Selective Targeting and Tissue Penetration to the Retina by a Systemically Administered Vascular Homing Peptide in Oxygen Induced Retinopathy (OIR)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peptide Synthesis
2.2. Mice and Mouse OIR Model
2.3. Peptide Targeting Study
2.4. Immunohistochemistry
2.5. Quantitative Analysis of Immunostaining
2.6. Statistical Analysis
3. Results
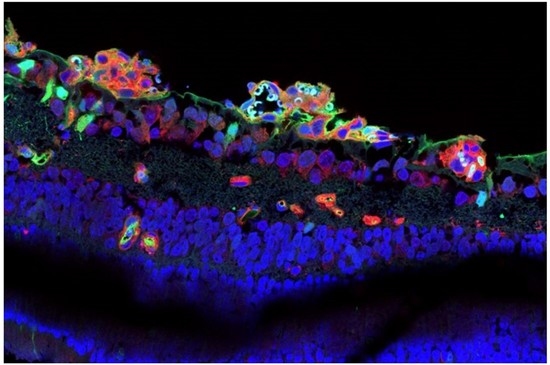
3.1. CAR Peptide Homes to Preretinal Tufts in OIR
3.2. CAR Peptide Homes to Bronchopulmonary Dysplasia in OIR Mice
3.3. CAR Homes to Pathological Angiogenesis in OIR Induced in R-Ras Knockout (KO) Mice
3.4. CAR Peptide Penetrates into the Retina in OIR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vähätupa, M.; Järvinen, T.A.H.; Uusitalo-Järvinen, H. Exploration of Oxygen-Induced Retinopathy Model to Discover New Therapeutic Drug Targets in Retinopathies. Front. Pharmacol. 2020, 11, 873. [Google Scholar] [CrossRef]
- Campochiaro, P.A. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog. Retin. Eye Res. 2015, 49, 67–81. [Google Scholar] [CrossRef] [Green Version]
- Mukwaya, A.; Jensen, L.; Lagali, N. Relapse of pathological angiogenesis: Functional role of the basement membrane and potential treatment strategies. Exp. Mol. Med. 2021, 53, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Ko, J.; Ju, C.; Eltzschig, H.K. Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [PubMed] [Green Version]
- D’Amico, A.G.; Maugeri, G.; Bucolo, C.; Saccone, S.; Federico, C.; Cavallaro, S.; D'Agata, V. Nap Interferes with Hypoxia-Inducible Factors and VEGF Expression in Retina of Diabetic Rats. J. Mol. Neurosci. 2017, 61, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. 2014, 9, 47–71. [Google Scholar] [CrossRef] [PubMed]
- Yeo, E.J. Hypoxia and aging. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Mishra, A. Angiogenic neovessels promote tissue hypoxia. Proc. Natl. Acad. Sci. USA 2016, 113, 10458–10460. [Google Scholar] [CrossRef] [Green Version]
- Sivaprasad, S.; Prevost, T.; Vasconcelos, J.C.; Riddell, A.; Murphy, C.; Kelly, J.; Bainbridge, J.; Tudor-Edwards, R.; Hopkins, D.; Hykin, P.; et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): A multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet 2017, 389, 2193–2203. [Google Scholar]
- Gross, J.G.; Glassman, A.R.; Jampol, L.M.; Inusah, S.; Aiello, L.P.; Antoszyk, A.N.; Baker, C.W.; Berger, B.B.; Bressler, N.M.; Browning, D.; et al. Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA 2015, 314, 2137–2146. [Google Scholar]
- Arrigo, A.; Bandello, F. Molecular Features of Classic Retinal Drugs, Retinal Therapeutic Targets and Emerging Treatments. Pharmaceutics 2021, 13, 1102. [Google Scholar] [CrossRef]
