Advanced Characterization of Imiquimod-Induced Psoriasis-Like Mouse Model
Abstract
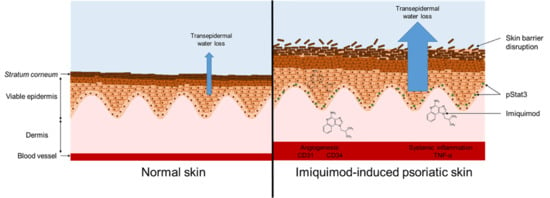
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Imiquimod Induced Psoriasis
2.3. Quantification of Imiquimod in Skin
2.4. Psoriasis Area Severity Index (PASI) Score Evaluation
2.5. Ear Thickness Measurements
2.6. Histology
2.7. Phosphorylated Signal Transducer and Activator of Transcription 3 (pSTAT3) Staining
2.8. Trans-Epidermal Water Loss (TEWL)
2.9. Vascular Endothelial Markers Staining
2.10. Quantification of Inflammatory Cytokines in Serum and Skin
2.11. Spleen Length and Mass
2.12. Lymph Nodes Staining
2.13. Statistical Analysis
3. Results
3.1. Relation of Imiquimod Concentration to the Severity of Symptoms
3.2. Severity of Skin Inflammation
3.3. Ear Thickness Measurements
3.4. Increased Epidermal Thickness and Infiltration of Keratinocytes
3.5. Expression of pSTAT3 Proteins
3.6. Skin Barrier Disruption
3.7. Dermal Hypervascularity and Angiogenesis
3.8. Inflammatory Cytokines
3.9. Systemic Inflammatory Response
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, A.; Leon, A.; Butler, D.C.; Reichenberg, J. Quality-of-life effects of common dermatological diseases. Semin. Cutan. Med. Surg. 2013, 32, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.-H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- El Malki, K.; Karbach, S.H.; Huppert, J.; Zayoud, M.; Reissig, S.; Schüler, R.; Nikolaev, A.; Karram, K.; Münzel, T.; Kuhlmann, C.R.W.; et al. An alternative pathway of imiquimod-induced psoriasis-like skin inflammation in the absence of interleukin-17 receptor a signaling. J. Investig. Dermatol. 2013, 133, 441–451. [Google Scholar] [CrossRef] [Green Version]
- van der Fits, L.; Mourits, S.; Voerman, J.S.A.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.-M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef] [PubMed]
- Chamcheu, J.C.; Chaves-Rodriquez, M.-I.; Adhami, V.M.; Siddiqui, I.A.; Wood, G.S.; Longley, B.J.; Mukhtar, H. Upregulation of PI3K/AKT/mTOR, FABP5 and PPARβ/δ in Human Psoriasis and Imiquimod-induced Murine Psoriasiform Dermatitis Model. Acta Derm. Venereol. 2016, 96, 854–856. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, A.; Al-Harbi, N.O.; Al-Harbi, M.M.; El-Sherbeeny, A.M.; Ahmad, S.F.; Siddiqui, N.; Ansari, M.A.; Zoheir, K.M.A.; Attia, S.M.; Al-Hosaini, K.A.; et al. Imiquimod-induced psoriasis-like skin inflammation is suppressed by BET bromodomain inhibitor in mice through RORC/IL-17A pathway modulation. Pharmacol. Res. 2015, 99, 248–257. [Google Scholar] [CrossRef]
- Lin, Y.-K.; Yang, S.-H.; Chen, C.-C.; Kao, H.-C.; Fang, J.-Y. Using Imiquimod-Induced Psoriasis-Like Skin as a Model to Measure the Skin Penetration of Anti-Psoriatic Drugs. PLoS ONE 2015, 10, e0137890. [Google Scholar] [CrossRef] [Green Version]
- Boisgard, A.-S.; Lamrayah, M.; Dzikowski, M.; Salmon, D.; Kirilov, P.; Primard, C.; Pirot, F.; Fromy, B.; Verrier, B. Innovative drug vehicle for local treatment of inflammatory skin diseases: Ex vivo and in vivo screening of five topical formulations containing poly(lactic acid) (PLA) nanoparticles. Eur. J. Pharm. Biopharm. 2017, 116, 51–60. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Z.; Wang, L.; Cun, D.; Tong, H.H.Y.; Yan, R.; Chen, X.; Wang, R.; Zheng, Y. Enhanced topical penetration, system exposure and anti-psoriasis activity of two particle-sized, curcumin-loaded PLGA nanoparticles in hydrogel. J. Control. Release 2017, 254, 44–54. [Google Scholar] [CrossRef]
