Formulation Strategies to Improve the Stability and Handling of Oral Solid Dosage Forms of Highly Hygroscopic Pharmaceuticals and Nutraceuticals
Abstract
:1. Introduction
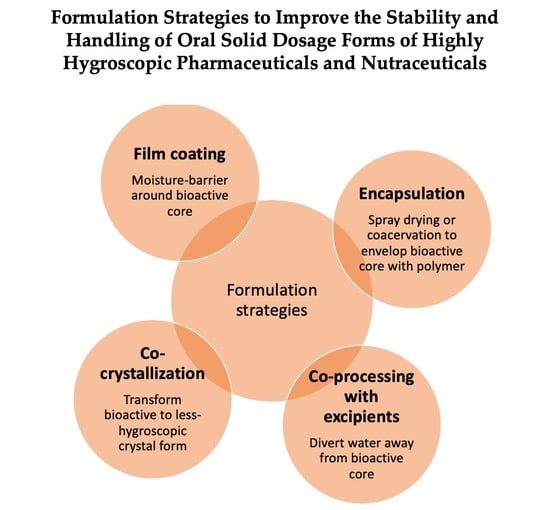
2. Formulation Strategies to Reduce or Control Hygroscopicity
2.1. Film Coating
2.1.1. Aqueous Solvent Coating
Water-Soluble Polymers
Combination of Water-Soluble and Water-Insoluble Polymers
Multi-Layer Coatings
2.1.2. Organic Solvent Coating
2.1.3. Dry Powder Coating
Polymer Coating
Sugar Coating
2.2. Encapsulation
2.2.1. Encapsulation by Spray Drying
Single Polymer as Wall Materials
Combination of Polymers as Wall Materials
2.2.2. Encapsulation by Coacervation
Complex Coacervation
Double-Emulsion Complex Coacervation for Hydrophilic Bioactives
Coacervation by Gelation
2.3. Co-Processing with Excipients
2.3.1. Amorphous and Low-Crystallinity Excipients
2.3.2. Hydrophilic Excipients
2.3.3. High Tg Excipients
2.3.4. Non-Hygroscopic and Inclusion Complex-Forming Excipients
2.4. Crystal Engineering
2.4.1. Co-Crystallization by Solvent Evaporation
2.4.2. Co-Crystallization by Liquid-Assisted Grinding
2.4.3. Co-Crystallization by Solvent Evaporation and Liquid-Assisted Grinding
2.4.4. Co-Crystallization by Neat Grinding
2.4.5. Co-Crystallization by Melt Crystallization
3. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| β-CD | β-cyclodextrin |
| ALG | Alginate |
| AMAP | Metacetamol |
| ANC | Anthocyanin |
| API-IL | Active Pharmaceutical Ingredient—Ionic Liquid |
| AS | Aspartame |
| BCl | Berberine Chloride |
| CA | Citric Acid |
| CMC | Carboxymethylcellulose |
| CS | Chitosan |
| EC | Ethyl Cellulose |
| EP | Epalrestat |
| FDA | Food and Drug Administration |
| FLR | Calcium Silicate |
| GA | Gum Arabic |
| GaA | Gallic Acid |
| GE | Gelatin |
| GRAS | Generally Recognised As Safe |
| HPC | Hydroxypropyl Cellulose |
| HPMC | Hydroxypropyl Methyl Cellulose |
| i-Car | i-Carrageenan |
| L-HPC | Low-substituted Hydroxypropylcellulose |
| LAC | Shellac |
| LCC | Low Crystallinity Cellulose |
| LVFX | Levofloxacin |
| MD | Maltodextrin |
| MET | Metformin |
| METCl | Metformin Chloride |
| Mw | Molecular Weight |
| OSDRC | One-step dry-coated |
| PLA | Poly(lactic acid) |
| PMMA | Poly(methacrylate-methylmethacrylate) |
| PS | Polystyrene |
| PSAA | Polymeric Surface-active Agent |
| PVA | Polyvinyl Alcohol |
| PVP | Poly(vinylpyrrolidone) |
| RH | Relative Humidity |
| SA | Stearic Acid |
| SPI | Soy Protein Isolate |
| TCM | Traditional Chinese Medicine |
| Tg | Glass transition temperature |
| THEDES | Therapeutic Deep Eutectic Solvents |
| VEM | Vemurafenib |
| W/O | Water/Oil |
| W/O/W | Water/Oil/Water |
| WPC | Whey Protein Concentrate |
| WPI | Whey Protein Isolate |
References
- Newman, A.W.; Reutzel-Edens, S.M.; Zografi, G. Characterization of the “hygroscopic” properties of active pharmaceutical ingredients. J. Pharm. Sci. 2008, 97, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Rajabi-Siahboomi, A.R.; Levina, M.; Upadhye, S.B.; Teckoe, J. Excipient Selection in Oral Solid Dosage Formulations Containing Moisture Sensitive Drugs. In Excipient Applications in Formulation Design and Drug Delivery; Springer: Cham, Switzerland, 2015; pp. 385–421. [Google Scholar]
- Thakur, T.S.; Thakuria, R. Crystalline Multicomponent Solids: An Alternative for Addressing the Hygroscopicity Issue in Pharmaceutical Materials. Cryst. Growth Des. 2020, 20, 6245–6265. [Google Scholar] [CrossRef]
- Helmenstine, A. Hygroscopic Definition and Examples. Available online: https://sciencenotes.org/hygroscopic-definition-and-examples/ (accessed on 15 August 2022).