- Gross, J.G.; Glassman, A.R.; Liu, D.; Sun, J.K.; Antoszyk, A.N.; Baker, C.W.; Bressler, N.M.; Elman, M.J.; Ferris, F.L., III; Gardner, T.W.; et al. Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 1138–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholson, L.; Crosby-Nwaobi, R.; Vasconcelos, J.C.; Prevost, A.T.; Ramu, J.; Riddell, A.; Bainbridge, J.W.; Hykin, P.G.; Sivaprasad, S. Mechanistic Evaluation of Panretinal Photocoagulation Versus Aflibercept in Proliferative Diabetic Retinopathy: CLARITY Substudy. Investig. Opthalmol. Vis. Sci. 2018, 59, 4277–4284. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Woo, S.J. Ocular Drug Delivery to the Retina: Current Innovations and Future Perspectives. Pharmaceutics 2021, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Thareja, A.; Hughes, H.; Alvarez-Lorenzo, C.; Hakkarainen, J.; Ahmed, Z. Penetration Enhancers for Topical Drug Delivery to the Ocular Posterior Segment—A Systematic Review. Pharmaceutics 2021, 13, 276. [Google Scholar] [CrossRef]
- Fresta, C.G.; Fidilio, A.; Caruso, G.; Caraci, F.; Giblin, F.J.; Leggio, G.M.; Salomone, S.; Drago, F.; Bucolo, C. A New Human Blood–Retinal Barrier Model Based on Endothelial Cells, Pericytes, and Astrocytes. Int. J. Mol. Sci. 2020, 21, 1636. [Google Scholar] [CrossRef] [Green Version]
- Puglia, C.; Santonocito, D.; Ostacolo, C.; Sommella, E.M.; Campiglia, P.; Carbone, C.; Drago, F.; Pignatello, R.; Bucolo, C. Ocular Formulation Based on Palmitoylethanolamide-Loaded Nanostructured Lipid Carriers: Technological and Pharmacological Profile. Nanomaterials 2020, 10, 287. [Google Scholar] [CrossRef] [Green Version]
- Spadaro, A.; Rao, M.; Lorenti, M.; Romano, M.R.; Augello, A.; Eandi, C.M.; Platania, C.B.M.; Drago, F.; Bucolo, C. New Brilliant Blue G Derivative as Pharmacological Tool in Retinal Surgery. Front. Pharmacol. 2020, 11, 708. [Google Scholar] [CrossRef]
- Järvinen, T.A.H.; Pemmari, T. Systemically Administered, Target-Specific, Multi-Functional Therapeutic Recombinant Proteins in Regenerative Medicine. Nanomaterials 2020, 10, 226. [Google Scholar] [CrossRef] [Green Version]
- Pasqualini, R.; Ruoslahti, E. Organ targeting in vivo using phage display peptide libraries. Nature 1996, 380, 364–366. [Google Scholar] [CrossRef]
- Ruoslahti, E. Vascular zip codes in angiogenesis and metastasis. Biochem. Soc. Trans. 2004, 32, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, T.A.H.; Rashid, J.; May, U.; Valmari, T.; Ahsan, F. Systemic targeted delivery of multi-functional recombinant proteins and nanoparticles in regenerative medicine. ACS Biomater. Sci. Eng. 2017, 3, 1273–1282. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.E.; Wesolowski, E.; McLellan, A.; Kostyk, S.K.; D'Amato, R.; Sullivan, R.; D'Amore, P.A. Oxygen-induced retinopathy in the mouse. Investig. Opthalmol. Vis. Sci. 1994, 35, 101–111. [Google Scholar]
- Stahl, A.; Connor, K.; Sapieha, P.; Chen, J.; Dennison, R.J.; Krah, N.M.; Seaward, M.R.; Willett, K.L.; Aderman, C.M.; Guerin, K.I.; et al. The mouse retina as an angiogenesis model. Investig. Opthalmol. Vis. Sci. 2010, 51, 2813–2826. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, T.A.; Ruoslahti, E. Molecular changes in the vasculature of injured tissues. Am. J. Pathol. 2007, 171, 702–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Järvinen, T.A.; Ruoslahti, E. Target-seeking antifibrotic compound enhances wound healing and suppresses scar formation in mice. Proc. Natl. Acad. Sci. USA 2010, 107, 21671–21676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickramasinghe, L.C.; Lau, M.; Deliyanti, D.; Gottschalk, T.A.; van Wijngaarden, P.; Talia, D.; Johnson, C.; Wilkinson-Berka, J.L.; Tsantikos, E.; Hibbs, M.L. Lung and Eye Disease Develop Concurrently in Supplemental Oxygen–Exposed Neonatal Mice. Am. J. Pathol. 2020, 190, 1801–1812. [Google Scholar] [CrossRef]