- Bahramizadeh, M.; Bahramizadeh, M.; Kiafar, B.; Jafarian, A.H.; Nikpoor, A.R.; Hatamipour, M.; Esmaily, H.; Rezaeemehr, Z.; Golmohammadzadeh, S.; Moosavian, S.A.; et al. Development, characterization and evaluation of topical methotrexate-entrapped deformable liposome on imiquimod-induced psoriasis in a mouse model. Int. J. Pharm. 2019, 569, 118623. [Google Scholar] [CrossRef]
- Sathe, P.; Saka, R.; Kommineni, N.; Raza, K.; Khan, W. Dithranol-loaded nanostructured lipid carrier-based gel ameliorate psoriasis in imiquimod-induced mice psoriatic plaque model. Drug Dev. Ind. Pharm. 2019, 45, 826–838. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, G.; Wang, W.; Liu, R.; Liao, L.; Cheng, N.; Li, W.; Zhang, W.; Ding, D. Cyclodextrin-Modified CeO2 Nanoparticles as a Multifunctional Nanozyme for Combinational Therapy of Psoriasis. Int. J. Nanomed. 2020, 15, 2515–2527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raychaudhuri, S.K.; Maverakis, E.; Raychaudhuri, S.P. Diagnosis and classification of psoriasis. Autoimmun. Rev. 2014, 13, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Gardien, K.L.M.; Baas, D.C.; de Vet, H.C.W.; Middelkoop, E. Transepidermal water loss measured with the Tewameter TM300 in burn scars. Burns 2016, 42, 1455–1462. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Froiio, F.; Mancuso, A.; Iannone, M.; Fresta, M.; Fiorito, S.; Celia, C.; Paolino, D. In vitro and in vivo trans-epidermal water loss evaluation following topical drug delivery systems application for pharmaceutical analysis. J. Pharm. Biomed. Anal. 2020, 186, 113295. [Google Scholar] [CrossRef]
- Danilenko, D.M. Review paper: Preclinical models of psoriasis. Vet. Pathol. 2008, 45, 563–575. [Google Scholar] [CrossRef]
- Heidenreich, R.; Röcken, M.; Ghoreschi, K. Angiogenesis drives psoriasis pathogenesis. Int. J. Exp. Pathol. 2009, 90, 232–248. [Google Scholar] [CrossRef]
- Ilan, N.; Madri, J.A. PECAM-1: Old friend, new partners. Curr. Opin. Cell Biol. 2003, 15, 515–524. [Google Scholar] [CrossRef]
- Cao, G.; O’Brien, C.D.; Zhou, Z.; Sanders, S.M.; Greenbaum, J.N.; Makrigiannakis, A.; DeLisser, H.M. Involvement of human PECAM-1 in angiogenesis and in vitro endothelial cell migration. Am. J. Physiol. Cell Physiol. 2002, 282, C1181–C1190. [Google Scholar] [CrossRef]
- Manole, C.; Gherghiceanu, M.; Simionescu, O. Telocyte dynamics in psoriasis. J. Cell Mol. Med. 2015, 19, 1504–1519. [Google Scholar] [CrossRef]
- Zhang, K.; Li, X.; Yin, G.; Liu, Y.; Tang, X. Functional characterization of T cells differentiated in vitro from bone marrow-derived CD34 cells of psoriatic patients with family history. Exp. Dermatol. 2010, 19, e128–e135. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.M.; Azim, Z.A. Immunohistochemical study of osteopontin, Ki-67, and CD34 of psoriasis in Mansoura, Egypt. Indian J. Pathol. Microbiol. 2012, 55, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, M.; Shamshiri, A.; Zavattaro, E.; Khazaei, S.; Rezaei, M.; Mahmoodi, R.; Sadeghi, M. Immunohistochemical expression of P53, Ki-67, and CD34 in psoriasis and psoriasiform dermatitis. Biomedicine 2019, 9, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sano, S.; Chan, K.S.; Carbajal, S.; Clifford, J.; Peavey, M.; Kiguchi, K.; Itami, S.; Nickoloff, B.J.; DiGiovanni, J. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat. Med. 2005, 11, 43–49. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Schwartz, D.M.; Villarino, A.V.; Gadina, M.; McInnes, I.B.; Laurence, A. The JAK-STAT pathway: Impact on human disease and therapeutic intervention. Annu. Rev. Med. 2015, 66, 311–328. [Google Scholar] [CrossRef] [Green Version]
