Dendrimer-Coated Gold Nanoparticles for Efficient Folate-Targeted mRNA Delivery In Vitro
Abstract
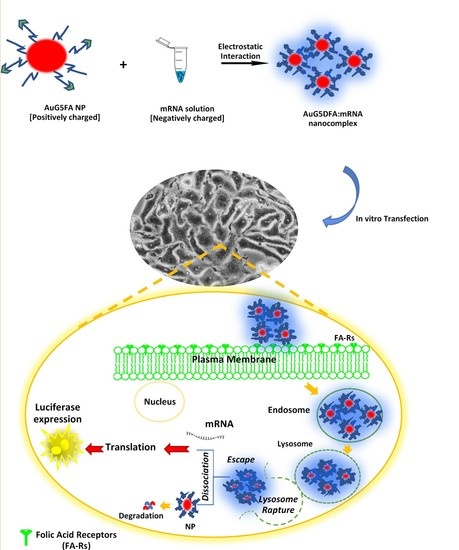
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Gold Nanoparticles (AuNPs)
2.3. Modification of PAMAM G5D with Folic Acid (FA)
2.4. Formulation of Dendrimer-Coated AuNPs (Au:G5D NPs, and Folic-Acid-Targeted, Dendrimer-Coated AuNPs (Au:G5D:FA NPs)
2.5. Ultra-Violet (UV) and Proton Nuclear Magnetic Resonance (1H NMR) Spectroscopy
2.6. Transmission Electron Microscopy (TEM) and Nanoparticle Tracking Analysis (NTA)
2.7. Nanocomplex Preparation and Binding Studies
2.7.1. Band Shift Assay
2.7.2. Ethidium Bromide Displacement Assay
2.7.3. RNase A Protection Assay
2.8. Cell Culture-Based Assays
2.8.1. MTT Cell Viability Assay
2.8.2. Apoptosis Assay
2.8.3. Transfection and Competition Assays
2.9. Statistical Analysis
3. Results
3.1. UV-Visible and 1H NMR Spectroscopy
3.2. Morphology, Size, and Zeta Potential of Nanoparticles and Nanocomplexes
3.3. The Band Shift Assay
3.4. Ethidium Bromide Dye Displacement Assay
3.5. RNase A Digestion Assay
3.6. The MTT Assay
3.7. Apoptosis Assay
3.8. Transfection and Competition Assays
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCrudden, C.M.; McCarthy, H.O. Cancer Gene Therapy-Key Biological Concepts in the Design of Multifunctional Non-Viral Delivery Systems. In Gene Therapy—Tools and Potential Applications, 1st ed.; Martin, F., Ed.; InTechOpen: London, UK, 2013; pp. 213–235. [Google Scholar]
- Kreig, A.M. CpG motifs in Bacterial DNA and Their Immune Effects. Annu. Rev. Immunol. 2002, 20, 709–760. [Google Scholar] [CrossRef]
- Su, X.; Fricke, J.; Kavanagh, D.; Irvine, D.J. In vitro and in Vivo Mrna Delivery Using Lipid-Enveloped Ph-Responsive Polymer Nanoparticles. Mol. Pharm. 2011, 8, 774–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malone, R.W.; Felgner, P.L.; Verma, I.M. Cationic liposome-mediated RNA transfection. Proc. Natl. Acad. Sci. USA 1989, 86, 6077–6081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavernier, G.; Andries, O.; Demeester, J.; Sanders, N.N.; De Smedt, S.C.; Rejman, J. mRNA as Gene Therapeutic: How to Control Protein Expression. J. Control. Release 2011, 150, 238–247. [Google Scholar] [CrossRef]
- Saenz-Badillos, J.; Amin, S.; Granstein, R. RNA as a Tumor Vaccine: A Review of the Literature. Exp. Dermatol. 2001, 10, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Weide, B.; Carralot, J.-P.; Reese, A.; Scheel, B.; Eigentler, T.K.; Hoerr, I.; Rammensee, H.-G.; Garbe, C.; Pascolo, S. Results of the First Phase I/II Clinical Vaccination Trial with Direct Injection of Mrna. J. Immunother. 2008, 31, 180–188. [Google Scholar] [CrossRef]
- Weide, B.; Pascolo, S.; Scheel, B.; Derhovanessian, E.; Pflugfelder, A.; Eigentler, T.K.; Pawelec, G.; Hoerr, I.; Rammensee, H.-G.; Garbe, C. Direct Injection of Protamine-Protected Mrna: Results of a Phase 1/2 Vaccination Trial in Metastatic Melanoma Patients. J. Immunother. 2009, 32, 498–507. [Google Scholar] [CrossRef]
- Mockey, M.; Gonçalves, C.; Dupuy, F.P.; Lemoine, F.M.; Pichon, C.; Midoux, P. Mrna Transfection of Dendritic Cells: Synergistic Effect of ARCA Mrna Capping with Poly (A) Chains in Cis and in Trans for a High Protein Expression Level. Biochem. Biophys. Res. Commun. 2006, 340, 1062–1068. [Google Scholar] [CrossRef]