- Pilch, J.; Brown, D.M.; Komatsu, M.; Järvinen, T.A.H.; Yang, M.; Peters, D.; Hoffman, R.M.; Ruoslahti, E. Peptides selected for binding to clotted plasma accumulate in tumor stroma and wounds. Proc. Natl. Acad. Sci. USA 2006, 103, 2800–2804. [Google Scholar] [CrossRef] [Green Version]
- Toba, M.; Alzoubi, A.; O’Neill, K.; Abe, K.; Urakami, T.; Komatsu, M.; Alvarez, D.; Järvinen, T.A.; Mann, D.; Ruoslahti, E.; et al. A Novel Vascular Homing Peptide Strategy to Selectively Enhance Pulmonary Drug Efficacy in Pulmonary Arterial Hypertension. Am. J. Pathol. 2014, 184, 369–375. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, M.; Ruoslahti, E. R-Ras is a global regulator of vascular regeneration that suppresses intimal hyperplasia and tumor angiogenesis. Nat. Med. 2005, 11, 1346–1350. [Google Scholar] [CrossRef]
- Kummola, L.; Ortutay, Z.; Vähätupa, M.; Prince, S.; Uusitalo-Järvinen, H.; Järvinen, T.A.; Junttila, I.S. R-Ras deficiency does not affect papain-induced IgE production in mice. Immunity, Inflamm. Dis. 2017, 5, 280–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vähätupa, M.; Nättinen, J.; Jylhä, A.; Aapola, U.; Kataja, M.; Kööbi, P.; Järvinen, T.A.H.; Uusitalo, H.; Uusitalo-Järvinen, H. SWATH-MS Proteomic Analysis of Oxygen-Induced Retinopathy Reveals Novel Potential Therapeutic Targets. Investig. Opthalmol. Vis. Sci. 2018, 59, 3294–3306. [Google Scholar] [CrossRef] [Green Version]
- Vähätupa, M.; Jääskeläinen, N.; Cerrada-Gimenez, M.; Thapa, R.; Järvinen, T.; Kalesnykas, G.; Uusitalo-Järvinen, H. Oxygen-Induced Retinopathy Model for Ischemic Retinal Diseases in Rodents. J. Vis. Exp. 2020, e61482. [Google Scholar] [CrossRef] [PubMed]
- Urakami, T.; Järvinen, T.A.; Toba, M.; Sawada, J.; Ambalavanan, N.; Mann, D.; McMurtry, I.; Oka, M.; Ruoslahti, E.; Komatsu, M. Peptide-Directed Highly Selective Targeting of Pulmonary Arterial Hypertension. Am. J. Pathol. 2011, 178, 2489–2495. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Russo, V.; Zeglinski, M.; Sellers, S.L.; Wu, Z.; Oram, C.; Santacruz, S.; Merkulova, Y.; Turner, C.; Tauh, K.; et al. Recombinant Decorin Fusion Protein Attenuates Murine Abdominal Aortic Aneurysm Formation and Rupture. Sci. Rep. 2017, 7, 15857. [Google Scholar] [CrossRef] [PubMed]
- Salomaa, T.; Pemmari, T.; Määttä, J.; Kummola, L.; Salonen, N.; González-Rodriguez, M.; Parviainen, L.; Hiihtola, L.; Vähätupa, M.; Järvinen, T.A.H.; et al. IL-13Rα1 suppresses tumor progression in two-stage skin carcinogenesis model by regulating regulatory T cells. J. Investig. Dermatol. 2021. In Press. [Google Scholar]
- Iqbal, A.; May, U.; Prince, S.N.; Järvinen, T.A.; Heydemann, A. Systemically Administered Homing Peptide Targets Dystrophic Lesions and Delivers Transforming Growth Factor-β (TGFβ) Inhibitor to Attenuate Murine Muscular Dystrophy Pathology. Pharmaceutics 2021, 13, 1506. [Google Scholar] [CrossRef]
