossRef]
- Chelikani, P.K.; Haver, A.C.; Reidelberger, R.D. Comparison of the inhibitory effects of PYY(3-36) and PYY(1-36) on gastric emptying in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1064–R1070. [Google Scholar] [CrossRef] [Green Version]
- Moran, T.H.; Smedh, U.; Kinzig, K.P.; Scott, K.A.; Knipp, S.; Ladenheim, E.E. Peptide YY(3-36) inhibits gastric emptying and produces acute reductions in food intake in rhesus monkeys. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R384–R388. [Google Scholar] [CrossRef]
- Witte, A.B.; Gryback, P.; Holst, J.J.; Hilsted, L.; Hellstrom, P.M.; Jacobsson, H.; Schmidt, P.T. Differential effect of PYY1-36 and PYY3-36 on gastric emptying in man. Regul. Pept. 2009, 158, 57–62. [Google Scholar] [CrossRef]
- Bottcher, G.; Ahren, B.; Lundquist, I.; Sundler, F. Peptide YY: Intrapancreatic localization and effects on insulin and glucagon secretion in the mouse. Pancreas 1989, 4, 282–288. [Google Scholar] [CrossRef]
- Nieuwenhuizen, A.G.; Karlsson, S.; Fridolf, T.; Ahren, B. Mechanisms underlying the insulinostatic effect of peptide YY in mouse pancreatic islets. Diabetologia 1994, 37, 871–878. [Google Scholar] [CrossRef]
- Lafferty, R.A.; Gault, V.A.; Flatt, P.R.; Irwin, N. Effects of 2 novel PYY(1-36) analogues, (P(3)L(31)P(34))PYY(1-36) and PYY(1-36)(Lys(12)PAL), on pancreatic beta-cell function, growth, and survival. Clin. Med. Insights Endocrinol. Diabetes 2019, 12, 1179551419855626. [Google Scholar] [CrossRef] [Green Version]
- Boey, D.; Heilbronn, L.; Sainsbury, A.; Laybutt, R.; Kriketos, A.; Herzog, H.; Campbell, L.V. Low serum PYY is linked to insulin resistance in first-degree relatives of subjects with type 2 diabetes. Neuropeptides 2006, 40, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Boey, D.; Lin, S.; Karl, T.; Baldock, P.; Lee, N.; Enriquez, R.; Couzens, M.; Slack, K.; Dallmann, R.; Sainsbury, A.; et al. Peptide YY ablation in mice leads to the development of hyperinsulinaemia and obesity. Diabetologia 2006, 49, 1360–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahrén, B.; Larsson, H. Peptide YY does not inhibit glucose-stimulated insulin secretion in humans. Eur. J. Endocrinol. 1996, 134, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.Q.; Debreceni, T.L.; Burgess, J.E.; Bellon, M.; Wishart, J.; Standfield, S.; Malbert, C.H.; Horowitz, M. Impact of gastric emptying and small intestinal transit on blood glucose, intestinal hormones, glucose absorption in the morbidly obese. Int. J. Obes. (Lond) 2018, 42, 1556–1564. [Google Scholar] [CrossRef]
- Duchman, S.M.; Ryan, A.J.; Schedl, H.P.; Summers, R.W.; Bleier, T.L.; Gisolfi, C.V. Upper limit for intestinal absorption of a dilute glucose solution in men at rest. Med. Sci. Sports Exerc. 1997, 29, 482–488. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Inoue, R.; Matsumoto, M.; Yajima, T.; Ushida, K.; Iwanaga, T. Comparative expression of hexose transporters (SGLT1, GLUT1, GLUT2 and GLUT5) throughout the mouse gastrointestinal tract. Histochem. Cell Biol. 2011, 135, 183–194. [Google Scholar] [CrossRef]
- Zhang, X.; Young, R.L.; Bound, M.; Hu, S.; Jones, K.L.; Horowitz, M.; Rayner, C.K.; Wu, T. Comparative effects of proximal and distal small intestinal glucose exposure on glycemia, incretin hormone Ssecretion, and the incretin effect in health and type 2 diabetes. Diabetes Care 2019, 42, 520–528. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Sun, F.; Li, Z.; Xiang, J.; Ding, Y.; Lu, Z.; Tian, Y.; Chen, H.; Zhang, J.; Wang, Y.; et al. Reduction of intestinal electrogenic glucose absorption after duodenojejunal bypass in a mouse model. Obes. Surg. 2013, 23, 1361–1369. [Google Scholar] [CrossRef]