- Calautti, E.; Avalle, L.; Poli, V. Psoriasis: A STAT3-Centric View. Int. J. Mol. Sci. 2018, 19, 171. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Zhang, X.; Xu, M.; Zhang, F.; Tian, F.; Cui, J.; Xia, Y.; Liang, C.; Zhou, S.; Wei, H.; et al. Berberine downregulates CDC6 and inhibits proliferation via targeting JAK-STAT3 signaling in keratinocytes. Cell Death Dis. 2019, 10, 274. [Google Scholar] [CrossRef]
- Kuang, Y.-H.; Lu, Y.; Liu, Y.-K.; Liao, L.-Q.; Zhou, X.-C.; Qin, Q.-S.; Jia, X.-K.; Wu, L.-S.; Zhu, W.; Chen, X. Topical Sunitinib ointment alleviates Psoriasis-like inflammation by inhibiting the proliferation and apoptosis of keratinocytes. Eur. J. Pharmacol. 2018, 824, 57–63. [Google Scholar] [CrossRef]
- Miyoshi, K.; Takaishi, M.; Nakajima, K.; Ikeda, M.; Kanda, T.; Tarutani, M.; Iiyama, T.; Asao, N.; DiGiovanni, J.; Sano, S. Stat3 as a therapeutic target for the treatment of psoriasis: A clinical feasibility study with STA-21, a Stat3 inhibitor. J. Investig. Dermatol. 2011, 131, 108–117. [Google Scholar] [CrossRef] [Green Version]
- Bogen, S.; Pak, J.; Garifallou, M.; Deng, X.; Muller, W.A. Monoclonal antibody to murine PECAM-1 (CD31) blocks acute inflammation in vivo. J. Exp. Med. 1994, 179, 1059–1064. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Fu, L.-X.; Guo, Z.-P.; Yin, B.; Cao, N.; Qin, S. Involvement of high mobility group box-1 in imiquimod-induced psoriasis-like mice model. J. Dermatol. 2017, 44, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Marini, O.; Bevilacqua, D.; DeFranco, A.L.; Hou, B.; Lonardi, S.; Vermi, W.; Rodegher, P.; Panato, A.; Tagliaro, F.; et al. Role of MyD88 signaling in the imiquimod-induced mouse model of psoriasis: Focus on innate myeloid cells. J. Leukoc. Biol. 2017, 102, 791–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilliet, M.; Conrad, C.; Geiges, M.; Cozzio, A.; Thürlimann, W.; Burg, G.; Nestle, F.O.; Dummer, R. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch. Dermatol. 2004, 140, 1490–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Paula, D.; Martins, C.A.; Bentley, M.V.L.B. Development and validation of HPLC method for imiquimod determination in skin penetration studies. Biomed. Chromatogr. 2008, 22, 1416–1423. [Google Scholar] [CrossRef]
- Amsen, D.; de Visser, K.E.; Town, T. Approaches to determine expression of inflammatory cytokines. Methods Mol. Biol. 2009, 511, 107–142. [Google Scholar] [CrossRef] [Green Version]
- Mastelic Gavillet, B.; Eberhardt, C.S.; Auderset, F.; Castellino, F.; Seubert, A.; Tregoning, J.S.; Lambert, P.-H.; de Gregorio, E.; Del Giudice, G.; Siegrist, C.-A. MF59 Mediates Its B Cell Adjuvanticity by Promoting T Follicular Helper Cells and Thus Germinal Center Responses in Adult and Early Life. J. Immunol. 2015, 194, 4836–4845. [Google Scholar] [CrossRef] [Green Version]
- Lofano, G.; Mancini, F.; Salvatore, G.; Cantisani, R.; Monaci, E.; Carrisi, C.; Tavarini, S.; Sammicheli, C.; Rossi Paccani, S.; Soldaini, E.; et al. Oil-in-Water Emulsion MF59 Increases Germinal Center B Cell Differentiation and Persistence in Response to Vaccination. J. Immunol. 2015, 195, 1617–1627. [Google Scholar] [CrossRef] [Green Version]