- Edgar, G.; Swan, W.O. The factors determining the hygroscopic properties of soluble substances. I. The vapor pressures of saturated solutions. J. Am. Chem. Soc. 1922, 44, 570–577. [Google Scholar] [CrossRef]
- Khankaria, R.K.; Grant, D.J.W. Pharmaceutical hydrates. Thermochim. Acta 1995, 248, 61–79. [Google Scholar] [CrossRef]
- Poole, J.W.; Owen, G.; Silverio, J.; Freyhof, J.N.; Rosenman, S.B. Physiochemical factors influencing the absorption of the anhydrous and trihydrate forms of ampicillin. Curr. Ther. Res. Clin. Exp. 1968, 10, 292–303. [Google Scholar]
- Gouda, H.; Moustafa, M.A.; Al-Shora, H.I. Effect of storage on nitrofurantoin solid dosage forms. Int. J. Pharm. 1984, 18, 213–215. [Google Scholar] [CrossRef]
- Ebian, A.; Moustafa, R.; Abul-Enin, E. Nitrofurantoin. I. Effect of aging at different relative humidities and higher temperatures on the drug release and the physical properties of tablets. Egypt. J. Pharm. Sci. 1985, 26, 287–300. [Google Scholar]
- Otsuka, M.; Teraoka, R.; Matsuda, Y. Physicochemical Stability of Nitrofurantoin Anhydrate and Monohydrate Under Various Temperature and Humidity Conditions. Pharm. Res. 1991, 8, 1066–1068. [Google Scholar] [CrossRef]
- Cheow, W.S.; Kiew, T.Y.; Hadinoto, K. Combining inkjet printing and amorphous nanonization to prepare personalized dosage forms of poorly-soluble drugs. Eur. J. Pharm. Biopharm. 2015, 96, 314–321. [Google Scholar] [CrossRef]
- Bry-Air. Moisture and Humidity Control Solution in Pharmaceutical Industry. Available online: https://www.bryair.com/general-pharmaceutical/ (accessed on 15 August 2022).
- Emery, E.; Oliver, J.; Pugsley, T.; Sharma, J.; Zhou, J. Flowability of moist pharmaceutical powders. Powder Technol. 2009, 189, 409–415. [Google Scholar] [CrossRef]
- Arigo, A.; Jawahar, N.; Nikhitha, K.; Jubie, S. Effect of Hygroscopicity on pharmaceutical ingredients, methods to determine and overcome: An Overview. J. Pharm. Sci. Res. 2019, 11, 6–10. [Google Scholar]
- Roy, S.; Siddique, S.; Majumder, S.; Mohammed Abdul, M.I.; Ur Rahman, S.A.; Lateef, D.; Dan, S.; Bose, A. A systemic approach on understanding the role of moisture in pharmaceutical product degradation and its prevention: Challenges and perspectives. Biomed. Res. 2018, 29, 3336–3343. [Google Scholar] [CrossRef]
- Waterman, K.C.; MacDonald, B.C. Package selection for moisture protection for solid, oral drug products. J. Pharm. Sci. 2010, 99, 4437–4452. [Google Scholar] [CrossRef] [PubMed]
- Daliu, P.; Santini, A.; Novellino, E. From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert. Rev. Clin. Pharmacol. 2019, 12, 1–7. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, Y.; Zhu, J.; Chow, K.; Shi, K. An update on electrostatic powder coating for pharmaceuticals. Particuology 2017, 31, 1–7. [Google Scholar] [CrossRef]
- Aguilar-Toala, J.E.; Quintanar-Guerrero, D.; Liceaga, A.M.; Zambrano-Zaragoza, M.L. Encapsulation of bioactive peptides: A strategy to improve the stability, protect the nutraceutical bioactivity and support their food applications. RSC Adv. 2022, 12, 6449–6458. [Google Scholar] [CrossRef]
- Maderuelo, C.; Lanao, J.M.; Zarzuelo, A. Enteric coating of oral solid dosage forms as a tool to improve drug bioavailability. Eur. J. Pharm. Sci. 2019, 138, 105019. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef]
- Varshosaz, J.; Ghassami, E.; Ahmadipour, S. Crystal Engineering for Enhanced Solubility and Bioavailability of Poorly Soluble Drugs. Curr. Pharm. Des. 2018, 24, 2473–2496. [Google Scholar] [CrossRef]
- Yang, Q.; Yuan, F.; Xu, L.; Yan, Q.; Yang, Y.; Wu, D.; Guo, F.; Yang, G. An Update of Moisture Barrier Coating for Drug Delivery. Pharmaceutics 2019, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Kiew, T.Y.; Hadinoto, K. Amorphous nanodrugs prepared by complexation with polysaccharides: Carrageenan versus dextran sulfate. Carbohydr. Polym. 2015, 117, 549–558. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Liu, J.; Wang, W.; Yang, Q.; Yang, G. Deep eutectic solvents: Recent advances in fabrication approaches and pharmaceutical applications. Int. J. Pharm. 2022, 622, 121811. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, Q.; Chen, Z.; Wu, W.; Lu, Y.; Qi, J. Ionic liquids as a useful tool for tailoring active pharmaceutical ingredients. J. Control. Release 2021, 338, 268–283. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, D.; Chen, W.; Fu, L.; Mu, T. Water absorption by deep eutectic solvents. Phys. Chem. Chem. Phys. 2019, 21, 2601–2610. [Google Scholar] [CrossRef]