- Gupta, N.; Patel, B.; Nahar, K.; Ahsan, F. Cell permeable peptide conjugated nanoerythrosomes of fasudil prolong pulmonary arterial vasodilation in PAH rats. Eur. J. Pharm. Biopharm. 2014, 88, 1046–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, N.; Ibrahim, H.M.; Ahsan, F. Peptide–Micelle Hybrids Containing Fasudil for Targeted Delivery to the Pulmonary Arteries and Arterioles to Treat Pulmonary Arterial Hypertension. J. Pharm. Sci. 2014, 103, 3743–3753. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Al-Saikhan, F.I.; Patel, B.; Rashid, J.; Ahsan, F. Fasudil and SOD packaged in peptide-studded-liposomes: Properties, pharmacokinetics and ex-vivo targeting to isolated perfused rat lungs. Int. J. Pharm. 2015, 488, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Nahar, K.; Absar, S.; Gupta, N.; Kotamraju, V.R.; McMurtry, I.F.; Oka, M.; Komatsu, M.; Nozik-Grayck, E.; Ahsan, F. Peptide-Coated Liposomal Fasudil Enhances Site Specific Vasodilation in Pulmonary Arterial Hypertension. Mol. Pharm. 2014, 11, 4374–4384. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Rashid, J.; Nozik-Grayck, E.; McMurtry, I.F.; Stenmark, K.R.; Ahsan, F. Cocktail of Superoxide Dismutase and Fasudil Encapsulated in Targeted Liposomes Slows PAH Progression at a Reduced Dosing Frequency. Mol. Pharm. 2017, 14, 830–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keshavarz, A.; Alobaida, A.; McMurtry, I.F.; Nozik-Grayck, E.; Stenmark, K.R.; Ahsan, F. CAR, a Homing Peptide, Prolongs Pulmonary Preferential Vasodilation by Increasing Pulmonary Retention and Reducing Systemic Absorption of Liposomal Fasudil. Mol. Pharm. 2019, 16, 3414–3429. [Google Scholar] [CrossRef] [PubMed]
- Rashid, J.; Nahar, K.; Raut, S.; Keshavarz, A.; Ahsan, F. Fasudil and DETA NONOate, Loaded in a Peptide-Modified Liposomal Carrier, Slow PAH Progression upon Pulmonary Delivery. Mol. Pharm. 2018, 15, 1755–1765. [Google Scholar] [CrossRef]
- Sawada, J.; Urakami, T.; Li, F.; Urakami, A.; Zhu, W.; Fukuda, M.; Li, D.Y.; Ruoslahti, E.; Komatsu, M. Small GTPase R-Ras Regulates Integrity and Functionality of Tumor Blood Vessels. Cancer Cell 2012, 22, 235–249. [Google Scholar] [CrossRef] [Green Version]
- Vähätupa, M.; Prince, S.; Vataja, S.; Mertimo, T.; Kataja, M.; Kinnunen, K.; Marjomäki, V.; Uusitalo, H.; Komatsu, M.; Järvinen, T.A.; et al. Lack of R-Ras Leads to Increased Vascular Permeability in Ischemic Retinopathy. Investig. Opthalmol. Vis. Sci. 2016, 57, 4898–4909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahar, K.; Rashid, J.; Absar, S.; Al-Saikhan, F.I.; Ahsan, F. Liposomal Aerosols of Nitric Oxide (NO) Donor as a Long-Acting Substitute for the Ultra-Short-Acting Inhaled NO in the Treatment of PAH. Pharm. Res. 2016, 33, 1696–1710. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Hu, L.; Huang, H.; Yu, Y.; Wang, J.; Yu, Y.; Li, K.; Li, Y.; Tian, T.; Chen, F. CAR (CARSKNKDC) Peptide Modified ReNcell-Derived Extracellular Vesicles as a Novel Therapeutic Agent for Targeted Pulmonary Hypertension Therapy. Hypertension 2020, 76, 1147–1160. [Google Scholar] [CrossRef]
