Transportan Peptide Stimulates the Nanomaterial Internalization into Mammalian Cells in the Bystander Manner through Macropinocytosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Mice
2.2. Preparation of Nanoparticles
2.3. Dynamic Light Scatter (DLS)
2.4. Cellular Uptake Study
2.5. Physical Interaction Assay
2.6. In Vitro Imaging
2.7. In Vitro EXT Knockdown Experiment
2.8. Transmission Electron Microscopy (TEM)
2.9. Ex Vivo Tissue Uptake
2.10. Statistical Analysis
3. Results
3.1. Bystander Activities of CPP Classes
3.2. Cellular Uptake of TP and Bystander AgNPs
3.3. TP-Induced Bystander Uptake of NPs
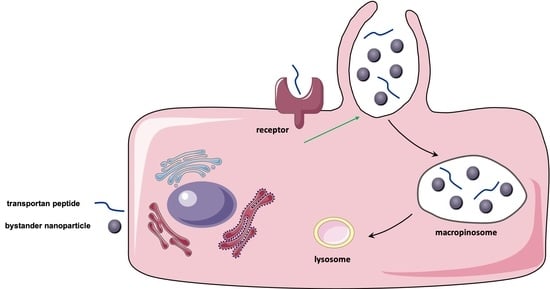
3.4. TP-Induced Bystander Uptake Mediated by Receptor-Dependent Macropinocytosis
3.5. TP-Induced Bystander Uptake under Physiological Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Björnmalm, M.; Thurecht, K.J.; Michael, M.; Scott, A.M.; Caruso, F. Bridging Bio–Nano Science and Cancer Nanomedicine. ACS Nano 2017, 11, 9594–9613. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nat. Cell Biol. 2003, 422, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Guidotti, G.; Brambilla, L.; Rossi, D. Cell-Penetrating Peptides: From Basic Research to Clinics. Trends Pharmacol. Sci. 2017, 38, 406–424. [Google Scholar] [CrossRef]
- Copolovici, D.M.; Langel, K.; Eriste, E.; Langel, Ü. Cell-Penetrating Peptides: Design, Synthesis, and Applications. ACS Nano 2014, 8, 1972–1994. [Google Scholar] [CrossRef]
- Koren, E.; Torchilin, V.P. Cell-penetrating peptides: Breaking through to the other side. Trends Mol. Med. 2012, 18, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Fretz, M.M.; Penning, N.A.; Al-Taei, S.; Futaki, S.; Takeuchi, T.; Nakase, I.; Storm, G.; Jones, A.T. Temperature-, concentration- and cholesterol-dependent translocation of L- and D-octa-arginine across the plasma and nuclear membrane of CD34+ leukaemia cells. Biochem. J. 2007, 403, 335–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosuge, M.; Takeuchi, T.; Nakase, I.; Jones, A.T.; Futaki, S. Cellular Internalization and Distribution of Arginine-Rich Peptides as a Function of Extracellular Peptide Concentration, Serum, and Plasma Membrane Associated Proteoglycans. Bioconjug. Chem. 2008, 19, 656–664. [Google Scholar] [CrossRef]
- Ben-Dov, N.; Korenstein, R. The uptake of HIV Tat peptide proceeds via two pathways which differ from macropinocytosis. Biochim. Biophys. Acta BBA Biomembr. 2015, 1848, 869–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, C.-Y.; Delaroche, D.; Burlina, F.; Alves, I.D.; Chassaing, G.; Sagan, S. Translocation and Endocytosis for Cell-penetrating Peptide Internalization. J. Biol. Chem. 2009, 284, 33957–33965. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, M.; Wikström, S.; Johansson, M. Cell surface adherence and endocytosis of protein transduction domains. Mol. Ther. 2003, 8, 143–150. [Google Scholar] [CrossRef]
- Palm-Apergi, C.; Lönn, P.; Dowdy, S.F. Do cell-penetrating peptides actually “penetrate” cellular membranes? Mol. Ther. 2012, 20, 695–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bechara, C.; Sagan, S. Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett. 2013, 587, 1693–1702. [Google Scholar] [CrossRef]
- Ruoslahti, E. Tumor penetrating peptides for improved drug delivery. Adv. Drug Deliv. Rev. 2017, 110–111, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, I.M.; Wadia, J.S.; Dowdy, S.F. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J. Control. Release 2005, 102, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Girard, O.M.; Hanahan, D.; Mattrey, R.F.; Ruoslahti, E. Tissue-Penetrating Delivery of Compounds and Nanoparticles into Tumors. Cancer Cell 2009, 16, 510–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Greenwald, D.R.; Ruoslahti, E. Coadministration of a Tumor-Penetrating Peptide Enhances the Efficacy of Cancer Drugs. Science 2010, 328, 1031–1035. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Tang, T.; Pang, H.-B. Cellular internalization of bystander nanomaterial induced by TAT-nanoparticles and regulated by extracellular cysteine. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-X.; Pang, H.-B. Macropinocytosis as a cell entry route for peptide-functionalized and bystander nanoparticles. J. Control. Release 2021, 329, 1222–1230. [Google Scholar] [CrossRef]