- Van Tendeloo, V.F.; Ponsaerts, P.; Berneman, Z.N. mRNA-Based Gene Transfer as a Tool for Gene and Cell Therapy. Curr. Opin. Mol. Ther. 2007, 9, 423–431. [Google Scholar]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Mol. Ther. 2019, 27, 710–728. [Google Scholar] [CrossRef] [Green Version]
- Xionga, H.; Liua, S.; Weia, T.; Chenga, Q.; Siegwarta, D.J. The Theranostic Dendrimer-Based Lipid Nanoparticles Containing Pegylated BODIPY Dyes for Tumor Imaging and Systemic Mrna Delivery in Vivo. J. Control. Release 2020, 325, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Chaplot, S.P.; Rupenthal, I.D. Dendrimers for Gene Delivery–A Potential Approach for Ocular Therapy? J. Pharm. Pharmacol. 2014, 66, 542–556. [Google Scholar] [CrossRef] [PubMed]
- Pillay, N.S.; Daniels, A.; Singh, M. Folate-Targeted Transgenic Activity of Dendrimer Functionalized Selenium Nanoparticles in vitro. Int. J. Mol. Sci. 2020, 21, 7177. [Google Scholar] [CrossRef]
- Mbatha, L.S.; Singh, M. Starburst Poly(amidoamine) Dendrimer Grafted Gold Nanoparticles as a Scaffold for Folic Acid-Targeted Plasmid DNA Delivery in vitro. J. Nanosci. Nanotechnol. 2019, 19, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Mbatha, L.S.; Maiyo, F.C.; Singh, M. Dendrimer Functionalized Folate-Targeted Gold Nanoparticles for Luciferase Gene Silencing in vitro: A Proof of Principle Study. Acta Pharm. 2019, 69, 49–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, T.; Hou, W.; Cao, X.; Wen, S.; Shen, M.; Shi, X. Dendrimer-Entrapped Gold Nanoparticles Modified with Folic Acid for Targeted Gene Delivery Applications. Biomater. Sci. 2013, 1, 1172–1180. [Google Scholar] [CrossRef]
- Luo, D.; Haverstick, K.; Belcheva, N.; Han, E.; Saltzman, W.M. Poly (ethylene glycol)-Conjugated PAMAM Dendrimer for Biocompatible, High-Efficiency DNA Delivery. Macromolecules 2002, 35, 3456–3462. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, Y.-B.; Choi, J.S.; Lee, Y.; Kim, T.-I.; Kim, H.J.; Yoon, J.K.; Kim, K.; Park, J.-S. Polyplexes Assembled with Internally Quaternized PAMAM-OH Dendrimer and Plasmid DNA Have a Neutral Surface and Gene Delivery Potency. Bioconjug. Chem. 2003, 14, 1214–1221. [Google Scholar] [CrossRef]
- Kim, T.-I.; Seo, H.J.; Choi, J.S.; Jang, H.-S.; Baek, J.-U.; Kim, K.; Park, J.-S. PAMAM-PEG-PAMAM: Novel Triblock Copolymer as a Biocompatible and Efficient Gene Delivery Carrier. Biomacromolecules 2004, 5, 2487–2492. [Google Scholar] [CrossRef]
- Shan, Y.; Luo, T.; Peng, C.; Sheng, R.; Cao, A.; Coa, X.; Shen, M.; Guo, R.; Tomas, H.; Shi, X. Gene Delivery Using Dendrimer-Entrapped Gold Nanoparticles as Nonviral Vectors. Biomaterials 2012, 33, 3025–3035. [Google Scholar] [CrossRef]
- Yuan, X.; Wen, S.; Shen, M.; Shi, X. Dendrimer-Stabilized Silver Nanoparticles Enable Efficient Colorimetric Sensing of Mercury Ions in Aqueous Solution. Anal. Methods 2013, 5, 5486–5492. [Google Scholar] [CrossRef]
- Figueroa, E.R.; Lin, A.Y.; Yan, J.; Luo, L.; Foster, A.E.; Drezek, R.A. Optimization of PAMAM-Gold Nanoparticle Conjugation for Gene Therapy. Biomaterials 2014, 35, 1725–1734. [Google Scholar] [CrossRef] [Green Version]