- Joshi, S.; Petereit, H.-U. Film coatings for taste masking and moisture protection. Int. J. Pharm. 2013, 457, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.; Tanaka, S.; Tamura, K.; Sakata, Y. Hygroscopicity of a sugarless coating layer formed by the interaction between mannitol and poly(vinyl alcohol) (PVA). Colloids Surf. B Biointerfaces 2014, 123, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wu, F.; Hong, Y.; Shen, L.; Lin, X.; Feng, Y. Improvements in sticking, hygroscopicity, and compactibility of effervescent systems by fluid-bed coating. RSC Adv. 2019, 9, 31594–31608. [Google Scholar] [CrossRef]
- Mwesigwa, E.; Buckton, G.; Basit, A.W. The hygroscopicity of moisture barrier film coatings. Drug Dev. Ind. Pharm. 2005, 31, 959–968. [Google Scholar] [CrossRef]
- Bley, O.; Siepmann, J.; Bodmeier, R. Importance of glassy-to-rubbery state transitions in moisture-protective polymer coatings. Eur. J. Pharm. Biopharm. 2009, 73, 146–153. [Google Scholar] [CrossRef]
- Patel, P.; Dave, A.; Vasava, A.; Patel, P. Formulation and characterization of sustained release dosage form of moisture sensitive drug. Int. J. Pharm. Investig. 2015, 5, 92. [Google Scholar] [CrossRef] [PubMed]
- Li, X.N.; Guo, H.X.; Heinamaki, J. Aqueous coating dispersion (pseudolatex) of zein improves formulation of sustained-release tablets containing very water-soluble drug. J. Colloid Interface Sci. 2010, 345, 46–53. [Google Scholar] [CrossRef]
- Penhasi, A.; Elias, M.; Eshtauber, E.; Naiman-Nissenboim, H.; Reuveni, A.; Baluashvili, I. A novel hybrid solid dispersion film coat as a moisture barrier for pharmaceutical applications. J. Drug Deliv. Sci. Technol. 2017, 40, 105–115. [Google Scholar] [CrossRef]
- El Mabrouki, H.; Kaukhova, I.E. Formulation and Development of Aqueous Film Coating for Moisture Protection of Hygroscopic Herniaria glabra L. Tablets. Turk. J. Pharm Sci 2022, 19, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, S.; Ohno, Y.; Makino, T.; Kashihara, T. Development and evaluation of the tablets coated with the novel formulation termed thin-layer sugarless coated tablets. Int. J. Pharm. 2004, 278, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Tsai, T.R.; Cheng, C.J.; Cham, T.M.; Lai, T.F.; Chuo, W.H. Formulation design of a highly hygroscopic drug (pyridostigmine bromide) for its hygroscopic character improvement and investigation of in vitro/in vivo dissolution properties. Drug Dev. Ind. Pharm. 2007, 33, 403–416. [Google Scholar] [CrossRef]
- Min, M.-H.; Park, J.-H.; Hur, J.-H.; Shin, H.-C.; Cho, Y.; Kim, D.-D. Formulation and bioequivalence studies of choline alfoscerate tablet comparing with soft gelatin capsule in healthy male volunteers. Drug Des. Dev. Ther. 2019, 13, 1049. [Google Scholar] [CrossRef]
- Pearnchob, N.; Siepmann, J.; Bodmeier, R. Pharmaceutical applications of shellac: Moisture-protective and taste-masking coatings and extended-release matrix tablets. Drug Dev. Ind. Pharm. 2003, 29, 925–938. [Google Scholar] [CrossRef]
- Reven, S.; Homar, M.; Peternel, L.; Kristl, J.; Žagar, E. Preparation and Characterization of Tablet Formulation based on Solid Dispersion of Glimepiride and Poly(ester amide) Hyperbranched Polymer. Pharm. Dev. Technol. 2011, 18, 323–332. [Google Scholar] [CrossRef]
- Satturwar, P.M.; Fulzele, S.V.; Joshi, S.B.; Dorle, A.K. Evaluation of the Film-Forming Property of Hydrogenated Rosin. Drug Dev. Ind. Pharm. 2003, 29, 877–884. [Google Scholar] [CrossRef]
- Huang, Y.T.; Tsai, T.R.; Cheng, C.J.; Cham, T.M.; Lai, T.F.; Chuo, W.H. Formulation design of an HPMC-based sustained release tablet for pyridostigmine bromide as a highly hygroscopic model drug and its in vivo/in vitro dissolution properties. Drug Dev. Ind. Pharm. 2007, 33, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Ziera, K.-I.; Schultzec, W.; Leopold, C.S. Combination of a hot-melt subcoating and an enteric coating for moisture protection of hygroscopic Sennae fructus tablets. Pharm. Dev. Technol. 2019, 24, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Ando, M.; Ito, R.; Ozeki, Y.; Nakayama, Y.; Nabeshima, T. Evaluation of a novel sugar coating method for moisture protective tablets. Int. J. Pharm. 2006, 336, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Luangtana-Anan, M.; Soradech, S.; Saengsod, S.; Nunthanid, J.; Limmatvapirat, S. Enhancement of Moisture Protective Properties and Stability of Pectin through Formation of a Composite Film: Effects of Shellac and Plasticizer. J. Food Sci. 2017, 82, 2915–2925. [Google Scholar] [CrossRef]
- Saikh, M.A.A. Aqueous Film Coating the Current Trend. J. Drug Deliv. Ther. 2021, 11, 212–224. [Google Scholar] [CrossRef]
- Gaikwad, S.S.; Kshirsagar, S.J. Review on Tablet in Tablet techniques. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 1. [Google Scholar] [CrossRef]
- FiorMarkets. Global Food Sugar Coating Market to Witness Steady Expansion Growth of USD 247.07 Million by 2030. Available online: https://www.globenewswire.com/news-release/2022/05/24/2449720/0/en/Global-Food-Sugar-Coating-Market-to-Witness-Steady-Expansion-Growth-of-USD-247-07-Million-By-2030.html (accessed on 15 August 2022).