- Kean, T.J.; Duesler, L.; Young, R.G.; Dadabayev, A.; Olenyik, A.; Penn, M.; Wagner, J.; Fink, D.J.; Caplan, A.I.; Dennis, J.E. Development of a peptide-targeted, myocardial ischemia-homing, mesenchymal stem cell. J. Drug Target. 2012, 20, 23–32. [Google Scholar] [CrossRef]
- De Rossi, G.; Vähätupa, M.; Cristante, E.; Arokiasamy, S.; Liyanage, S.E.; May, U.; Pellinen, L.; Uusitalo-Järvinen, H.; Bainbridge, J.W.; Järvinen, T.A.H.; et al. Pathological Angiogenesis Requires Syndecan-4 for Efficient VEGFA-Induced VE-Cadherin Internalization. Arterioscler. Thromb. Vasc. Biol. 2021, 49, 1374–1389. [Google Scholar] [CrossRef]
- Gopal, S.; Arokiasamy, S.; Pataki, C.; Whiteford, J.R.; Couchman, J.R. Syndecan receptors: Pericellular regulators in development and inflammatory disease. Open Biol. 2021, 11, 200377. [Google Scholar] [CrossRef] [PubMed]
- Corti, F.; Wang, Y.; Rhodes, J.M.; Atri, D.; Archer-Hartmann, S.; Zhang, J.; Zhuang, Z.W.; Chen, D.; Wang, T.; Wang, Z.; et al. N-terminal syndecan-2 domain selectively enhances 6-O heparan sulfate chains sulfation and promotes VEGFA165-dependent neovascularization. Nat. Commun. 2019, 10, 1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.N.; Yan, M.; Chan, A.M. A thirty-year quest for a role of R-Ras in cancer: From an oncogene to a multitasking GTPase. Cancer Lett. 2017, 403, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Sawada, J.; Komatsu, M. Normalization of tumor vasculature by R-Ras. Cell Cycle 2012, 11, 4285–4286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Vuori, K.; Wang, H.-G.; Reed, J.C.; Ruoslahti, E. Integrin activation by R-ras. Cell 1996, 85, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Weber, S.M.; Carroll, S.L. The Role of R-Ras Proteins in Normal and Pathologic Migration and Morphologic Change. Am. J. Pathol. 2021, 191, 1499–1510. [Google Scholar] [CrossRef]
- May, U.; Prince, S.; Vähätupa, M.; Laitinen, A.M.; Nieminen, K.; Uusitalo-Jarvinen, H.; Järvinen, T.A.H. Resistance of R-Ras knockout mice to skin tumour induction. Sci. Rep. 2015, 5, 11663. [Google Scholar] [CrossRef] [Green Version]
- Ketomäki, T.; Vähätupa, M.; May, U.; Pemmari, T.; Ruikka, E.; Hietamo, J.; Kaipiainen, P.; Barker, H.; Parkkila, S.; Uusitalo-Järvinen, H.; et al. R-Ras regulates vascular permeability, but not overall healing in skin wounds. Exp. Dermatol. 2019, 28, 202–206. [Google Scholar] [CrossRef] [Green Version]
- Järvinen, T.A. Neovascularisation in tendinopathy: From eradication to stabilisation? Br. J. Sports Med. 2020, 54, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Ichimiya, H.; Maeda, K.; Enomoto, A.; Weng, L.; Takahashi, M.; Murohara, T. Girdin/GIV regulates transendothelial permeability by controlling VE-cadherin trafficking through the small GTPase, R-Ras. Biochem. Biophys. Res. Commun. 2015, 461, 260–267. [Google Scholar] [CrossRef]
- Li, F.; Sawada, J.; Komatsu, M. R-Ras-Akt axis induces endothelial lumenogenesis and regulates the patency of regenerating vasculature. Nat. Commun. 2017, 8, 1720. [Google Scholar] [CrossRef]