- Jurowich, C.F.; Rikkala, P.R.; Thalheimer, A.; Wichelmann, C.; Seyfried, F.; Sander, V.; Kreissl, M.; Germer, C.T.; Koepsell, H.; Otto, C. Duodenal-jejunal bypass improves glycemia and decreases SGLT1-mediated glucose absorption in rats with streptozotocin-induced type 2 diabetes. Ann. Surg. 2013, 258, 89–97. [Google Scholar] [CrossRef]
- de Jonge, C.; Rensen, S.S.; Verdam, F.J.; Vincent, R.P.; Bloom, S.R.; Buurman, W.A.; le Roux, C.W.; Schaper, N.C.; Bouvy, N.D.; Greve, J.W. Endoscopic duodenal-jejunal bypass liner rapidly improves type 2 diabetes. Obes. Surg. 2013, 23, 1354–1360. [Google Scholar] [CrossRef]
- Koehestanie, P.; de Jonge, C.; Berends, F.J.; Janssen, I.M.; Bouvy, N.D.; Greve, J.W. The effect of the endoscopic duodenal-jejunal bypass liner on obesity and type 2 diabetes mellitus, a multicenter randomized controlled trial. Ann. Surg. 2014, 260, 984–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobo, M.V.; Huerta, L.; Ruiz-Velasco, N.; Teixeiro, E.; de la Cueva, P.; Celdran, A.; Martin-Hidalgo, A.; Vega, M.A.; Bragado, R. Localization of the lipid receptors CD36 and CLA-1/SR-BI in the human gastrointestinal tract: Towards the identification of receptors mediating the intestinal absorption of dietary lipids. J. Histochem. Cytochem. 2001, 49, 1253–1260. [Google Scholar] [CrossRef] [Green Version]
- Ockner, R.K.; Manning, J.A. Fatty acid-binding protein in small intestine. Identification, isolation, and evidence for its role in cellular fatty acid transport. J. Clin. Investig. 1974, 54, 326–338. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Yang, Y.; Braunstein, E.; Georgeson, K.E.; Harmon, C.M. Gut expression and regulation of FAT/CD36: Possible role in fatty acid transport in rat enterocytes. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E916–E923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nassir, F.; Wilson, B.; Han, X.; Gross, R.W.; Abumrad, N.A. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J. Biol. Chem. 2007, 282, 19493–19501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, D.V.; Drover, V.A.; Knopfel, M.; Dhanasekaran, P.; Hauser, H.; Phillips, M.C. Influence of class B scavenger receptors on cholesterol flux across the brush border membrane and intestinal absorption. J. Lipid Res. 2009, 50, 2235–2244. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.L.; Clark, S.B.; Holt, P.R. Transmucosal triglyceride transport rates in proximal and distal rat intestine in vivo. J. Lipid Res. 1975, 16, 251–257. [Google Scholar]
- Nauli, A.M.; Nassir, F.; Zheng, S.; Yang, Q.; Lo, C.M.; Vonlehmden, S.B.; Lee, D.; Jandacek, R.J.; Abumrad, N.A.; Tso, P. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology 2006, 131, 1197–1207. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.; Moulson, C.L.; Newberry, E.P.; Lin, M.H.; Xie, Y.; Kennedy, S.M.; Miner, J.H.; Davidson, N.O. Fatty acid transport protein 4 is dispensable for intestinal lipid absorption in mice. J. Lipid Res. 2009, 50, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Masson, C.J.; Plat, J.; Mensink, R.P.; Namiot, A.; Kisielewski, W.; Namiot, Z.; Fullekrug, J.; Ehehalt, R.; Glatz, J.F.; Pelsers, M.M. Fatty acid- and cholesterol transporter protein expression along the human intestinal tract. PLoS ONE 2010, 5, e10380. [Google Scholar] [CrossRef]
