Biobased Ionic Liquids as Multitalented Materials in Lipidic Drug Implants
Abstract
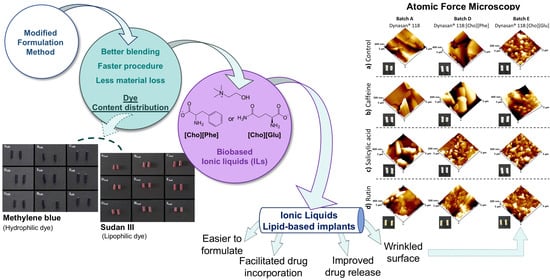
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Implants Preparation
2.3. Dye Content Distribution
2.4. Drug Content
2.4.1. Implants Containing Caffeine
2.4.2. Implants Containing Salicylic Acid or Rutin
2.5. In Vitro Drug Release
2.6. Water Content and Lipidic Erosion
2.7. Atomic Force Microscopy (AFM)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Dye Content Distribution
3.2. Implants Containing Each Drugs
3.2.1. Drug Content
3.2.2. In Vitro Drug Release, Water Content, and Lipidic Erosion




3.2.3. Atomic Force Microscopy (AFM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Shi, Y.; Li, L.C. Current advances in sustained-release systems for parenteral drug delivery. Expert Opin. Drug Deliv. 2005, 2, 1039–1058. [Google Scholar] [CrossRef]
- García-Estrada, P.; García-Bon, M.A.; López-Naranjo, E.J.; Basaldúa-Pérez, D.N.; Santos, A.; Navarro-Partida, J. Polymeric Implants for the Treatment of Intraocular Eye Diseases: Trends in Biodegradable and Non-Biodegradable Materials. Pharmaceutics 2021, 13, 701. [Google Scholar] [CrossRef] [PubMed]
- Kreye, F.; Siepmann, F.; Siepmann, J. Lipid implants as drug delivery systems. Expert Opin. Drug Deliv. 2008, 5, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Koennings, S.; Garcion, E.; Faisant, N.; Menei, P.; Benoit, J.P.; Goepferich, A. In vitro investigation of lipid implants as a controlled release system for interleukin-18. Int. J. Pharm. 2006, 314, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pillai, J. Implantable drug delivery systems: An overview. Nanostructures Eng. Cells Tissues Organs 2018, 473–511. [Google Scholar] [CrossRef]
- Kreye, F.; Siepmann, F.; Willart, J.F.; Descamps, M.; Siepmann, J. Drug release mechanisms of cast lipid implants. Eur. J. Pharm. Biopharm. 2011, 78, 394–400. [Google Scholar] [CrossRef]
- Kreye, F.; Siepmann, F.; Zimmer, A.; Willart, J.F.; Descamps, M.; Siepmann, J. Controlled release implants based on cast lipid blends. Eur. J. Pharm. Sci. 2011, 43, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Guse, C.; Koennings, S.; Kreye, F.; Siepmann, F.; Goepferich, A.; Siepmann, J. Drug release from lipid-based implants: Elucidation of the underlying mass transport mechanisms. Int. J. Pharm. 2006, 314, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Nicolai, M.; Amaral, V.; Antunes, C.; Shuwisitkul, D.; Portugal Mota, J. Lipid-Based Implants: Impact of Formulation Parameters. Adv. Mater. Res. 2014, 1060, 87–90. [Google Scholar] [CrossRef]
- Koennings, S.; Sapin, A.; Blunk, T.; Menei, P.; Goepferich, A. Towards controlled release of BDNF—Manufacturing strategies for protein-loaded lipid implants and biocompatibility evaluation in the brain. J. Control. Release 2007, 119, 163–172. [Google Scholar] [CrossRef]
- Gouveia, W.; Jorge, T.F.; Martins, S.; Meireles, M.; Carolino, M.; Cruz, C.; Almeida, T.V.; Araújo, M.E.M. Toxicity of ionic liquids prepared from biomaterials. Chemosphere 2014, 104, 51–56. [Google Scholar] [CrossRef]
- Caparica, R.; Júlio, A.; Baby, A.R.; Eduarda, M.E.A.; Fernandes, A.S.; Costa, J.G.; Santos de Almeida, T. Choline-Amino Acid Ionic Liquids as Green Functional Excipients to Enhance Drug Solubility. Pharmaceutics 2018, 10, 288. [Google Scholar] [CrossRef] [Green Version]