- Giroldi, M.; Grambusch, I.M.; Neutzling Lehn, D.; Volken de Souza, C.F. Encapsulation of dairy protein hydrolysates: Recent trends and future prospects. Dry. Technol. 2021, 39, 1513–1528. [Google Scholar] [CrossRef]
- Peighambardoust, S.H.; Golshan Tafti, A.; Hesari, J. Application of spray drying for preservation of lactic acid starter cultures: A review. Trends Food Sci. Technol. 2011, 22, 215–224. [Google Scholar] [CrossRef]
- Santos, D.; Maurício, A.C.; Sencadas, V.; Santos, J.D.; Fernandes, M.H.; Gomes, P.S. Spray Drying: An Overview. In Biomaterials—Physics and Chemistry—New Edition; IntechOpen: London, UK, 2018. [Google Scholar]
- Sarabandi, K.; Gharehbeglou, P.; Jafari, S.M. Spray-drying encapsulation of protein hydrolysates and bioactive peptides: Opportunities and challenges. Dry. Technol. 2019, 38, 577–595. [Google Scholar] [CrossRef]
- Yu, H.; Teo, J.; Chew, J.W.; Hadinoto, K. Dry powder inhaler formulation of high-payload antibiotic nanoparticle complex intended for bronchiectasis therapy: Spray drying versus spray freeze drying preparation. Int. J. Pharm. 2016, 499, 38–46. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation Efficiency of Food Flavours and Oils during Spray Drying. Dry. Technol. 2008, 26, 816–835. [Google Scholar] [CrossRef]
- Ortiz, S.E.M.; Mauri, A.; Monterrey-Quintero, E.; Trindade, M.A.; Santana, A.S.; Favaro-Trindade, C.S. Production and properties of casein hydrolysate microencapsulated by spray drying with soybean protein isolate. LWT-Food Sci. Technol. 2008, 42, 919–923. [Google Scholar] [CrossRef]
- Rocha, G.A.; Trindade, M.A.; Netto, F.M.; Favaro-Trindade, C.S. Microcapsules of a Casein Hydrolysate: Production, Characterization, and Application in Protein Bars. Food Sci. Technol. Int. 2009, 15, 407–413. [Google Scholar] [CrossRef]
- Sarabandi, K.; Mahoonak, A.S.; Hamishekar, H.; Ghorbani, M.; Jafari, S.M. Microencapsulation of casein hydrolysates_ Physicochemical, antioxidant and microstructure properties. J. Food Eng. 2018, 237, 86–95. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Jafari, S.M.; Sarabandi, K.; Mohammadi, M.; Heshmati, M.K.; Pezeshki, A. Influence of spray drying encapsulation on the retention of antioxidant properties and microstructure of flaxseed protein hydrolysates. Colloids Surf. B Biointerfaces 2019, 178, 421–429. [Google Scholar] [PubMed]
- Kurozawa, L.E.; Park, K.J.; Hubinger, M.D. Effect of carrier agents on the physicochemical properties of a spray dried chicken meat protein hydrolysate. J. Food Eng. 2009, 94, 326–333. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Corke, H. Production and Properties of Spray-dried Amaranthus Betacyanin Pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, G.R.; González-García, R.; Grajales-Lagunes, A.; Ruiz-Cabrera, M.A.; Abud-Archila, M. Spray-Drying of Cactus Pear Juice (Opuntia streptacantha): Effect on the Physicochemical Properties of Powder and Reconstituted Product. Dry. Technol. 2005, 23, 955–973. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of process conditions on the physicochemical properties of acßai (Euterpe oleraceae Mart.) powder produced by spray drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Chen, Q.; Bi, J.; Zhou, Y.; Liu, X.; Wu, X.; Chen, R. Multiobjective optimization of spray drying of jujube (Zizyphus jujuba miller) powder using response surface methodology. Food Bioprocess Technol. 2014, 7, 1807–1818. [Google Scholar] [CrossRef]
- Tsolaki, E.; Stocker, M.W.; Healy, A.M.; Ferguson, S. Formulation of ionic liquid APIs via spray drying processes to enable conversion into single and two-phase solid forms. Int. J. Pharm. 2021, 603, 120669. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Mao, X.-Y.; Li, F.-F.; Zhang, D.; Leng, X.-J.; Ren, F.-Z.; Teng, G.-X. The improving effect of spray-drying encapsulation processon the bitter taste and stability of whey protein hydrolysate. Eur. Food Res. Technol. 2012, 235, 91–97. [Google Scholar] [CrossRef]
- Favaro-Trindade, C.S.; Santana, A.S.; Monterrey-Quintero, E.S.; Trindade, M.A.; Netto, F.M. The use of spray drying technology to reduce bitter taste of casein hydrolysate. Food Hydrocoll. 2010, 24, 336–340. [Google Scholar] [CrossRef]
- Ahmed, M.; Akter, M.S.; Eun, J.B. Impact of alpha-amylase and maltodextrin on physicochemical, functional and antioxidant capacity of spray-dried purple sweet potato flour. J. Sci. Food Agric. 2010, 90, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.-J.; Mao, X.-Y.; Wang, Q.; Yang, S.; Zhang, D.; Chen, S.-W.; Li, Y.-H. Effect of spray drying and freeze drying on the immunomodulatory activity, bitter taste and hygroscopicity of hydrolysate derived from whey protein concentrate. LWT—Food Sci. Technol. 2014, 56, 296–302. [Google Scholar] [CrossRef]
- Wang, H.; Tong, X.; Yuan, Y.; Peng, X.; Zhang, Q.; Zhang, S.; Xie, C.; Zhang, X.; Yan, S.; Xu, J.; et al. Effect of Spray-Drying and Freeze-Drying on the Properties of Soybean Hydrolysates. J. Chem. 2020, 2020, 1759–1769. [Google Scholar] [CrossRef]
- Mendanha, D.V.; Molina Ortiz, S.E.; Favaro-Trindade, C.S.; Mauri, A.; Monterrey-Quintero, E.S.; Thomazini, M. Microencapsulation of casein hydrolysate by complex coacervation with SPI/pectin. Food Res. Int. 2009, 42, 1099–1104. [Google Scholar] [CrossRef]
- Xiao, J.-X.; Huang, G.-Q.; Wang, S.-Q.; Sun, Y.-T. Microencapsulation of capsanthin by soybean protein isolate-chitosan coacervation and microcapsule stability evaluation. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Gomez-Mascaraque, L.G.; Llavata-Cabrero, B.; Martinez-Sanz, M.; Fabra, M.J.; Lopez-Rubio, A. Self-assembled gelatin-iota-carrageenan encapsulation structures for intestinal-targeted release applications. J. Colloid. Interface Sci. 2018, 517, 113–123. [Google Scholar] [CrossRef]