- Pang, H.-B.; Braun, G.B.; Ruoslahti, E. Neuropilin-1 and heparan sulfate proteoglycans cooperate in cellular uptake of nanoparticles functionalized by cationic cell-penetrating peptides. Sci. Adv. 2015, 1, e1500821. [Google Scholar] [CrossRef] [Green Version]
- Pang, H.-B.; Braun, G.B.; Friman, T.; Aza-Blanc, P.; Ruidiaz, M.E.; Sugahara, K.N.; Teesalu, T.; Ruoslahti, E. An endocytosis pathway initiated through neuropilin-1 and regulated by nutrient availability. Nat. Commun. 2014, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pooga, M.; Hällbrink, M.; Zorko, M.; Langel, Ü. Cell penetration by transportan. FASEB J. 1998, 12, 67–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wierzbicki, P.M.; Kogut-Wierzbicka, M.; Ruczynski, J.; Siedlecka-Kroplewska, K.; Kaszubowska, L.; Rybarczyk, A.; Alenowicz, M.; Rekowski, P.; Kmiec, Z. Protein and siRNA delivery by transportan and transportan 10 into colorectal cancer cell lines. Folia Histochem. Cytobiol. 2015, 52, 270–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepe, D.; Carvalho, V.F.; McCall, M.; De Lemos, D.P.; Lopes, L.B. Transportan in nanocarriers improves skin localization and antitumor activity of paclitaxel. Int. J. Nanomed. 2016, 11, 2009–2019. [Google Scholar] [CrossRef] [Green Version]
- Pooga, M.; Kut, C.; Kihlmark, M.; Hällbrink, M.; Fernaeus, S.; Raid, R.; Land, T.; Hallberg, E.; Bartfai, T.; Langel, Ü. Cellular translocation of proteins by transportan. FASEB J. 2001, 15, 1451–1453. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Wei, Y.; Yang, Q.; Yang, Y.; Sailor, M.J.; Pang, H.-B. Rapid chelator-free radiolabeling of quantum dots for in vivo imaging. Nanoscale 2019, 11, 22248–22254. [Google Scholar] [CrossRef]
- Braun, G.B.; Friman, T.; Pang, H.-B.; Pallaoro, A.; De Mendoza, T.H.; Willmore, A.-M.A.; Kotamraju, V.R.; Mann, A.P.; She, Z.-G.; Sugahara, K.N.; et al. Etchable plasmonic nanoparticle probes to image and quantify cellular internalization. Nat. Mater. 2014, 13, 904–911. [Google Scholar] [CrossRef] [Green Version]
- Cho, E.C.; Xie, J.; Wurm, P.A.; Xia, Y. Understanding the Role of Surface Charges in Cellular Adsorption versus Internalization by Selectively Removing Gold Nanoparticles on the Cell Surface with a I2/KI Etchant. Nano Lett. 2009, 9, 1080–1084. [Google Scholar] [CrossRef]
- Tang, T.; Wei, Y.; Kang, J.; She, Z.-G.; Kim, D.; Sailor, M.J.; Ruoslahti, E.; Pang, H.-B. Tumor-specific macrophage targeting through recognition of retinoid X receptor beta. J. Control. Release 2019, 301, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, L.; Machado, M.; Sanches-Vaz, M.; Prudêncio, M.; Vale, N.; Gomes, P. Coupling the cell-penetrating peptides transportan and transportan 10 to primaquine enhances its activity against liver-stage malaria parasites. MedChemComm 2018, 10, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezzat, K.; Helmfors, H.; Tudoran, O.; Juks, C.; Lindberg, S.; Padari, K.; El-Andaloussi, S.; Pooga, M.; Langel, Ü. Scavenger receptor-mediated uptake of cell-penetrating peptide nanocomplexes with oligonucleotides. FASEB J. 2011, 26, 1172–1180. [Google Scholar] [CrossRef]