- Mutch, D.M.; Anderle, P.; Fiaux, M.; Mansourian, R.; Vidal, K.; Wahli, W.; Williamson, G.; Roberts, M.A. Regional variations in ABC transporter expression along the mouse intestinal tract. Physiol. Genom. 2004, 17, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Bojsen-Moller, K.N.; Jacobsen, S.H.; Dirksen, C.; Jorgensen, N.B.; Reitelseder, S.; Jensen, J.E.; Kristiansen, V.B.; Holst, J.J.; van Hall, G.; Madsbad, S. Accelerated protein digestion and amino acid absorption after Roux-en-Y gastric bypass. Am. J. Clin. Nutr. 2015, 102, 600–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dirksen, C.; Graff, J.; Fuglsang, S.; Rehfeld, J.F.; Holst, J.J.; Madsen, J.L. Energy intake, gastrointestinal transit, and gut hormone release in response to oral triglycerides and fatty acids in men with and without severe obesity. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G332–G337. [Google Scholar] [CrossRef] [PubMed]
- Little, T.J.; Feltrin, K.L.; Horowitz, M.; Smout, A.J.; Rades, T.; Meyer, J.H.; Pilichiewicz, A.N.; Wishart, J.; Feinle-Bisset, C. Dose-related effects of lauric acid on antropyloroduodenal motility, gastrointestinal hormone release, appetite, and energy intake in healthy men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1090–R1098. [Google Scholar] [CrossRef] [Green Version]
- Pilichiewicz, A.N.; Chaikomin, R.; Brennan, I.M.; Wishart, J.M.; Rayner, C.K.; Jones, K.L.; Smout, A.J.; Horowitz, M.; Feinle-Bisset, C. Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E743–E753. [Google Scholar] [CrossRef] [Green Version]
- Hutchison, A.T.; Feinle-Bisset, C.; Fitzgerald, P.C.; Standfield, S.; Horowitz, M.; Clifton, P.M.; Luscombe-Marsh, N.D. Comparative effects of intraduodenal whey protein hydrolysate on antropyloroduodenal motility, gut hormones, glycemia, appetite, and energy intake in lean and obese men. Am. J. Clin. Nutr. 2015, 102, 1323–1331. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, X.; Trahair, L.G.; Bound, M.J.; Little, T.J.; Deacon, C.F.; Horowitz, M.; Jones, K.L.; Rayner, C.K. Small intestinal glucose delivery affects the lowering of blood glucose by acute vildagliptin in type 2 diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 4769–4778. [Google Scholar] [CrossRef]
- Ma, J.; Pilichiewicz, A.N.; Feinle-Bisset, C.; Wishart, J.M.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effects of variations in duodenal glucose load on glycaemic, insulin, and incretin responses in type 2 diabetes. Diabet Med. 2012, 29, 604–608. [Google Scholar] [CrossRef]
- Trahair, L.G.; Horowitz, M.; Rayner, C.K.; Gentilcore, D.; Lange, K.; Wishart, J.M.; Jones, K.L. Comparative effects of variations in duodenal glucose load on glycemic, insulinemic, and incretin responses in healthy young and older subjects. J. Clin. Endocrinol. Metab. 2012, 97, 844–851. [Google Scholar] [CrossRef]