- Vogt, A.; Wischke, C.; Neffe, A.T.; Ma, N.; Alexiev, U.; Lendlein, A. Nanocarriers for drug delivery into and through the skin-Do existing technologies match clinical challenges? J. Control. Release 2016, 242, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Nino, M.; Calabrò, G.; Santoianni, P. Topical delivery of active principles: The field of dermatological research. Dermatol. Online J. 2010, 16, 4. [Google Scholar]
- Wikramanayake, T.C.; Stojadinovic, O.; Tomic-Canic, M. Epidermal Differentiation in Barrier Maintenance and Wound Healing. Adv. Wound Care 2014, 3, 272–280. [Google Scholar] [CrossRef]
- Takahashi, T.; Koga, Y.; Kainoh, M. Anti-IL-12/IL-23p40 antibody ameliorates dermatitis and skin barrier dysfunction in mice with imiquimod-induced psoriasis-like dermatitis. Eur. J. Pharmacol. 2018, 828, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.P.; Desai, P.R.; Patel, A.R.; Singh, M.S. Skin permeating nanogel for the cutaneous co-delivery of two anti-inflammatory drugs. Biomaterials 2012, 33, 1607–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietrzak, A.T.; Zalewska, A.; Chodorowska, G.; Krasowska, D.; Michalak-Stoma, A.; Nockowski, P.; Osemlak, P.; Paszkowski, T.; Roliński, J.M. Cytokines and anticytokines in psoriasis. Clin. Chim. Acta 2008, 394, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Yazici, A.C.; Tursen, U.; Apa, D.D.; Ikizoglu, G.; Api, H.; Baz, K.; Tasdelen, B. The changes in expression of ICAM-3, Ki-67, PCNA, and CD31 in psoriatic lesions before and after methotrexate treatment. Arch. Dermatol. Res. 2005, 297, 249–255. [Google Scholar] [CrossRef]
- Itoh, T.; Hatano, R.; Komiya, E.; Otsuka, H.; Narita, Y.; Aune, T.M.; Dang, N.H.; Matsuoka, S.; Naito, H.; Tominaga, M.; et al. Biological Effects of IL-26 on T Cell-Mediated Skin Inflammation, Including Psoriasis. J. Investig. Dermatol. 2019, 139, 878–889. [Google Scholar] [CrossRef] [Green Version]
- Creamer, D.; Allen, M.H.; Sousa, A.; Poston, R.; Barker, J.N. Localization of endothelial proliferation and microvascular expansion in active plaque psoriasis. Br. J. Dermatol. 1997, 136, 859–865. [Google Scholar] [CrossRef]
- Coimbra, S.; Figueiredo, A.; Castro, E.; Rocha-Pereira, P.; Santos-Silva, A. The roles of cells and cytokines in the pathogenesis of psoriasis. Int. J. Dermatol. 2012, 51, 389–395, quiz 395–398. [Google Scholar] [CrossRef]
- Hjuler, K.F.; Gormsen, L.C.; Vendelbo, M.H.; Egeberg, A.; Nielsen, J.; Iversen, L. Systemic Inflammation and Evidence of a Cardio-splenic Axis in Patients with Psoriasis. Acta Derm. Venereol. 2018, 98, 390–395. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.-M.; Ma, M.; Li, H.; Qi, Q.; Liu, Y.-T.; Yan, Y.-X.; Shen, Y.-F.; Yang, X.-Q.; Zhu, F.-H.; He, S.-J.; et al. Topical administration of reversible SAHH inhibitor ameliorates imiquimod-induced psoriasis-like skin lesions in mice via suppression of TNF-α/IFN-γ-induced inflammatory response in keratinocytes and T cell-derived IL-17. Pharmacol. Res. 2018, 129, 443–452. [Google Scholar] [CrossRef]
- Grine, L.; Dejager, L.; Libert, C.; Vandenbroucke, R.E. An inflammatory triangle in psoriasis: TNF, type I IFNs and IL-17. Cytokine Growth Factor Rev. 2015, 26, 25–33. [Google Scholar] [CrossRef]