- Rocha-Selmi, G.A.; Bozza, F.T.; Thomazini, M.; Bolini, H.M.; Favaro-Trindade, C.S. Microencapsulation of aspartame by double emulsion followed by complex coacervation to provide protection and prolong sweetness. Food Chem. 2013, 139, 72–78. [Google Scholar] [CrossRef]
- Shaddel, R.; Hesari, J.; Azadmard-Damirchi, S.; Hamishehkar, H.; Fathi-Achachlouei, B.; Huang, Q. Double emulsion followed by complex coacervation as a promising method for protection of black raspberry anthocyanins. Food Hydrocoll. 2018, 77, 803–816. [Google Scholar] [CrossRef]
- Shaddel, R.; Hesari, J.; Azadmard-Damirchi, S.; Hamishehkar, H.; Fathi-Achachlouei, B.; Huang, Q. Use of gelatin and gum Arabic for encapsulation of black raspberry anthocyanins by complex coacervation. Int. J. Biol. Macromol. 2018, 107, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Kanha, N.; Regenstein, J.M.; Surawang, S.; Pitchakarn, P.; Laokuldilok, T. Properties and kinetics of the in vitro release of anthocyanin-rich microcapsules produced through spray and freeze-drying complex coacervated double emulsions. Food Chem. 2021, 340, 127950. [Google Scholar] [CrossRef]
- Loca, D.; Sevostjanovs, E.; Makrecka, M.; Zharkova-Malkova, O.; Berzina-Cimdina, L.; Tupureina, V.; Sokolova, M. Microencapsulation of mildronate in biodegradable and non-biodegradable polymers. J. Microencapsul. 2014, 31, 246–253. [Google Scholar] [CrossRef]
- Huang, X.; Ganzle, M.; Zhang, H.; Zhao, M.; Fang, Y.; Nishinari, K. Microencapsulation of probiotic lactobacilli with shellac as moisture barrier and to allow controlled release. J. Sci. Food Agric. 2021, 101, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheow, W.S.; Hadinoto, K. Dry powder inhaler formulation of lipid-polymer hybrid nanoparticles via electrostatically-driven nanoparticle assembly onto microscale carrier particles. Int. J. Pharm. 2012, 434, 49–58. [Google Scholar] [CrossRef]
- Airaksinen, S.; Karjalainen, M.; Kivikero, N.; Westermarck, S.; Shevchenko, A.; Rantanen, J.; Yliruusi, J. Excipient Selection Can Significantly Affect Solid-State Phase Transformationin Formulation During Wet Granulation. Aaps Pharmscitech 2004, 6, E311–E322. [Google Scholar] [CrossRef]
- Jin, S.; Lee, C.H.; Lim, D.Y.; Lee, J.; Park, S.J.; Song, I.S.; Choi, M.K. Improved Hygroscopicity and Bioavailability of Solid Dispersion of Red Ginseng Extract with Silicon Dioxide. Pharmaceutics 2021, 13, 1022. [Google Scholar] [CrossRef]
- Mihranyan, A.; Stromme, M.; Ek, R. Influence of cellulose powder structure on moisture-induced degradation of acetylsalicylic acid. Eur. J. Pharm. Sci. 2006, 27, 220–225. [Google Scholar] [CrossRef]
- Heidarian, M.; Mihranyan, A.; Stromme, M.; Ek, R. Influence of water-cellulose binding energy on stability of acetylsalicylic acid. Int. J. Pharm. 2006, 323, 139–145. [Google Scholar] [CrossRef]
- Hirai, N.; Ishikawa, K.; Takahashi, K. Improvement of the agitation granulation method to prepare granules containing a high content of a very hygroscopic drug. J. Pharm. Pharmacol. 2006, 58, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Moribe, K.; Sekiya, N.; Fujito, T.; Yamamoto, M.; Higashi, K.; Yokohama, C.; Tozuka, Y.; Yamamoto, K. Stabilization mechanism of limaprost in solid dosage form. Int. J. Pharm. 2007, 338, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.H.; Wong, S.Y.; Law, M.W.; Chu, K.K.; Chow, A.H. Anti-hygroscopic effect of dextrans in herbal formulations. Int. J. Pharm. 2008, 363, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Bernal, C.-A.; Aragón, M.; Baena, Y. Dry powder formulation from fruits of Physalis peruviana L. standardized extract with hypoglycemic activity. Powder Technol. 2016, 301, 839–847. [Google Scholar] [CrossRef]
- Sablani, S.S.; Shrestha, A.K.; Bhandari, B.R. A new method of producing date powder granules: Physicochemical characteristics of powder. J. Food Eng. 2007, 87, 416–421. [Google Scholar] [CrossRef]
- Iftekhar, Q.U.A.; Fatima, S.; Naveed, S.; Usman, S.; Ishaq, S.; Rehman, H. Formulation Development of Betahistine Dihydrochloride by using Co-processed QuickTab™: A Strategy to Shelf Stable Tablets of Hygroscopic Drug. Lat. Am. J. Pharm. 2020, 39, 1240–1245. [Google Scholar]
- Maeda, H.; Iga, Y.; Nakayama, H. Characterization of inclusion complexes of betahistine with β-cyclodextrin and evaluation of their anti-humidity properties. J. Incl. Phenom. Macrocycl. Chem. 2016, 86, 337–342. [Google Scholar] [CrossRef]
- Guo, M.; Sun, X.; Chen, J.; Cai, T. Pharmaceutical cocrystals: A review of preparations, physicochemical properties and applications. Acta Pharm. Sin. B 2021, 11, 2537–2564. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, Q.; Zhu, B.; Zhang, Z.; Ma, X.; Dai, W.; Gong, X.; Ren, G.; Mei, X. Drug–Drug Cocrystals Provide Significant Improvements of Drug Properties in Treatment with Progesterone. Cryst. Growth Des. 2020, 20, 3053–3063. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Chen, J.-m.; Wang, Y.-X.; Sun, C.C. Novel Salt-Cocrystals of Berberine Hydrochloride with Aliphatic Dicarboxylic Acids: Odd−Even Alternation in Physicochemical Properties. Mol. Pharm. 2021, 18, 1758–1767. [Google Scholar] [CrossRef]