- Pilichiewicz, A.N.; Papadopoulos, P.; Brennan, I.M.; Little, T.J.; Meyer, J.H.; Wishart, J.M.; Horowitz, M.; Feinle-Bisset, C. Load-dependent effects of duodenal lipid on antropyloroduodenal motility, plasma CCK and PYY, and energy intake in healthy men. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R2170–R2178. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Rayner, C.K.; Horowitz, M. Inter-regulation of gastric emptying and incretin hormone secretion: Implications for postprandial glycemic control. Biomark. Med. 2016, 10, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Laferrere, B.; Heshka, S.; Wang, K.; Khan, Y.; McGinty, J.; Teixeira, J.; Hart, A.B.; Olivan, B. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007, 30, 1709–1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dirksen, C.; Jorgensen, N.B.; Bojsen-Moller, K.N.; Kielgast, U.; Jacobsen, S.H.; Clausen, T.R.; Worm, D.; Hartmann, B.; Rehfeld, J.F.; Damgaard, M.; et al. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. Int. J. Obes. 2013, 37, 1452–1459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, N.Q.; Debreceni, T.L.; Bambrick, J.E.; Bellon, M.; Wishart, J.; Standfield, S.; Rayner, C.K.; Horowitz, M. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption and postprandial symptoms after gastric bypass. Obesity 2014, 22, 2003–2009. [Google Scholar] [CrossRef]
- Mingrone, G.; Nolfe, G.; Gissey, G.C.; Iaconelli, A.; Leccesi, L.; Guidone, C.; Nanni, G.; Holst, J.J. Circadian rhythms of GIP and GLP1 in glucose-tolerant and in type 2 diabetic patients after biliopancreatic diversion. Diabetologia 2009, 52, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Salinari, S.; Bertuzzi, A.; Asnaghi, S.; Guidone, C.; Manco, M.; Mingrone, G. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care 2009, 32, 375–380. [Google Scholar] [CrossRef] [Green Version]
- Powell, D.R.; Smith, M.; Greer, J.; Harris, A.; Zhao, S.; DaCosta, C.; Mseeh, F.; Shadoan, M.K.; Sands, A.; Zambrowicz, B.; et al. LX4211 increases serum glucagon-like peptide 1 and peptide YY levels by reducing sodium/glucose cotransporter 1 (SGLT1)-mediated absorption of intestinal glucose. J. Pharm. Exp. Ther. 2013, 345, 250–259. [Google Scholar] [CrossRef]
- Oguma, T.; Nakayama, K.; Kuriyama, C.; Matsushita, Y.; Yoshida, K.; Hikida, K.; Obokata, N.; Tsuda-Tsukimoto, M.; Saito, A.; Arakawa, K.; et al. Intestinal sodium glucose cotransporter 1 inhibition enhances glucagon-like peptide-1 secretion in normal and diabetic rodents. J. Pharm. Exp. Ther. 2015, 354, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Dobbins, R.L.; Greenway, F.L.; Chen, L.; Liu, Y.; Breed, S.L.; Andrews, S.M.; Wald, J.A.; Walker, A.; Smith, C.D. Selective sodium-dependent glucose transporter 1 inhibitors block glucose absorption and impair glucose-dependent insulinotropic peptide release. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G946–G954. [Google Scholar] [CrossRef]
- He, Y.L.; Haynes, W.; Meyers, C.D.; Amer, A.; Zhang, Y.; Mahling, P.; Mendonza, A.E.; Ma, S.; Chutkow, W.; Bachman, E. The effects of licogliflozin, a dual SGLT1/2 inhibitor, on body weight in obese patients with or without diabetes. Diabetes Obes. Metab. 2019, 21, 1311–1321. [Google Scholar] [CrossRef] [Green Version]
- Qualmann, C.; Nauck, M.A.; Holst, J.J.; Orskov, C.; Creutzfeldt, W. Glucagon-like peptide 1 (7-36 amide) secretion in response to luminal sucrose from the upper and lower gut. A study using alpha-glucosidase inhibition (acarbose). Scand. J. Gastroenterol. 1995, 30, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.Y.; Yang, J.H.; Shan, C.Y.; Zhou, H.T.; Xu, Y.G.; Wang, Y.; Ren, H.Z.; Chang, B.C.; Chen, L.M. Effects of 24-week treatment with acarbose on glucagon-like peptide 1 in newly diagnosed type 2 diabetic patients: A preliminary report. Cardiovasc. Diabetol. 