- Greaves, D.R.; Gordon, S. Thematic review series: The Immune System and Atherogenesis. Recent insights into the biology of macrophage scavenger receptors. J. Lipid Res. 2005, 46, 11–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, P.C.; Giljohann, D.A.; Daniel, W.L.; Zheng, D.; Prigodich, A.E.; Mirkin, C.A. Scavenger Receptors Mediate Cellular Uptake of Polyvalent Oligonucleotide-Functionalized Gold Nanoparticles. Bioconjug. Chem. 2010, 21, 2250–2256. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Kim, M.; Kim, H.-J.; Lee, Y.; Seo, Y.; Pham, C.D.; Lee, J.; Byun, S.J.; Kwon, M.-H. Heparan sulfate proteoglycans (HSPGs) and chondroitin sulfate proteoglycans (CSPGs) function as endocytic receptors for an internalizing anti-nucleic acid antibody. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Mooij, H.L.; Cabrales, P.; Moens, S.J.B.; Xu, D.; Udayappan, S.D.; Tsai, A.G.; van der Sande, M.A.J.; de Groot, E.; Intaglietta, M.; Kastelein, J.J.P.; et al. Loss of Function in Heparan Sulfate Elongation Genes EXT1 and EXT 2 Results in Improved Nitric Oxide Bioavailability and Endothelial Function. J. Am. Heart Assoc. 2014, 3, e001274. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, M.; Birch, D.; Nielsen, H.M. Applications and Challenges for Use of Cell-Penetrating Peptides as Delivery Vectors for Peptide and Protein Cargos. Int. J. Mol. Sci. 2016, 17, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, S.B.; Pereira, M.P.; Kelley, S.O. Recent advances in the use of cell-penetrating peptides for medical and biological applications. Adv. Drug Deliv. Rev. 2009, 61, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Pourmousa, M.; Wong-Ekkabut, J.; Patra, M.; Karttunen, M. Molecular Dynamic Studies of Transportan Interacting with a DPPC Lipid Bilayer. J. Phys. Chem. B 2013, 117, 230–241. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, D.T.; Nakatsuji, T.; Yamasaki, K.; Kobzik, L.; Gallo, R.L. HSV-1 exploits the innate immune scavenger receptor MARCO to enhance epithelial adsorption and infection. Nat. Commun. 2013, 4, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurrikoff, K.; Gestin, M.; Langel, Ü. Recent in vivo advances in cell-penetrating peptide-assisted drug delivery. Expert Opin. Drug Deliv. 2016, 13, 373–387. [Google Scholar] [CrossRef] [PubMed]





| CPPs | Classes | Sequences | Normalized AgNP Uptake |
|---|---|---|---|
| RPAR | Cationic | RPARPAR | 1.01 ± 0.01 |
| TAT | Cationic | YGRKKRRQRRR | 4.50 ± 0.23 |
| R9 | Cationic | RRRRRRRRR | 5.26 ± 0.48 |
| Transportan | Amphiphilic | GWTLNSAGYLLGKINLKALAALAKKIL | 12.01 ± 0.18 |
| MAP | Amphiphilic | KLALKLALKALKAALKLA | 0.92 ± 0.06 |
| PFV | Hydrophobic | PFVYLI | 0.95 ± 0.05 |
| Angiopep-2 | Hydrophobic | TFFYGGSRGKRNNFKTEEY | 0.91 ± 0.04 |
| HAI | Hydrophobic | HAIYPRH | 0.98 ± 0.03 |
| Nanoparticles | Surface Coating | Z-Ave (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|
| AgNP-555 | CF555, PEG2000 | 83.57 ± 0.16 | 0.100 ± 0.009 | −26.3 ± 0.4 |
| AgNP-647 | CF647, PEG2000 | 100.40 ± 0.48 | 0.131 ± 0.005 | −14.7 ± 0.6 |
| AuNP | CF647, BSA | 63.12 ± 0.47 | 0.253 ± 0.007 | −30.2 ± 1.2 |
| Au50 | BSA | 46.41 ± 1.31 | 0.306 ± 0.002 | −11.9 ± 1.4 |
| IONP | CF647, Dextran | 57.30 ± 5.96 | 0.247 ± 0.090 | 2.7 ± 0.2 |
| QD | CF647, PEG2000 | 32.93 ± 1.06 | 0.331 ± 0.069 | −11.9 ± 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-X.; Wei, Y.; Zhong, R.; Li, L.; Pang, H.-B. Transportan Peptide Stimulates the Nanomaterial Internalization into Mammalian Cells in the Bystander Manner through Macropinocytosis. Pharmaceutics 2021, 13, 552. https://doi.org/10.3390/pharmaceutics13040552
Li Y-X, Wei Y, Zhong R, Li L, Pang H-B. Transportan Peptide Stimulates the Nanomaterial Internalization into Mammalian Cells in the Bystander Manner through Macropinocytosis. Pharmaceutics. 2021; 13(4):552. https://doi.org/10.3390/pharmaceutics13040552
Chicago/Turabian StyleLi, Yue-Xuan, Yushuang Wei, Rui Zhong, Ling Li, and Hong-Bo Pang. 2021. "Transportan Peptide Stimulates the Nanomaterial Internalization into Mammalian Cells in the Bystander Manner through Macropinocytosis" Pharmaceutics 13, no. 4: 552. https://doi.org/10.3390/pharmaceutics13040552
APA StyleLi, Y. -X., Wei, Y., Zhong, R., Li, L., & Pang, H. -B. (2021). Transportan Peptide Stimulates the Nanomaterial Internalization into Mammalian Cells in the Bystander Manner through Macropinocytosis. Pharmaceutics, 13(4), 552. https://doi.org/10.3390/pharmaceutics13040552