- Watanabe, T.; Ito, M.; Suzuki, H.; Terada, K.; Noguchi, S. Reduced deliquescency of isosorbide by cocrystallization and mechanisms for hygroscopicity. Int. J. Pharm. 2021, 607, 120959. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-N.; Li, Y.-J.; Chang, L.; Liu, L.-X.; Feng, Y.-R.; Wu, L.-L.; Zhang, L.; Zhang, Y.-J. Improving hygroscopic stability of palmatine chloride by forming a pharmaceutical salt cocrystal of palmatine chloride-gallic acid with neutral molecule. J. Drug Deliv. Sci. Technol. 2021, 66, 102839. [Google Scholar] [CrossRef]
- Sun, J.; Jia, L.; Wang, M.; Liu, Y.; Li, M.; Han, D.; Gong, J. Novel Drug−Drug Multicomponent Crystals of Epalrestat−Metformin: Improved Solubility and Photostability of Epalrestat andReduced Hygroscopicity of Metformin. Cryst. Growth Des. 2022, 22, 1005–1016. [Google Scholar] [CrossRef]
- Khajir, S.; Shayanfar, A.; Monajjemzadeh, F.; Jouyban, A. Crystal engineering of valproic acid and carbamazepine to improve hygroscopicity and dissolution profile. Drug Dev. Ind. Pharm. 2022, 47, 1674–1679. [Google Scholar] [CrossRef]
- Huang, G.-L.; Yang, L.; Ren, B.-Y.; Lv, X.-Y.; Song, L.-Y.; Dai, X.-L.; Chen, J.-M. Simultaneously improving the physicochemical and pharmacokinetic properties of vemurafenib through cocrystallization strategy. J. Drug Deliv. Sci. Technol. 2022, 70, 103230. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Gao, Z. Crystal structure, dissolution and hygroscopicity of a novel cocrystal hydrate of berberine hydrochloride with L(+)-lactic acid. Pharmazie 2020, 75, 483–487. [Google Scholar] [CrossRef]
- Lu, Q.; Dun, J.; Chen, J.M.; Liu, S.; Sun, C.C. Improving solid-state properties of berberine chloride through forming a salt cocrystal with citric acid. Int. J. Pharm. 2019, 554, 14–20. [Google Scholar] [CrossRef]
- Shinozaki, T.; Ono, M.; Higashi, K.; Moribe, K. A Novel Drug-Drug Cocrystal of Levofloxacin and Metacetamol: Reduced Hygroscopicity and Improved Photostability of Levofloxacin. J. Pharm. Sci. 2019, 108, 2383–2390. [Google Scholar] [CrossRef]
- de Maere d’Aertrycke, J.B.; Robeyns, K.; Willocq, J.; Leyssens, T. Cocrystallization as a tool to solve deliquescence issues: The case of l-lactic acid. J. Cryst. Growth 2017, 472, 3–10. [Google Scholar] [CrossRef]






| API/ Nutraceuticals | Coating Technique | Film/ Coating Material | Key Findings | Ref. |
|---|---|---|---|---|
| Aqueous solvent coating | ||||
| L-cysteine | Pan-coating | Sugarless layer: Polyvinyl Alcohol (PVA) and mannitol (sweetener) | At 25 °C/75%RH, moisture absorption rates increased in this order: uncoated tablets (~7%) > PVA-film-coated tablet > sugarless layer-coated tablets (0.25%). It was lowest at mannitol:PVA concentration of 15:2.5 and 15:4 (w/w). | [30] |
| Citric acid/Sodium bicarbonate effervescent tablets | Fluid bed | Poly(vinylpyrrolidone) (PVP) | Moisture absorption rate constants (within 15 min) of coated tablets (0.34–0.50) was lower than uncoated granules (0.67) and commercial vitamin C ET (0.71), showing decreased hygroscopicity. | [31] |
| Lactose monohydrate, microcrystalline cellulose, pre-gelatinized starch, magnesium stearate and colloidal silica | Fluid bed | Eudragit® L 30D-55 PVA-based Opadry AMB® HPMC-based Sepifilm™ LP 014 | Sepifilm and Opadry films were more hygroscopic than Eudragit film. Eudragit film was the most effective coating for limiting moisture sorption. | [32] |
| Freeze-dried garlic powder | Pan-coating | Amorphous polymers: HPMC-based Methocel® E5 Eudragit® E PO Presence of crystalline in polymer: PVA-based Opadry AMB® (Anti-plasticizing agent: PVP) | Opadry AMB® was chosen due to its most promising moisture-protective ability at its glassy state. The addition of anti-plasticizing agent increased water uptake instead, and was deemed unsuitable to enhance the polymer’s performance. | [33] |
| Ranitidine Hydrochloride | Pan-coating | Eudragit® E PO and Eudragit® RLPO | Eudragit RLPO 10% and Eudragit E PO 10% coated tablets absorbed less moisture at all the tested conditions. In 350 h at 75%RH, it absorbed 15–20% compared with uncoated tablets at 45–50%. In 200 h at room temperature (RT), it absorbed 4–6% moisture compared with uncoated tablets at 8–10%. In 170 h at 75%RH, it absorbed 10–15% compared with marketed formulation RANTEC 300 at 35–40%. | [34] |
| Metoprolol tartrate tablets | Pan-coating | Water-insoluble Zein Plasticizer: PEG400 or glycerol | Water vapor permeability of aqueous-based coating was lower than organic-based with PEG400. | [35] |
| Calcium chloride tablets | Fluid bed | Lipophilic substance: Stearic acid (SA) Water-soluble film forming polymer: Hydroxypropyl cellulose (HPC) Polymeric surface-active agent (PSAA) | This combination of polymers helped to lower water permeability of the film compared to individual component films, where HPC:SA:PSAA at ratio of 62:25:10 had the lowest water vapor transmission rate at 60 g/m2 day. The uncoated tablets had the highest moisture absorption through the test period compared to coated tablets. HPC:SA:PSAA at ratio of 62:25:10 had the highest sealing capability, with a weight gain of only 3.5% in RT/75%RH after 168 h compared with 10% for uncoated tablets. | [36] |
| Herniara glabra L. extract | Fluid bed | Lipophilic/enteric substance: Shellac Water-soluble film forming polymer: Hydroxypropyl methyl cellulose (HPMC) Plasticizer: Stearic acid (SA), PEG1500, PEG400 Pigment: Titanium dioxide | Combination formulation of 25% HPMC, 20% shellac, 10% PEG 1500, 29.6% PEG400, 5% tween80, 10% titanium oxide and 0.4% acid red 2 offered good protection against moisture compared with the core tablets, with its weight gain decreasing from 16.1% in core tablets to 5.7% at 75%RH, and from 18.2% to 7.5% at 90%RH after around 110 h. The long-term and accelerated stability studies showed stability of formulation. | [37] |