2013, 12, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanis, L.; Hausken, T.; Gentilcore, D.; Rigda, R.S.; Rayner, C.K.; Feinle-Bisset, C.; Horowitz, M.; Jones, K.L. Comparative effects of glucose and xylose on blood pressure, gastric emptying and incretin hormones in healthy older subjects. Br. J. Nutr. 2011, 105, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.A.; Reaven, G.; Olefsky, J. Plasma glucose and insulin responses to orally administered simple and complex carbohydrates. Diabetes 1976, 25, 741–747. [Google Scholar] [CrossRef]
- Wu, T.; Bound, M.J.; Zhao, B.Y.R.; Standfield, S.D.; Bellon, M.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effects of a D-xylose preload with or without sitagliptin on gastric emptying, glucagon-like peptide-1, and postprandial glycemia in type 2 diabetes. Diabetes Care 2013, 36, 1913–1918. [Google Scholar] [CrossRef] [Green Version]
- Damci, T.; Yalin, S.; Balci, H.; Osar, Z.; Korugan, U.; Ozyazar, M.; Ilkova, H. Orlistat augments postprandial increases in glucagon-like peptide 1 in obese type 2 diabetic patients. Diabetes Care 2004, 27, 1077–1080. [Google Scholar] [CrossRef] [Green Version]
- Ellrichmann, M.; Kapelle, M.; Ritter, P.R.; Holst, J.J.; Herzig, K.H.; Schmidt, W.E.; Schmitz, F.; Meier, J.J. Orlistat inhibition of intestinal lipase acutely increases appetite and attenuates postprandial glucagon-like peptide-1-(7-36)-amide-1, cholecystokinin, and peptide YY concentrations. J. Clin. Endocrinol. Metab. 2008, 93, 3995–3998. [Google Scholar] [CrossRef] [Green Version]
- Enc, F.Y.; Ones, T.; Akin, H.L.; Dede, F.; Turoglu, H.T.; Ulfer, G.; Bekiroglu, N.; Haklar, G.; Rehfeld, J.F.; Holst, J.J.; et al. Orlistat accelerates gastric emptying and attenuates GIP release in healthy subjects. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G482–G489. [Google Scholar] [CrossRef]
- O’Donovan, D.; Horowitz, M.; Russo, A.; Feinle-Bisset, C.; Murolo, N.; Gentilcore, D.; Wishart, J.M.; Morris, H.A.; Jones, K.L. Effects of lipase inhibition on gastric emptying of, and on the glycaemic, insulin and cardiovascular responses to, a high-fat/carbohydrate meal in type 2 diabetes. Diabetologia 2004, 47, 2208–2214. [Google Scholar] [CrossRef] [Green Version]
- Chaikomin, R.; Wu, K.L.; Doran, S.; Meyer, J.H.; Jones, K.L.; Feinle-Bisset, C.; Horowitz, M.; Rayner, C.K. Effects of mid-jejunal compared to duodenal glucose infusion on peptide hormone release and appetite in healthy men. Regul. Pept. 2008, 150, 38–42. [Google Scholar] [CrossRef]
- Wu, T.; Thazhath, S.S.; Marathe, C.S.; Bound, M.J.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Comparative effect of intraduodenal and intrajejunal glucose infusion on the gut-incretin axis response in healthy males. Nutr. Diabetes 2015, 5, e156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigda, R.S.; Trahair, L.G.; Little, T.J.; Wu, T.; Standfield, S.; Feinle-Bisset, C.; Rayner, C.K.; Horowitz, M.; Jones, K.L. Regional specificity of the gut-incretin response to small intestinal glucose infusion in healthy older subjects. Peptides 2016, 86, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Poppitt, S.D.; Shin, H.S.; McGill, A.T.; Budgett, S.C.; Lo, K.; Pahl, M.; Duxfield, J.; Lane, M.; Ingram, J.R. Duodenal and ileal glucose infusions differentially alter gastrointestinal peptides, appetite response, and food intake: A tube feeding study. Am. J. Clin. Nutr 2017, 106, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Mangan, A.M.; Al Najim, W.; McNamara, N.; Martin, W.P.; Antanaitis, A.; Bleiel, S.B.; Kent, R.M.; le Roux, C.W.; Docherty, N.G. Effect of macronutrient type and gastrointestinal release site on PYY response in normal healthy subjects. J. Clin. Endocrinol. Metab. 2019, 104, 3661–3669. [Google Scholar] [CrossRef]