- Oladimeji, O.; Akinyelu, A.; Singh, M. Co-polymer Functionalised Gold Nanoparticles Show Efficient Mitochondrial Targeted Drug Delivery in Cervical Carcinoma Cells. J. Biomed. Nanotechnol. 2020, 16, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Akinyelu, A.; Oladimeji, O.; Singh, M. Lactobionic Acid-Chitosan Functionalized Gold Coated Poly(lactide-co-glycolide) Nanoparticles for Hepatocyte Targeted Gene Delivery. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 045017. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Singh, M. Assessing Nucleic acid: Cationic Nanoparticle Interaction for Gene Delivery. In Bio-Carrier Vectors; Narayan, K., Ed.; Methods in Molecular Biology Series; Springer-Nature: New York, NY, USA, 2021; Volume 2211, pp. 43–55. [Google Scholar]
- Maiyo, F.; Singh, M. Folate-Targeted mRNA Delivery Using Chitosan Functionalized Selenium Nanoparticles: Potential in Cancer Immunotherapy. Pharmaceuticals 2019, 12, 164. [Google Scholar] [CrossRef] [Green Version]
- Singh, D.; Singh, M. Hepatocellular-Targeted mRNA Delivery Using Functionalized Selenium Nanoparticles in Vitro. Pharmaceutics 2021, 13, 298. [Google Scholar] [CrossRef]
- Maiyo, F.; Moodley, R.; Singh, M. Cytotoxicity, Antioxidant and Apoptosis Studies of Quercetin-3-O-Glucoside and 4-(Β-D-Glucopyranosyl-1→4-A-L-Rhamnopyranosyloxy)-Benzyl Isothiocyanate from Moringa Oleifera. Anti-Cancer Agents Med. Chem. 2016, 16, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.; Mallia, A.; Gartner, F.; Provenzano, M.; Fujimoto, E.; Goeke, N.; Olson, B.; Klenk, D. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.; Aveyard, J.; Fernig, G.D.G. Determination of Size and Concentration of Gold Nanoparticles from UV—Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Turner, J.L.; Wooley, K.L. Folic Acid-Conjugated Nanostructured Materials Designed for Cancer Cell Targeting. Chem. Commun. 2003, 19, 2400–2401. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, F.; Lavernia, E. On the Analysis of Grain Size in Bulk Nanocrystalline Materials via X-ray Diffraction. Metall. Mater. Trans. A 2003, 34, 1349–1355. [Google Scholar] [CrossRef]
- Mansoori, G.A.; Brandenburg, K.S.; Shakeri-Zadeh, A. A Comparative Study of Two Folate-Conjugated Gold Nanoparticles for Cancer Nanotechnology Applications. Cancers 2010, 2, 1911–1928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.Y.; Thomas, T.P.; Desai, A.; Zong, H.; Leroueil, P.R.; Majoros, I.J.; Baker, J.R. Targeted Dendrimeric Anticancer Prodrug: A Methotrexate-Folic Acid-Poly (Amidoamine) Conjugate and a Novel, Rapid,“One Pot” Synthetic Approach. Bioconjug. Chem. 2010, 21, 489–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, J.L.; Oliviera, H.; Pandita, D.; Rodrigues, J.; Pêgo, A.P.; Granja, P.L.; Tomas, H. Functionalization of Poly(Amidoamine) Dendrimers with Hydrophobic Chains for Improved Gene Delivery in Mesenchymal Stem Cells. J. Control. Release 2010, 144, 55–64. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, N.; Chen, L.; Meng, X.; Liu, Y.; Li, Y.; Wang, J. Synthesis and Characterization of DOX-Conjugated Dendrimer-Modified Magnetic Iron Oxide Conjugates for Magnetic Resonance Imaging, Targeting, and Drug Delivery. J. Mater. Chem. 2012, 22, 9594–9601. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siwowska, K.; Schmid, R.M.; Cohrs, S.; Schibli, R.; Müller, C. Folate Receptor-Positive Gynecological Cancer Cells: In Vitro and in Vivo Characterization. Pharmaceuticals 2017, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, H.; Bao, G. Physical Principles of Nanoparticle Cellular Endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef] [Green Version]