| Vitamin C, E, B2, calcium pantothenate, L-cysteine | Pan-coating | Sugarless layer: Undercoating (UC) 2%: HPMC 10%, Purified Water (PW) 90% Build-up coating (BC) 38%: Erythritol 18.2–22%, talc 10.6%, TiO2 0.8%, MCC 0–3.8%, powdered acacia 4.6%, PW 62% Syrup coating (SC) 5%: Erythritol 34.2%, PEG6000 3.8%, PW 62% Polishing: Mixture of waxes, Carnauba wax and white beeswax | Lower hygroscopicity of the sugarless coated tablets was confirmed by the stability of the actives after storage at 40 °C/75%RH for 6 months under closed condition, where >95% drug content remained compared to <90% in sugar-coated tablets. Stability of the actives after storage at 40 °C/75%RH for 1 month under open conditions left >95% drug content in sugarless coated tablets, which was higher than uncoated tablets with <85% drug remaining. The stability and therefore hygroscopicity of sugarless coated tablets were superior to uncoated tablets, and similar to sugar-coated tablets. | [38] |
| Pyridostigmine Bromide | Fluid bed | Seal layer coating: Opadry II Sustained release layer coating: Surelease® Waterproof layer coating: Opadry II HP | At 25 °C/60%RH, 30 °C/65%RH and 40 °C/75%RH, the uncoated core pellets had their moisture absorption potential increased after 4 h, in contrast to the insignificant increase in moisture absorption of coated pellets up to 4 weeks. Coated pellets’ hygroscopicity was significantly less compared to pure PB which rapidly transformed from solid to liquid state within 10 min. | [39] |
| Choline Alfoscerate | Organic solvent coating (subcoating) Aqueous solvent coating (outer coating) | Hydrophobic substance: HPMC-based Opadry I® Hydrophilic substance: PVA-based Opadry AMB® | Film-coated core tablets maintained their appearance for 30 days in 60%RH. Visual observation of the chosen formulation before and after 3 months exhibited no change, compared with a deformed commercial capsule that was packaged in Zymax blister film. | [40] |
| Organic solvent coating | ||||
| Aspirin (Acetylsalicylic Acid) | Pan-coating | Shellac | Shellac-coated tablets had significantly lower water uptake rates at 12.2% than HPMC-coated tablets at 19.2% at 100%RH. The difference was less pronounced at 75%RH with uptake rates at 3.0% compared with HPMC-coated tablets at 4.2%. This shows that shellac had higher potential for moisture protection than HPMC especially at high RH. However, the difference between the observed stability of drug was not as pronounced. Much lower shellac coating levels were needed for similar moisture protection as HPMC-coated tablets. | [41] |
| Solid dispersion of glimepiride and poly(ester amide) hyperbranched polymer | Pan-coating | HPMC phthalate Tablet core mixed with lactose (filler) and magnesium stearate (lubricant) | After 24 h in 75%RH, the increase in weight of solid dispersion powder at 29.5% (deliquescent) was significantly larger compared with the tablet core at 3.0% (hygroscopic) and coated tablets at 1.7% (slightly hygroscopic). | [42] |
| Diclofenac | Fluid bed | Hydrophobic substance: Hydrogenated Rosin (HR) Plasticizer: Hydrophilic: Glycerol (GLY) Hydrophobic: Dibutyl sebacate (DBS) | Hydrophilic plasticizer GLY was incompatible with hydrophobic HR due to their opposing natures. Flexible films cannot be formed as they cannot blend uniformly, resulting in brittle non-uniform films. HR films plasticized with DBS gave extremely low rates of water vapor transmission rates (10−5 g cm/cm2/24 h), which was very low compared with shellac. | [43] |
| Dry powder coating | ||||
| Pyridostigmine Bromide | Direct compression | Hydroxypropyl methyl cellulose (HPMC) Hydrophobic excipient with core: Avicel pH 102 | HPMC-coated drug improved the hygroscopicity of pure drug slightly but it remained hygroscopic, as it softened in 2–3 days under ambient condition in comparison to the pure drug which transformed from solid to liquid state in 10 min. To improve hygroscopicity, water-insoluble excipient (Avicel pH 102) was added into the HPMC coating. The tablets did not show significant increase in moisture absorption up to 2 weeks at all conditions at 25 °C/60%RH, 30 °C/65%RH and 40 °C/75%RH, | [44] |
| Sennae fructus tablets | Dry powdered lipids coating: Hot-melt coating Coating of enteric coating: Fluid bed | Lipids: Medium chain triglyceride (MCT), Stearic acid (SA), Precirol® ATO 5 (Pr), Compritol® 888 ATO (Cp) Aq enteric coating: Eudragit® L 30D-55 (EuL55) | The moisture permeability of the lipids follow this order: SA > MCT > Pr ~ Cp Only with Pr did the addition of EuL55 reduce moisture absorption. Other subcoatings of lipids with the addition of EuL55 showed no difference in their moisture absorption compared with just EuL55. The maximum reduction in hygroscopicity compared with tablet cores quantifies to 98% at 33%RH/RT, 96% at 43%RH/RT (Cp10 + EuL55 10) and 85% at 75%RH/RT (Pr10 + EuL55 10). | [45] |
| Fructose | One step dry-coated tablets (OSDRC) by compression | Crystallized compressed amorphous sucrose Plasticizer: Hydroxypropyl cellulose (HPC) and poly(vinylpyrrolidone) (PVP) | OSDRC tablets’ water vapor adsorption at 25 °C/75% in 15 h amount to a weight change of <1.0% in comparison to 5.5–6.0% in HPMC-coated tablets, showing its superior moisture-protective properties. | [46] |
| Drug/ Nutraceutical | Technique | Wall Material | Key Findings | Ref. |
|---|---|---|---|---|
| Single polymer | ||||
| Casein Hydrolysate | Spray-drying | Soy Protein Isolate (SPI) | Encapsulation increased hygroscopicity from 53 g/100 g to 106.99 g/100 g and 102.12 g/100 g, respectively, with SPI:hydrolysate ratios 70:30 and 80:20. | [57] |