- Maljaars, P.W.; Symersky, T.; Kee, B.C.; Haddeman, E.; Peters, H.P.; Masclee, A.A. Effect of ileal fat perfusion on satiety and hormone release in healthy volunteers. Int. J. Obes. 2008, 32, 1633–1639. [Google Scholar] [CrossRef] [Green Version]
- van Avesaat, M.; Troost, F.J.; Ripken, D.; Hendriks, H.F.; Masclee, A.A. Ileal brake activation: Macronutrient-specific effects on eating behavior? Int. J. Obes. 2015, 39, 235–243. [Google Scholar] [CrossRef]
- Christiansen, C.B.; Gabe, M.B.N.; Svendsen, B.; Dragsted, L.O.; Rosenkilde, M.M.; Holst, J.J. The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G53–G65. [Google Scholar] [CrossRef] [Green Version]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015, 39, 424–429. [Google Scholar] [CrossRef] [Green Version]
- Freeland, K.R.; Wolever, T.M. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-alpha. Br. J. Nutr. 2010, 103, 460–466. [Google Scholar] [CrossRef] [Green Version]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canfora, E.E.; van der Beek, C.M.; Jocken, J.W.E.; Goossens, G.H.; Holst, J.J.; Olde Damink, S.W.M.; Lenaerts, K.; Dejong, C.H.C.; Blaak, E.E. Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: A randomized crossover trial. Sci. Rep. 2017, 7, 2360. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.B.; Trammell, S.A.J.; Wewer Albrechtsen, N.J.; Schoonjans, K.; Albrechtsen, R.; Gillum, M.P.; Kuhre, R.E.; Holst, J.J. Bile acids drive colonic secretion of glucagon-like-peptide 1 and peptide-YY in rodents. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G574–G584. [Google Scholar] [CrossRef]
- Petersen, N.; Reimann, F.; Bartfeld, S.; Farin, H.F.; Ringnalda, F.C.; Vries, R.G.; van den Brink, S.; Clevers, H.; Gribble, F.M.; de Koning, E.J. Generation of L cells in mouse and human small intestine organoids. Diabetes 2014, 63, 410–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudling, M.; Camilleri, M.; Graffner, H.; Holst, J.J.; Rikner, L. Specific inhibition of bile acid transport alters plasma lipids and GLP-1. BMC Cardiovasc Disord. 2015, 15, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; Bound, M.J.; Standfield, S.D.; Gedulin, B.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Effects of rectal administration of taurocholic acid on glucagon-like peptide-1 and peptide YY secretion in healthy humans. Diabetes Obes. Metab. 2013, 15, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Adrian, T.E.; Gariballa, S.; Parekh, K.A.; Thomas, S.A.; Saadi, H.; Al Kaabi, J.; Nagelkerke, N.; Gedulin, B.; Young, A.A. Rectal taurocholate increases L cell and insulin secretion, and decreases blood glucose and food intake in obese type 2 diabetic volunteers. Diabetologia 2012, 55, 2343–2347. [Google Scholar] [CrossRef]
- Marathe, C.S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care 2013, 36, 1396–1405. [Google Scholar] [CrossRef] [Green Version]
- Watson, L.E.; Xie, C.; Wang, X.; Li, Z.; Phillips, L.K.; Sun, Z.; Jones, K.L.; Horowitz, M.; Rayner, C.K.; Wu, T. Gastric emptying in patients with well-controlled type 2 diabetes compared with young and older control subjects without diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 3311–3319. [Google Scholar] [CrossRef]