- de Almeida, T.S.; Júlio, A.; Saraiva, N.; Fernandes, A.S.; Araújo, M.E.M.; Baby, A.R.; Rosado, C.; Mota, J.P. Choline- versus imidazole-based ionic liquids as functional ingredients in topical delivery systems: Cytotoxicity, solubility, and skin permeation studies. Drug Dev. Ind. Pharm. 2017, 43, 1858–1865. [Google Scholar] [CrossRef]
- Czekanski, L.; de Almeida, T.S.; Portugal Mota, J.; Rijo, P.; Araújo, M.E.M. Synthesis of benzoazole ionic liquids and evaluation of their antimicrobial activity. J. Biomed. Biopharm. Res. 2014, 11, 227–235. [Google Scholar] [CrossRef]
- Caparica, R.; Júlio, A.; Fernandes, F.; Araújo, M.E.M.; Costa, J.G.; de Almeida, T.S. Upgrading the topical delivery of poorly soluble drugs using ionic liquids as a versatile tool. Int. J. Mol. Sci. 2021, 22, 4338. [Google Scholar] [CrossRef]
- Silva, A.T.; Teixeira, C.; Marques, E.F.; Prudêncio, C.; Gomes, P.; Ferraz, R. Surfing the Third Wave of Ionic Liquids: A Brief Review on the Role of Surface-Active Ionic Liquids in Drug Development and Delivery. ChemMedChem 2021, 16, 1–9. [Google Scholar] [CrossRef]
- Shmukler, L.E.; Fedorova, I.V.; Fadeeva, Y.A.; Safonova, L.P. The physicochemical properties and structure of alkylammonium protic ionic liquids of RnH4-nNX (n = 1–3) family. A mini–review. J. Mol. Liq. 2021, 321, 114350. [Google Scholar] [CrossRef]
- Pedro, S.N.; Freire, C.S.R.; Silvestre, A.J.D.; Freire, M.G. The role of ionic liquids in the pharmaceutical field: An overview of relevant applications. Int. J. Mol. Sci. 2020, 21, 8298. [Google Scholar] [CrossRef]
- Marrucho, I.M.; Branco, L.C.; Rebelo, L.P.N. Ionic liquids in pharmaceutical applications. Annu. Rev. Chem. Biomol. Eng. 2014, 5, 527–546. [Google Scholar] [CrossRef]
- Mitkare, S.S.; Lakhane, K.G.; Kokulwar, P.U. Ionic liquids: Novel Applications in Drug Delivery. Res. J. Pharm. Technol. 2013, 6, 1274–1278. [Google Scholar]
- Sidat, Z.; Marimuthu, T.; Kumar, P.; du Toit, L.C.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Ionic liquids as potential and synergistic permeation enhancers for transdermal drug delivery. Pharmaceutics 2019, 11, 96. [Google Scholar] [CrossRef] [Green Version]
- Caparica, R.; Júlio, A.; Araújo, M.E.M.; Baby, A.R.; Fonte, P.; Costa, J.G.; de Almeida, T.S. Anticancer activity of rutin and its combination with ionic liquids on renal cells. Biomolecules 2020, 10, 233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamshina, J.L.; Barber, P.S.; Rogers, R.D. Ionic liquids in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 1367–1381. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, R.; Branco, L.C.; Prudêncio, C.; Noronha, J.P.; Petrovski, Ž. Ionic liquids as active pharmaceutical ingredients. ChemMedChem 2011, 6, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Dobler, D.; Schmidts, T.; Klingenhoefer, I.; Runkel, F. Ionic liquids as ingredients in topical drug delivery systems. Int. J. Pharm. 2012, 441, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Zech, O.; Thomaier, S.; Bauduin, P.; Rück, T.; Touraud, D.; Kunz, W. Microemulsions with an ionic liquid surfactant and room temperature ionic liquids as polar pseudo-phase. J. Phys. Chem. B 2009, 113, 465–473. [Google Scholar] [CrossRef]
- Zhu, W.; Guo, C.; Yu, A.; Gao, Y.; Cao, F.; Zhai, G. Microemulsion-based hydrogel formulation of penciclovir for topical delivery. Int. J. Pharm. 2009, 378, 152–158. [Google Scholar] [CrossRef]
