Dermal Delivery of Niacinamide—In Vivo Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Study Design
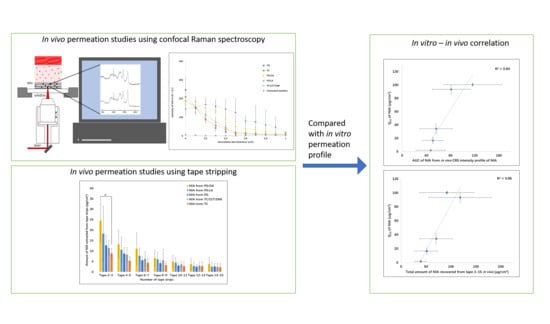
2.3. Confocal Raman Spectroscopy (CRS) Studies
2.4. Tape Stripping (TS) and Transepidermal Water Loss (TEWL) Measurement
2.5. Data Analysis
3. Results and Discussion
3.1. CRS Studies
3.2. Depth Profiling of PG in the SC
3.3. Tape Stripping (TS) and Transepidermal Water Loss (TEWL)
3.4. In Vitro–In Vivo Correlation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Hadgraft, J.; Lane, M.E. Advanced topical formulations (ATF). Int. J. Pharm. 2016, 514, 52–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Supe, S.; Takudage, P. Methods for evaluating penetration of drug into the skin: A review. Skin Res. Technol. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, R.J.; Maibach, H.I. Absorption of Some Organic Compounds Through the Skin in Man. J. Investig. Dermatol. 1970, 54, 399–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronaugh, R.L.; Franz, T.J. Vehicle effects on percutaneous absorption: In vivo and in vitro comparisons with human skin. Br. J. Dermatol. 1986, 115, 1–11. [Google Scholar] [CrossRef]
- Mavon, A.; Miquel, C.; Lejeune, O.; Payre, B.; Moretto, P. In vitro percutaneous absorption and in vivo stratum corneum distribution of an organic and a mineral sunscreen. Skin Pharmacol. Physiol. 2007, 20, 10–20. [Google Scholar] [CrossRef]
- Binder, L.; Kulovits, E.M.; Petz, R.; Ruthofer, J.; Baurecht, D.; Klang, V.; Valenta, C. Penetration monitoring of drugs and additives by ATR-FTIR spectroscopy/tape stripping and confocal Raman spectroscopy–A comparative study. Eur. J. Pharm. Biopharm. 2018, 130, 214–223. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry: Topical Dermatological Drug Products NDAs and ANDAs-in vivo Bioavailability, Bioequivalence, in vitro Release, and Associated Studies; Draft Guidance; Silver Spring: Montgomery County, MD, USA, 1998.
- FDA. Draft Guidance for Industry on Topical Dermatological Drug Product NDAs and ANDAs-in vivo Bioavailability, Bioequivalence, in vitro Release and Associated Studies; Withdrawal; Silver Spring: Montgomery County, MD, USA, 2002; pp. 35122–35123.
- Bashir, S.J.; Chew, A.L.; Anigbogu, A.; Dreher, F.; Maibach, H.I. Physical and physiological effects of stratum corneum tape stripping. Skin Res. Technol. 2001, 7, 40–48. [Google Scholar] [CrossRef]
- Abd, E.; Yousef, S.A.; Pastore, M.N.; Telaprolu, K.; Mohammed, Y.H.; Namjoshi, S.; Grice, J.E.; Roberts, M.S. Skin models for the testing of transdermal drugs. Clin. Pharmacol. 2016, 8, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Holmgaard, R.; Nielsen, J.B. Dermal Absorption of Pesticides: Evaluation of Variability and Prevention; Danish Environmental Protection Agency: Copenhagen, Denmark, 2009. [Google Scholar]
- Gotter, B.; Faubel, W.; Neubert, R.H. Optical methods for measurements of skin penetration. Skin Pharmacol. Physiol. 2008, 21, 156–165. [Google Scholar] [CrossRef]
- Caspers, P.J.; Lucassen, G.W.; Wolthuis, R.; Bruining, H.A.; Puppels, G.J. In vitro and in vivo Raman spectroscopy of human skin. Biospectroscopy 1998, 4, S31–S39. [Google Scholar] [CrossRef]
- Caspers, P.; Lucassen, G.; Bruining, H.A.; Puppels, G. Automated depth-scanning confocal Raman microspectrometer for rapidin vivo determination of water concentration profiles in human skin. J. Raman Spectrosc. 2000, 31, 813–818. [Google Scholar] [CrossRef]