- Vaporciyan, A.A.; DeLisser, H.M.; Yan, H.C.; Mendiguren, I.I.; Thom, S.R.; Jones, M.L.; Ward, P.A.; Albelda, S.M. Involvement of platelet-endothelial cell adhesion molecule-1 in neutrophil recruitment in vivo. Science 1993, 262, 1580–1582. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Shi, Y.; Wang, F.; Yang, H.; Han, S.; Bai, Y. Altered circulating T follicular helper cell subsets in patients with psoriasis vulgaris. Immunol. Lett. 2017, 181, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Yanaba, K.; Kamata, M.; Ishiura, N.; Shibata, S.; Asano, Y.; Tada, Y.; Sugaya, M.; Kadono, T.; Tedder, T.F.; Sato, S. Regulatory B cells suppress imiquimod-induced, psoriasis-like skin inflammation. J. Leukoc. Biol. 2013, 94, 563–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terhorst, D.; Chelbi, R.; Wohn, C.; Malosse, C.; Tamoutounour, S.; Jorquera, A.; Bajenoff, M.; Dalod, M.; Malissen, B.; Henri, S. Dynamics and Transcriptomics of Skin Dendritic Cells and Macrophages in an Imiquimod-Induced, Biphasic Mouse Model of Psoriasis. J. Immunol. 2015, 195, 4953–4961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, P.; Narasimhan, B.; Wang, Q. Biocompatible nanoparticles and vesicular systems in transdermal drug delivery for various skin diseases. Int. J. Pharm. 2019, 555, 49–62. [Google Scholar] [CrossRef]
- Pradhan, M.; Alexander, A.; Singh, M.R.; Singh, D.; Saraf, S.; Saraf, S. Understanding the prospective of nano-formulations towards the treatment of psoriasis. Biomed. Pharmacother. 2018, 107, 447–463. [Google Scholar] [CrossRef]












| Ctrl | D2 | D3 | D4 | D6 | D8 | |||
|---|---|---|---|---|---|---|---|---|
| IMQ concentration | 0 | + | + | + | ++++ | ++++ | ||
| Physical/ Histological Markers | PASI score | 0 | 0 | + | ++ | ++++ | ++++ | |
| Ear thickness | - | - | - | - | ++ | ++++ | ||
| Epidermal thickness | - | - | + | ++ | ++ | ++++ | ||
| pSTAT3 signal | 0 | 0 | 0 | 0 | ++ | ++ | ||
| TEWL | Back | - | + | + | +++ | ++++ | ++++ | |
| Ear | - | - | - | + | +++ | +++ | ||
| Dermal vascularity | - | - | - | + | ++ | ++ | ||
| Systemic Toxicity | TNF-α serum | - | + | - | - | - | - | |
| Spleen | Length | - | + | +++ | +++ | +++ | ++++ | |
| Mass | - | - | + | ++ | +++ | +++ | ||
| Lymph nodes | Area | - | - | + | + | ++ | + | |
| Germinal Centers | 0 | 0 | 0 | 0 | 0 | + | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabeen, M.; Boisgard, A.-S.; Danoy, A.; El Kholti, N.; Salvi, J.-P.; Boulieu, R.; Fromy, B.; Verrier, B.; Lamrayah, M. Advanced Characterization of Imiquimod-Induced Psoriasis-Like Mouse Model. Pharmaceutics 2020, 12, 789. https://doi.org/10.3390/pharmaceutics12090789
Jabeen M, Boisgard A-S, Danoy A, El Kholti N, Salvi J-P, Boulieu R, Fromy B, Verrier B, Lamrayah M. Advanced Characterization of Imiquimod-Induced Psoriasis-Like Mouse Model. Pharmaceutics. 2020; 12(9):789. https://doi.org/10.3390/pharmaceutics12090789
Chicago/Turabian StyleJabeen, Mehwish, Anne-Sophie Boisgard, Alix Danoy, Naima El Kholti, Jean-Paul Salvi, Roselyne Boulieu, Bérengère Fromy, Bernard Verrier, and Myriam Lamrayah. 2020. "Advanced Characterization of Imiquimod-Induced Psoriasis-Like Mouse Model" Pharmaceutics 12, no. 9: 789. https://doi.org/10.3390/pharmaceutics12090789
APA StyleJabeen, M., Boisgard, A. -S., Danoy, A., El Kholti, N., Salvi, J. -P., Boulieu, R., Fromy, B., Verrier, B., & Lamrayah, M. (2020). Advanced Characterization of Imiquimod-Induced Psoriasis-Like Mouse Model. Pharmaceutics, 12(9), 789. https://doi.org/10.3390/pharmaceutics12090789