- Júlio, A.; Costa Lima, S.A.; Reis, S.; de Almeida, T.S.; Fonte, P. Development of ionic liquid-polymer nanoparticle hybrid systems for delivery of poorly soluble drugs. J. Drug Deliv. Sci. Technol. 2019, 56, 1–23. [Google Scholar] [CrossRef]
- Júlio, A.; Caparica, R.; Costa Lima, S.A.; Fernandes, A.S.; Rosado, C.; Prazeres, D.M.F.; Reis, S.; de Almeida, T.S.; Fonte, P. Ionic liquid-polymer nanoparticle hybrid systems as new tools to deliver poorly soluble drugs. Nanomaterials 2019, 9, 1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Almeida, T.S.; Júlio, A.; Mota, J.P.; Rijo, P.; Reis, C.P. An emerging integration between ionic liquids ans nanotechnology: General uses and future prospects in drug delivery. Ther. Deliv. 2017, 6, 461–473. [Google Scholar] [CrossRef]
- Luo, Q.; Pentzer, E. Encapsulation of Ionic Liquids for Tailored Applications. ACS Appl. Mater. Interfaces 2020, 12, 5169–5176. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Herman, A.P. Caffeine’s mechanisms of action and its cosmetic use. Ski. Pharmacol. Physiol. 2012, 26, 8–14. [Google Scholar] [CrossRef]
- Lupi, O.; Semenovitch, I.J.; Treu, C.; Bottino, D.; Bouskela, E. Evaluation of the effects of caffeine in the microcirculation and edema on thighs and buttocks using the orthogonal polarization spectral imaging and clinical parameters. J. Cosmet. Dermatol. 2007, 6, 102–107. [Google Scholar] [CrossRef]
- Velasco, M.V.R.; Tano, C.N.; Machado-Santelli, G.M.; Consiglieri, V.O.; Kaneko, T.M.; Baby, A.R. Effects of caffeine and siloxanetriol alginate caffeine, as anticellulite agents, on fatty tissue: Histological evaluation. J. Cosmet. Dermatol. 2008, 7, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W.; Tian, M.; Manohar, M.; Harraz, M.M.; Park, S.W.; Schroeder, F.C.; Snyder, S.H.; Klessig, D.F. Human GAPDH is a target of aspirin’s primary metabolite salicylic acid and its derivatives. PLoS ONE 2015, 10, e0143447. [Google Scholar] [CrossRef]
- Ferré, S. Mechanisms of the psychostimulant effects of caffeine: Implications for substance use disorders. Psychopharmacology 2016, 233, 1963–1979. [Google Scholar] [CrossRef] [Green Version]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolahdouzan, M.; Hamadeh, M.J. The neuroprotective effects of caffeine in neurodegenerative diseases. CNS Neurosci. Ther. 2017, 23, 272–290. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin: Therapeutic potential and recent advances in drug delivery. Expert Opin. Investig. Drugs 2013, 22, 1063–1079. [Google Scholar] [CrossRef] [PubMed]
- Bari, H. A prolonged release parenteral drug delivery system—An overview. Int. J. Pharm. Sci. Rev. Res. 2010, 3, 1–11. [Google Scholar]
- Pezzini, B.R.; Silva, M.A.S.; Ferraz, H.G. Formas farmacêuticas sólidas orais de liberação prolongada: Sistemas monolíticos e multiparticulados. Rev. Bras. Ciencias Farm 2007, 43, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Gowda, D.V.; Aravind Ram, A.S.; Venkatesh, M.P.; Khan, M.S. Development and evaluation of clozapine pellets for controlled release. Int. J. Res. Ayurveda Pharm. 2012, 3, 611–618. [Google Scholar]
- Asmus, L.R.; Gurny, R.; Möller, M. Solutions for lipophilic drugs: A biodegradable polymer acting as solvent, matrix, and carrier to solve drug delivery issues. Int. J. Artif. Organs 2011, 34, 238–242. [Google Scholar] [CrossRef]