- Boncheva, M.; De Sterke, J.; Caspers, P.J.; Puppels, G.J. Depth profiling of Stratum corneum hydration in vivo: A comparison between conductance and confocal Raman spectroscopic measurements. Exp. Dermatol. 2009, 18, 870–876. [Google Scholar] [CrossRef]
- Pudney, P.; Mélot, M.; Caspers, P.; Pol, A.; Puppels, G. An In Vivo Confocal Raman Study of the Delivery of Trans Retinol to the Skin. Appl. Spectrosc. 2007, 61, 804–811. [Google Scholar] [CrossRef]
- Mohammed, D.; Matts, P.J.; Hadgraft, J.; Lane, M.E. In Vitro–In Vivo Correlation in Skin Permeation. Pharm. Res. 2014, 31, 394–400. [Google Scholar] [CrossRef]
- Mateus, R.; Abdalghafor, H.; Oliveira, G.; Hadgraft, J.; Lane, M.E. A new paradigm in dermatopharmacokinetics-Confocal Raman spectroscopy. Int. J. Pharm. 2013, 444, 106–108. [Google Scholar] [CrossRef]
- Mateus, R.; Moore, D.J.; Hadgraft, J.; Lane, M.E. Percutaneous absorption of salicylic acid–in vitro and in vivo studies. Int. J. Pharm. 2014, 475, 471–474. [Google Scholar] [CrossRef]
- Caspers, P.J.; Nico, C.; Bakker Schut, T.C.; de Sterke, J.; Pudney, P.D.A.; Curto, P.R.; Illand, A.; Puppels, G.J. Method to quantify the in vivo skin penetration of topically applied materials based on confocal Raman spectroscopy. Trans. Biophotonics 2019, 1, e201900004. [Google Scholar] [CrossRef] [Green Version]
- Hakozaki, T.; Minwalla, L.; Zhuang, J.; Chhoa, M.; Matsubara, A.; Miyamoto, K.; Greatens, A.; Hillebrand, G.; Bissett, D.; Boissy, R. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br. J. Dermatol. 2002, 147, 20–31. [Google Scholar] [CrossRef]
- Shalita, A.R.; Smith, J.G.; Parish, L.C.; Sofman, M.S.; Chalker, D.K. Topical nicotinamide compared with clindamycin gel in the treatment of inflammatory acne vulgaris. Int. J. Dermatol. 1995, 34, 434–437. [Google Scholar] [CrossRef]
- Mohammed, D.; Crowther, J.M.; Matts, P.J.; Hadgraft, J.; Lane, M.E. Influence of niacinamide containing formulations on the molecular and biophysical properties of the stratum corneum. Int. J. Pharm. 2013, 441, 192–201. [Google Scholar] [CrossRef]
- Damian, D.L.; Halliday, G.M.; Taylor, C.A.; Barnetson, R.S. Ultraviolet radiation induced suppression of Mantoux reactions in humans. J. Investig. Dermatol. 1998, 110, 824–827. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Lane, M.E.; Hadgraft, J.; Heinrich, M.; Chen, T.; Lian, G.; Sinko, B. A comparison of the in vitro permeation of niacinamide in mammalian skin and in the Parallel Artificial Membrane Permeation Assay (PAMPA) model. Int. J. Pharm. 2019, 556, 142–149. [Google Scholar] [CrossRef]
- Zhang, Y.; Kung, C.P.; Sil, B.C. Topical Delivery of Niacinamide: Influence of Binary and Ternary Solvent Systems. Pharmaceutics 2019, 11, 668. [Google Scholar] [CrossRef] [Green Version]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Propylene Glycol, Tripropylene Glycol, and PPGs as Used in Cosmetics. Int. J. Toxicol. 2012, 31, 245S–260S. [Google Scholar] [CrossRef]
- Sullivan, D.W.; Gad, S.C.; Julien, M. A review of the nonclinical safety of Transcutol®, a highly purified form of diethylene glycol monoethyl ether (DEGEE) used as a pharmaceutical excipient. Food Chem. Toxicol. 2014, 72, 40–50. [Google Scholar] [CrossRef]
- CIR. Safety Assessment of Fatty Acids and Fatty Acid Salts as Used in Cosmetics; CIR: Washington, DC, USA, 2019.