- Boroumand, S.; Safari, M.; Shaabani, E.; Shirzad, M.; Faridi-Majidi, R. Selenium Nanoparticles: Synthesis, Characterization and Study of Their Cytotoxicity, Antioxidant and Antibacterial Activity. Mater. Res. Express 2019, 6, 0850d8. [Google Scholar] [CrossRef]
- Shi, X.; Sun, K.; Baker, J.R. Spontaneous Formation of Functionalized Dendrimer-Stabilized Gold Nanoparticles. J. Phys. Chem. C. 2008, 112, 8251–8258. [Google Scholar] [CrossRef] [Green Version]
- Azzam, T.; Domb, A.J. Current Developments in Gene Transfection Agents. Curr. Drug Deliv. 2004, 1, 165–193. [Google Scholar] [CrossRef]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-Dependent Internalization of Particles Via the Pathways of Clathrin-and Caveolae-Mediated Endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.; Aron, Y.; Thévenot, G.; François, D.; Monsigny, M.; Fajac, I. Potocytosis and Cellular Exit of Complexes as Cellular Pathways for Gene Delivery by Polycations. J. Gene Med. 2005, 7, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 2). Trop. J. Pharm. Res. 2013, 12, 265–273. [Google Scholar]
- Fratila, R.M.; Mitchell, S.G.; Del Pino, P.; Grazu, V.; De La Fuente, J.S.M. Strategies for the Biofunctionalization of Gold and Iron Oxide Nanoparticles. Langmuir 2014, 30, 15057–15071. [Google Scholar] [CrossRef]
- Mbatha, L.; Chakravorty, S.; de Koning, C.B.; van Otterlo, W.A.; Arbuthnot, P.; Ariatti, M.; Singh, M. Spacer Length: A Determining Factor in the Design of Galactosyl Ligands for Hepatoma Cell-Specific Liposomal Gene Delivery. Curr. Drug Deliv. 2016, 13, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-C.; Chang, C.-W. Complexation of Bioreducible Cationic Polymers with Gold Nanoparticles for Improving Stability in Serum and Application on Nonviral Gene Delivery. ACS Appl. Mater. Interfaces 2015, 7, 7724–7731. [Google Scholar] [CrossRef]
- Obata, Y.; Saito, S.; Takeda, N.; Takeoka, S. Plasmid DNA-Encapsulating Liposomes: Effect of a Spacer between the Cationic Head Group and Hydrophobic Moieties of the Lipids on Gene Expression Efficiency. Biochim. Biophys. Acta 2009, 1788, 1148–1158. [Google Scholar] [CrossRef] [Green Version]
- Pitard, B. Supramolecular Assemblies of DNA Delivery Systems. Somat. Cell Mol. Genet. 2002, 27, 5–15. [Google Scholar] [CrossRef]
- Daniels, A.; Singh, M.; Ariatti, M. Pegylated and Non-Pegylated siRNA Lipoplexes Formulated with Cholesteryl Cytofectins Promote Efficient Luciferase Knockdown in Hela Tat Luc Cells. Nucleos Nucleot Nucleic 2013, 32, 206–220. [Google Scholar] [CrossRef]
- Daniels, A.N.; Singh, M. Sterically Stabilized siRNA: Gold Nanocomplexes Enhance c-MYC Silencing in a Breast Cancer Cell Model. Nanomedicine 2019, 14, 1387–1401. [Google Scholar] [CrossRef] [PubMed]
- Nundkumar, N.; Singh, S.; Singh, M. Amino Acid Functionalized Hydrotalcites for Gene Silencing. J. Nanosci. Nanotechnol. 2020, 20, 3387–3397. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.; Daniels, A.; Ariatti, M.; Singh, M. Anti-c-MYC Cholesterol based Lipoplexes as Onco-Nanotherapeutic Agents in vitro. F1000 Res. 2020, 9, 770. [Google Scholar] [CrossRef]