- Hyun, D.C. A Polymeric Bowl for Multi-Agent Delivery. Macromol. Rapid Commun. 2015, 36, 1498–1504. [Google Scholar] [CrossRef] [PubMed]
- Kreye, F.; Siepmann, F.; Siepmann, J. Drug release mechanisms of compressed lipid implants. Int. J. Pharm. 2011, 404, 27–35. [Google Scholar] [CrossRef]
- Christian, P.; Ehmann, H.M.A.; Werzer, O.; Coclite, A.M. Wrinkle formation in a polymeric drug coating deposited via initiated chemical vapor deposition. Soft Matter 2016, 12, 9501–9508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; Alexandridis, P. Nanoparticles in ionic liquids: Interactions and organization. Phys. Chem. Chem. Phys. 2015, 17, 18238–18261. [Google Scholar] [CrossRef]
- Patil, A.B.; Bhanage, B.M. Shape selectivity using ionic liquids for the preparation of silver and silver sulphide nanomaterials. Phys. Chem. Chem. Phys. 2014, 16, 3027–3035. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wu, X.; Qi, J.; Zhu, Q.; Wu, W.; Lu, Y.; Chen, Z. Ionic liquids: Green and tailor-made solvents in drug delivery. Drug Discov. Today 2020, 25, 901–908. [Google Scholar] [CrossRef] [PubMed]




| Drug | Formulations | % w/w | ||||
|---|---|---|---|---|---|---|
| Dynasan® 118 | Gelucire® 50/02 | Sucrose | [Cho][Phe] | [Cho][Glu] | ||
| Controls (without drug) | A0 | 100 | - | - | - | - |
| B0 | 90 | 10 | - | - | - | |
| C0 | 90 | - | 10 | - | - | |
| D0 | 99.8 | - | - | 0.2 | - | |
| E0 | 99.8 | - | - | - | 0.2 | |
| F0 | 89.8 | 10 | - | 0.2 | - | |
| G0 | 89.8 | 10 | - | - | 0.2 | |
| H0 | 89.8 | - | 10 | 0.2 | - | |
| I0 | 89.8 | - | 10 | - | 0.2 | |
| Dye2.5% w/w (Methylene blue orSudan III) | ADye | 97.5 | - | - | - | - |
| BDye | 87.5 | 10 | - | - | - | |
| CDye | 87.5 | - | 10 | - | - | |
| DDye | 97.3 | - | - | 0.2 | - | |
| EDye | 97.3 | - | - | - | 0.2 | |
| FDye | 87.3 | 10 | - | 0.2 | - | |
| GDye | 87.3 | 10 | - | - | 0.2 | |
| HDye | 87.3 | - | 10 | 0.2 | - | |
| IDye | 87.3 | - | 10 | - | 0.2 | |
| Drug 10% w/w (caffeine, salicylic acid, or rutin) | ADrug | 90 | - | - | - | - |
| BDrug | 80 | 10 | - | - | - | |
| CDrug | 80 | - | 10 | - | - | |
| DDrug | 89.8 | - | - | 0.2 | - | |
| EDrug | 89.8 | - | - | - | 0.2 | |
| FDrug | 79.8 | 10 | - | 0.2 | - | |
| GDrug | 79.8 | 10 | - | - | 0.2 | |
| HDrug | 79.8 | - | 10 | 0.2 | - | |
| IDrug | 79.8 | - | 10 | - | 0.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Júlio, A.; Sultane, A.; Viana, A.S.; Mota, J.P.; Santos de Almeida, T. Biobased Ionic Liquids as Multitalented Materials in Lipidic Drug Implants. Pharmaceutics 2021, 13, 1163. https://doi.org/10.3390/pharmaceutics13081163
Júlio A, Sultane A, Viana AS, Mota JP, Santos de Almeida T. Biobased Ionic Liquids as Multitalented Materials in Lipidic Drug Implants. Pharmaceutics. 2021; 13(8):1163. https://doi.org/10.3390/pharmaceutics13081163
Chicago/Turabian StyleJúlio, Ana, Anaisa Sultane, Ana Silveira Viana, Joana Portugal Mota, and Tânia Santos de Almeida. 2021. "Biobased Ionic Liquids as Multitalented Materials in Lipidic Drug Implants" Pharmaceutics 13, no. 8: 1163. https://doi.org/10.3390/pharmaceutics13081163
APA StyleJúlio, A., Sultane, A., Viana, A. S., Mota, J. P., & Santos de Almeida, T. (2021). Biobased Ionic Liquids as Multitalented Materials in Lipidic Drug Implants. Pharmaceutics, 13(8), 1163. https://doi.org/10.3390/pharmaceutics13081163