- Iliopoulos, F.; Caspers, P.J.; Puppels, G.J.; Lane, M.E. Franz Cell Diffusion Testing and Quantitative Confocal Raman Spectroscopy: In Vitro-In Vivo Correlation. Pharmaceutics 2020, 12, 887. [Google Scholar] [CrossRef]
- Caspers, P.J.; Bruining, H.A.; Puppels, G.J.; Lucassen, G.W.; Carter, E.A. In Vivo Confocal Raman Microspectroscopy of the Skin: Noninvasive Determination of Molecular Concentration Profiles. J. Investig. Dermatol. 2001, 116, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Bielfeldt, S.; Schoder, V.; Ely, U.; Van Der Pol, A.; De Sterke, J.; Wilhelm, K.-P. Assessment of human stratum corneum thickness and its barrier properties by in-vivo confocal Raman spectroscopy. Int. J. Cosmet. Sci. 2009, 31, 479–480. [Google Scholar] [CrossRef]
- Crowther, J.M.; Sieg, A.; Blenkiron, P.; Marcott, C.; Matts, P.J.; Kaczvinsky, J.R.; Rawlings, A.V. Measuring the effects of topical moisturizers on changes in stratum corneum thickness, water gradients and hydration in vivo. Br. J. Dermatol. 2008, 159, 567–577. [Google Scholar] [CrossRef]
- Dąbrowska, A.K.; Adlhart, C.; Spano, F.; Rotaru, G.-M.; Derler, S.; Zhai, L.; Spencer, N.D.; Rossi, R.M. In vivo confirmation of hydration-induced changes in human-skin thickness, roughness and interaction with the environment. Biointerphases 2016, 11, 031015. [Google Scholar] [CrossRef] [Green Version]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Hoelgaard, A.; Møllgaard, B. Dermal drug delivery—Improvement by choice of vehicle or drug derivative. J. Control. Release 1985, 2, 111–120. [Google Scholar] [CrossRef]
- Haque, T.; Rahman, K.M.; Thurston, D.E.; Hadgraft, J.; Lane, M.E. Topical delivery of anthramycin I. Influence of neat solvents. Eur. J. Pharm. Sci. 2017, 104, 188–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kung, C.-P.; Zhang, Y.; Sil, B.C.; Hadgraft, J.; Lane, M.E.; Patel, B.; McCulloch, R. Investigation of binary and ternary solvent systems for dermal delivery of methadone. Int. J. Pharm. 2020, 586, 119538. [Google Scholar] [CrossRef]
- Lane, M.E.; Hadgraft, J.; Oliveira, G.; Vieira, R.; Mohammed, D.; Hirata, K. Rational formulation design. Int. J. Cosmet. Sci. 2012, 34, 496–501. [Google Scholar] [CrossRef] [Green Version]
- Walker, M.; Hadgraft, J. Oleic acid—a membrane “fluidiser” or fluid within the membrane? Int. J. Pharm. 1991, 71, R1–R4. [Google Scholar] [CrossRef]
- EMA. Propylene Glycol Used as an Excipient; EMA: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Wotton, P.K.; Møllgaard, B.; Hadgraft, J.; Hoelgaard, A. Vehicle effect on topical drug delivery. III. Effect of Azone on the cutaneous permeation of metronidazole and propylene glycol. Int. J. Pharm. 1985, 24, 19–26. [Google Scholar] [CrossRef]
- Ilić, T.; Pantelić, I.; Lunter, D.; Đorđević, S.; Marković, B.; Ranković, D.; Daniels, R.; Savić, S. Critical quality attributes, in vitro release and correlated in vitro skin permeation—in vivo tape stripping collective data for demonstrating therapeutic (non)equivalence of topical semisolids: A case study of “ready-to-use” vehicles. Int. J. Pharm. 2017, 528, 253–267. [Google Scholar] [CrossRef]
- Rougier, A.; Lotte, C.; Corcuff, P.; Maibach, H. Relationship between skin permeability and corneocyte size according to anatomic site, age, and sex in man. J. Soc. Cosmet. Chem. 1988, 39, 15–26. [Google Scholar]
- Machado, M.; Hadgraft, J.; Lane, M.E. Assessment of the variation of skin barrier function with anatomic site, age, gender and ethnicity. Int. J. Cosmet. Sci. 2010, 32, 397–409. [Google Scholar] [CrossRef]
- van der Molen, R.G.; Spies, F.; van ’t Noordende, J.M.; Boelsma, E.; Mommaas, A.M.; Koerten, H.K. Tape stripping of human stratum corneum yields cell layers that originate from various depths because of furrows in the skin. Arch. Dermatol. Res. 1997, 289, 514–518. [Google Scholar] [CrossRef]
- Hadgraft, J.; Lane, M.E. Drug crystallization–implications for topical and transdermal delivery. Expert Opin. Drug Deliv. 2016, 13, 817–830. [Google Scholar] [CrossRef]






| Solvent System | Amount of NIA (μg/cm2) |
|---|---|
| PG | 39.3 ± 12.2 |
| TC | 51.4 ± 23.2 |
| PG:OA (10:90) | 118.6 ± 63.2 |
| PG:LA (50:50) | 91.6 ± 54.8 |
| TC:CCT:DMI (50:25:25) | 70.2 ± 33.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Kung, C.-P.; Iliopoulos, F.; Sil, B.C.; Hadgraft, J.; Lane, M.E. Dermal Delivery of Niacinamide—In Vivo Studies. Pharmaceutics 2021, 13, 726. https://doi.org/10.3390/pharmaceutics13050726
Zhang Y, Kung C-P, Iliopoulos F, Sil BC, Hadgraft J, Lane ME. Dermal Delivery of Niacinamide—In Vivo Studies. Pharmaceutics. 2021; 13(5):726. https://doi.org/10.3390/pharmaceutics13050726
Chicago/Turabian StyleZhang, Yanling, Chin-Ping Kung, Fotis Iliopoulos, Bruno C. Sil, Jonathan Hadgraft, and Majella E. Lane. 2021. "Dermal Delivery of Niacinamide—In Vivo Studies" Pharmaceutics 13, no. 5: 726. https://doi.org/10.3390/pharmaceutics13050726
APA StyleZhang, Y., Kung, C. -P., Iliopoulos, F., Sil, B. C., Hadgraft, J., & Lane, M. E. (2021). Dermal Delivery of Niacinamide—In Vivo Studies. Pharmaceutics, 13(5), 726. https://doi.org/10.3390/pharmaceutics13050726