- Seimon, R.V.; Brennan, I.M.; Russo, A.; Little, T.J.; Jones, K.L.; Standfield, S.; Wishart, J.M.; Horowitz, M.; Feinle-Bisset, C. Gastric emptying, mouth-to-cecum transit, and glycemic, insulin, incretin, and energy intake responses to a mixed-nutrient liquid in lean, overweight, and obese males. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E294–E300. [Google Scholar] [CrossRef]
- Perano, S.J.; Rayner, C.K.; Kritas, S.; Horowitz, M.; Donaghue, K.; Mpundu-Kaambwa, C.; Giles, L.; Couper, J.J. Gastric Emptying Is More Rapid in Adolescents With Type 1 Diabetes and Impacts on Postprandial Glycemia. J. Clin. Endocrinol. Metab. 2015, 100, 2248–2253. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.L.; Horowitz, M.; Wishart, J.M.; Maddox, A.F.; Harding, P.E.; Chatterton, B.E. Relationships between gastric-emptying, intragastric meal distribution and blood-glucose concentrations in diabetes-mellitus. J. Nucl. Med. 1995, 36, 2220–2228. [Google Scholar] [PubMed]
- Watson, L.E.; Phillips, L.K.; Wu, T.; Bound, M.J.; Checklin, H.L.; Grivell, J.; Jones, K.L.; Clifton, P.M.; Horowitz, M.; Rayner, C.K. A whey/guar “preload” improves postprandial glycaemia and glycated haemoglobin levels in type 2 diabetes: A 12-week, single-blind, randomized, placebo-controlled trial. Diabetes Obes. Metab. 2019, 21, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, T.; Rink, L.; Sherr, J.L.; Herold, K.C. Acute metabolic effects of exenatide in patients with type 1 diabetes with and without residual insulin to oral and intravenous glucose challenges. Diabetes Care 2014, 37, 210–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; Little, T.J.; Bound, M.J.; Borg, M.; Zhang, X.; Deacon, C.F.; Horowitz, M.; Jones, K.L.; Rayner, C.K. A protein preload enhances the glucose-lowering efficacy of vildagliptin in type 2 diabetes. Diabetes Care 2016, 39, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Vilsbøll, T.; Krarup, T.; Madsbad, S.; Holst, J.J. Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul. Pept. 2003, 114, 115–121. [Google Scholar] [CrossRef]
- Alleleyn, A.M.; van Avesaat, M.; Troost, F.J.; Masclee, A.A. Gastrointestinal nutrient infusion site and eating behavior: Evidence for a proximal to distal gradient within the small intestine? Nutrients 2016, 8, 117. [Google Scholar] [CrossRef] [Green Version]
- Maljaars, P.W.; Peters, H.P.; Kodde, A.; Geraedts, M.; Troost, F.J.; Haddeman, E.; Masclee, A.A. Length and site of the small intestine exposed to fat influences hunger and food intake. Br. J. Nutr. 2011, 106, 1609–1615. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Checklin, H.L.; Wishart, J.M.; Stevens, J.E.; Jones, K.L.; Horowitz, M.; Meyer, J.H.; Rayner, C.K. A randomised trial of enteric-coated nutrient pellets to stimulate gastrointestinal peptide release and lower glycaemia in type 2 diabetes. Diabetologia 2013, 56, 1236–1242. [Google Scholar] [CrossRef] [Green Version]
- Calderon, G.; McRae, A.; Rievaj, J.; Davis, J.; Zandvakili, I.; Linker-Nord, S.; Burton, D.; Roberts, G.; Reimann, F.; Gedulin, B.; et al. Ileo-colonic delivery of conjugated bile acids improves glucose homeostasis via colonic GLP-1-producing enteroendocrine cells in human obesity and diabetes. EBioMedicine 2020, 55, 102759. [Google Scholar] [CrossRef]