- Elsabahy, M.; Nazarali, A.; Foldvari, M. Non-Viral Nucleic Acid Delivery: Key Challenges and Future Directions. Curr. Drug Deliv. 2011, 8, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Kambhampati, S.P.; Clunies-Ross, A.J.; Bhutto, I.; Mishra, M.K.; Edwards, M.; McLeod, D.S.; Kannan, R.M.; Lutty, G. Systemic and Intravitreal Delivery of Dendrimers to Activated Microglia/Macrophage in Ischemia/Reperfusion Mouse Retina. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4413–4424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bettinger, T.; Carlisle, R.C.; Read, M.L.; Ogris, M.; Seymour, L.W. Peptide-Mediated RNA Delivery: A Novel Approach for Enhanced Transfection of Primary and Post-Mitotic Cells. Nucleic Acids Res. 2001, 29, 3882–3891. [Google Scholar] [CrossRef] [Green Version]
- Srinivasarao, M.; Galliford, C.V.; Low, P.S. Principles in the Design of Ligand-Targeted Cancer Therapeutics and Imaging Agents. Nat. Rev. Drug Discov. 2015, 14, 203–219. [Google Scholar] [CrossRef]











| Nanoparticles/Nanocomplexes | NP:mRNA (w/w) Ratio | Mean Diameter (nm) ± SD | ζ Potential (mV) ± SD | Polydispersity Index |
|---|---|---|---|---|
| Au [16] | - | 65.9 ± 9.8 | −7.3 ± 1.6 | 0.022 |
| G5D [16] | - | 161.3 ± 11.9 | +87.2 ± 2.4 | 0.005 |
| Au:G5D [16] | - | 100.5 ± 44.1 | +20.9 ± 2.2 | 0.193 |
| G5D:FA [16] | - | 128.0 ± 1.20 | +71.2 ± 3.4 | 0.00009 |
| Au:G5D:FA [16] | - | 77.7 ± 12.5 | +29.0 ± 0.5 | 0.026 |
| Au:G5D-mRNA | 3:1 | 207.2 ± 35.5 # | −37.3 ± 0.1 *** | 0.029 |
| Au:G5D:FA-mRNA | 4:1 | 101.8 ± 36.9 # | −65.7 ± 1.4 *** | 0.131 |
| G5D-mRNA | 2:1 | 118.0 ± 6.20 # | −21.0 ± 0.5 *** | 0.028 |
| G5D:FA-mRNA | 4:1 | 265.2 ± 51.6 # | −25.8 + 0.0 *** | 0.038 |
| Cell Lines | Apoptotic Indices | ||||
|---|---|---|---|---|---|
| Cell Control | Nanocomplexes | ||||
| Au:G5D | Au:G5D:FA | G5D | G5D:FA | ||
| HEK293 | 0.0 | 0.03 ± 0.0001 | 0.04 ± 0.0004 | 0.07 ± 0.0010 | 0.08 ± 0.0020 |
| HepG2 | 0.0 | 0.06 ± 0.0015 | 0.04 ± 0.0018 | 0.08 ± 0.0012 | 0.09 ± 0.0011 |
| Caco-2 | 0.0 | 0.05 ± 0.0010 | 0.04 ± 0.0011 | 0.13 ± 0.0015 | 0.11 ± 0.0030 |
| MCF-7 | 0.0 | 0.04 ± 0.0011 | 0.06 ± 0.0003 | 0.25 ± 0.0030 | 0.23 ± 0.0010 |
| KB | 0.0 | 0.05 ± 0.0021 | 0.06 ± 0.0003 | 0.19 ± 0.0015 | 0.20 ± 0.0012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbatha, L.S.; Maiyo, F.; Daniels, A.; Singh, M. Dendrimer-Coated Gold Nanoparticles for Efficient Folate-Targeted mRNA Delivery In Vitro. Pharmaceutics 2021, 13, 900. https://doi.org/10.3390/pharmaceutics13060900
Mbatha LS, Maiyo F, Daniels A, Singh M. Dendrimer-Coated Gold Nanoparticles for Efficient Folate-Targeted mRNA Delivery In Vitro. Pharmaceutics. 2021; 13(6):900. https://doi.org/10.3390/pharmaceutics13060900
Chicago/Turabian StyleMbatha, Londiwe Simphiwe, Fiona Maiyo, Aliscia Daniels, and Moganavelli Singh. 2021. "Dendrimer-Coated Gold Nanoparticles for Efficient Folate-Targeted mRNA Delivery In Vitro" Pharmaceutics 13, no. 6: 900. https://doi.org/10.3390/pharmaceutics13060900
APA StyleMbatha, L. S., Maiyo, F., Daniels, A., & Singh, M. (2021). Dendrimer-Coated Gold Nanoparticles for Efficient Folate-Targeted mRNA Delivery In Vitro. Pharmaceutics, 13(6), 900. https://doi.org/10.3390/pharmaceutics13060900