- Herrera, J.L.; Komatsu, M. R-Ras Deficiency in Pericytes Causes Frequent Microphthalmia and Perturbs Retinal Vascular Development. J. Vasc. Res. 2021, 1–15. [Google Scholar] [CrossRef]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Greenwald, D.R.; Ruoslahti, E. Coadministration of a Tumor-Penetrating Peptide Enhances the Efficacy of Cancer Drugs. Science 2010, 328, 1031–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruoslahti, E. Tumor penetrating peptides for improved drug delivery. Adv. Drug Deliv. Rev. 2017, 110–111, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Nel, A.; Ruoslahti, E.; Meng, H. New Insights into “Permeability” as in the Enhanced Permeability and Retention Effect of Cancer Nanotherapeutics. ACS Nano 2017, 11, 9567–9569. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, T.A.H.; Ruoslahti, E. Generation of a multi-functional, target organ-specific, anti-fibrotic molecule by molecular engineering of the extracellular matrix protein, decorin. Br. J. Pharmacol. 2019, 176, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-X.; Pang, H.-B. Macropinocytosis as a cell entry route for peptide-functionalized and bystander nanoparticles. J. Control. Release 2021, 329, 1222–1230. [Google Scholar] [CrossRef]
- Pemmari, T.; Ivanova, L.; May, U.; Lingasamy, P.; Tobi, A.; Pasternack, A.; Prince, S.; Ritvos, O.; Makkapati, S.; Teesalu, T.; et al. Exposed CendR Domain in Homing Peptide Yields Skin-Targeted Therapeutic in Epidermolysis Bullosa. Mol. Ther. 2020, 28, 1833–1845. [Google Scholar] [CrossRef]
- Pang, H.-B.; Braun, G.B.; Ruoslahti, E. Neuropilin-1 and heparan sulfate proteoglycans cooperate in cellular uptake of nanoparticles functionalized by cationic cell-penetrating peptides. Sci. Adv. 2015, 1, e1500821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, H.-B.; Braun, G.B.; Friman, T.; Aza-Blanc, P.; Ruidiaz, M.E.; Sugahara, K.N.; Teesalu, T.; Ruoslahti, E. An endocytosis pathway initiated through neuropilin-1 and regulated by nutrient availability. Nat. Commun. 2014, 5, 4904. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vähätupa, M.; Salonen, N.; Uusitalo-Järvinen, H.; Järvinen, T.A.H. Selective Targeting and Tissue Penetration to the Retina by a Systemically Administered Vascular Homing Peptide in Oxygen Induced Retinopathy (OIR). Pharmaceutics 2021, 13, 1932. https://doi.org/10.3390/pharmaceutics13111932
Vähätupa M, Salonen N, Uusitalo-Järvinen H, Järvinen TAH. Selective Targeting and Tissue Penetration to the Retina by a Systemically Administered Vascular Homing Peptide in Oxygen Induced Retinopathy (OIR). Pharmaceutics. 2021; 13(11):1932. https://doi.org/10.3390/pharmaceutics13111932
Chicago/Turabian StyleVähätupa, Maria, Niklas Salonen, Hannele Uusitalo-Järvinen, and Tero A. H. Järvinen. 2021. "Selective Targeting and Tissue Penetration to the Retina by a Systemically Administered Vascular Homing Peptide in Oxygen Induced Retinopathy (OIR)" Pharmaceutics 13, no. 11: 1932. https://doi.org/10.3390/pharmaceutics13111932
APA StyleVähätupa, M., Salonen, N., Uusitalo-Järvinen, H., & Järvinen, T. A. H. (2021). Selective Targeting and Tissue Penetration to the Retina by a Systemically Administered Vascular Homing Peptide in Oxygen Induced Retinopathy (OIR). Pharmaceutics, 13(11), 1932. https://doi.org/10.3390/pharmaceutics13111932