- Fruhbeck, G. Bariatric and metabolic surgery: A shift in eligibility and success criteria. Nat. Rev. Endocrinol. 2015, 11, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Rubino, F.; Schauer, P.R.; Kaplan, L.M.; Cummings, D.E. Metabolic surgery to treat type 2 diabetes: Clinical outcomes and mechanisms of action. Annu. Rev. Med. 2010, 61, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, N.B.; Dirksen, C.; Bojsen-Møller, K.N.; Jacobsen, S.H.; Worm, D.; Hansen, D.L.; Kristiansen, V.B.; Naver, L.; Madsbad, S.; Holst, J.J. The exaggerated glucagon-like peptide-1 response is important for the improved β-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes 2013, 62, 3044–3052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svane, M.S.; Bojsen-Moller, K.N.; Nielsen, S.; Jorgensen, N.B.; Dirksen, C.; Bendtsen, F.; Kristiansen, V.B.; Hartmann, B.; Holst, J.J.; Madsbad, S. Effects of endogenous GLP-1 and GIP on glucose tolerance after Roux-en-Y gastric bypass surgery. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E505–E514. [Google Scholar] [CrossRef] [Green Version]
- Svane, M.S.; Jorgensen, N.B.; Bojsen-Moller, K.N.; Dirksen, C.; Nielsen, S.; Kristiansen, V.B.; Torang, S.; Wewer Albrechtsen, N.J.; Rehfeld, J.F.; Hartmann, B.; et al. Peptide YY and glucagon-like peptide-1 contribute to decreased food intake after Roux-en-Y gastric bypass surgery. Int. J. Obes. 2016, 40, 1699–1706. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Kinross, J.; Burcelin, R.; Gibson, G.; Jia, W.; Pettersson, S. Host-gut microbiota metabolic interactions. Science 2012, 336, 1262–1267. [Google Scholar] [CrossRef] [Green Version]
- Seck, E.H.; Senghor, B.; Merhej, V.; Bachar, D.; Cadoret, F.; Robert, C.; Azhar, E.I.; Yasir, M.; Bibi, F.; Jiman-Fatani, A.A.; et al. Salt in stools is associated with obesity, gut halophilic microbiota and Akkermansia muciniphila depletion in humans. Int. J. Obes. 2019, 43, 862–871. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef]
- Wahlstrom, A.; Sayin, S.I.; Marschall, H.U.; Backhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef] [Green Version]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Backhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Byrne, C.S.; Chambers, E.S.; Alhabeeb, H.; Chhina, N.; Morrison, D.J.; Preston, T.; Tedford, C.; Fitzpatrick, J.; Irani, C.; Busza, A.; et al. Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. Am. J. Clin. Nutr. 2016, 104, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, C.; Jones, K.L.; Rayner, C.K.; Wu, T. Enteroendocrine Hormone Secretion and Metabolic Control: Importance of the Region of the Gut Stimulation. Pharmaceutics 2020, 12, 790. https://doi.org/10.3390/pharmaceutics12090790
Xie C, Jones KL, Rayner CK, Wu T. Enteroendocrine Hormone Secretion and Metabolic Control: Importance of the Region of the Gut Stimulation. Pharmaceutics. 2020; 12(9):790. https://doi.org/10.3390/pharmaceutics12090790
Chicago/Turabian StyleXie, Cong, Karen L. Jones, Christopher K. Rayner, and Tongzhi Wu. 2020. "Enteroendocrine Hormone Secretion and Metabolic Control: Importance of the Region of the Gut Stimulation" Pharmaceutics 12, no. 9: 790. https://doi.org/10.3390/pharmaceutics12090790
APA StyleXie, C., Jones, K. L., Rayner, C. K., & Wu, T. (2020). Enteroendocrine Hormone Secretion and Metabolic Control: Importance of the Region of the Gut Stimulation. Pharmaceutics, 12(9), 790. https://doi.org/10.3390/pharmaceutics12090790