| Casein Hydrolysate | Spray-drying | Maltodextrin: MD 10DE MD 20DE | Hygroscopicity of encapsulated hydrolysate was significantly less than free hydrolysate, improving from 53 g/100 g to 13.93 g/100 g (10DE) and 13.13 g/100 g (20DE). | [58] |
| Casein hydrolysates | Spray-drying | Maltodextrin (MD) | The microencapsulation process by MD (60% MD) significantly reduced values of hygroscopicity. | [59] |
| Flaxseed protein hydrolysates | Spray-drying | Maltodextrin (MD) | Hygroscopicity of encapsulated hydrolysate was significantly less than free hydrolysate, improving from from 39.2% to 17.4% in powders produced with carrier ratio 3:1. | [60] |
| Chicken meat protein hydrolysate | Spray-drying | Maltodextrin (MD) Gum Arabic (GA) | Hygroscopicity of encapsulated hydrolysate was significantly less than free hydrolysate, improving from 40.9 g/100 g to 15.9 g/100 g with 30% MD and 21.2 g/100 g with 30% GA. | [61] |
| Amaranthus Betacyanin Pigments | Spray-drying | Maltodextrin: MD 10DE MD 15DE MD 20DE MD 25DE Starches: Corn starch Modified corn starch | Hygroscopic properties increased with decreasing Mw of the wall materials. Hygroscopicity improved from 44.6–46.0 g/100 g to 40.9 g/100 g at the lowest DE investigated (i.e., 10DE MD). Corn starch gave the lowest hygroscopicity at 24.6 g/100 g. | [62] |
| Cactus pear juice | Spray-drying | Maltodextrin: MD 10DE MD 20DE | Hygroscopicity varied from 36.30–48.93 g/100 g. The least hygroscopic powders were obtained at the highest MD concentrations and pressure. | [63] |
| Acai (Euterpe Oleraceae Mart.) | Spray-drying | Maltodextrin: MD 10DE | The lowest hygroscopicity values were obtained when the highest MD concentrations used. Lower inlet air temperature and higher feed flow led to lower hygroscopicity. | [64] |
| Jujube powder | Spray-drying | Maltodextrin (MD) | Hygroscopicity was significantly affected by weight ratio of MD and feed flow rate (FFR). Lower hygroscopicities were obtained at higher MD and FFR. The highest MD concentration resulted in the least hygroscopicity. The optimum formulation had a hygroscopicity of 18.59%. | [65] |
| API-ILs: 1. -butyl-3-methylimidazolium Ibuprofenate (BMIm Ibu) 1. -butyl-3-methyl imidazolium Warfarinate (BMIm War) Choline Ibuprofenate (Cho Ibu) Choline Warfarinate (Cho War) Propranolol Saccharin (Pro Sac) | Spray-drying | Ethyl Cellulose: EC4 EC10 Maltodextrin: MD 6D | The physical stabilities of API-ILs encapsulated were tested by storing them at 25 °C/50%RH for up to 14 days. API-ILs encapsulated by MD were found to rapidly absorb water and transform from fine powder into extremely viscous sticky treacle-like liquid. In contrast, API-ILs encapsulated by EC remained as fine white powder and appeared unchanged upon storage. | [66] |
| Mixed polymers | ||||
| Whey protein hydrolysate | Spray-drying | Maltodextrin and β-cyclodextrin (MD and β-CD) MD | Hygroscopicity of encapsulated hydrolysate was significantly less than free hydrolysate, improving from 64.31 g/100 g to 43.09 g/100 g for MD-encapsulated and 36.99 g/100 g for MD/β-CD (1:1 w/w) encapsulated hydrolysate (70% wall). | [67] |
| Casein hydrolysate | Spray-drying | Gelatin and Soy Protein Isolate (GE and SPI) | All formulations were less hygroscopic than free hydrolysate, with the lowest at 27.23 g/100 g (20% hydrolysate, 80% carrier, (wall materials: 40% GE, 60% SPI)) compared to 53 g/100 g for free hydrolysate. Variations in GE and SPI concentration did not cause significant differences in hygroscopicity. | [68] |
| Purple sweet potato | Spray-drying | Maltodextrin (MD) and α-amylase | Hygroscopic moisture of spray dried flours treated with MD was at 2.9–3.0 g/kg, exhibiting lower hygroscopicity than control at 3.3 g/kg. Hygroscopicity increased at higher levels of amylase. | [69] |
| Whey protein hydrolysate | Spray-drying Or Freeze-drying | Whey Protein Concentrate and Sodium Alginate (WPC and ALG) WPC | Hygroscopicity of encapsulated hydrolysate obtained via both methods were significantly less than free hydrolysate. For spray-drying, the hygroscopic moisture of free hydrolysate at 26.69 g/100 g was reduced to 20.31 g/100 g (WPC) and 20.93 g/100 g (WPC/ALG). For freeze-drying, the hygroscopic moisture of free hydrolysate at 31.09 g/100 g was reduced to 26.28 g/100 g (WPC) and 24.40 g/100 g (WPC/ALG). | [70] |
| Soybean hydrolysates | Spray-drying Or Freeze-drying | Soy Protein Isolate and Maltodextrin (SPI and MD) | Hygroscopicity of encapsulated hydrolysate obtained via both methods were significantly less than free hydrolysate. For spray-drying, the hygroscopic moisture of free hydrolysate at 39 g/100 g was reduced to 18–20 g/100 g. For freeze-drying, the hygroscopic moisture of free hydrolysate at 41 g/100 g was reduced to 27–28 g/100 g. | [71] |
| Drug/ Nutraceutical | Technique | Wall Material | Key Findings | Ref. |
|---|---|---|---|---|
| Capsanthin | Complex coacervation Freeze drying | Soybean Protein Isolate/Chitosan (SPI/CS) | Microencapsulated capsanthin had enhanced stability against low and medium RH where it had improved retention rates from 42.77%, 54.37%, and 56.69% to 81.01%, 80.71% and 73.79% in RH 33%, 58%, 68%, respectively. It was less effective in protecting capsanthin in high humidity of 98%RH where its retention rate worsened from 68.99% to 45.81%. | [73] |
| Polyphenols from grape Juice Extract (GJE) | Extrusion into solution for complex coacervation Freeze drying | Gelatin/i-Carageenan (GE/i-Car) | Water uptake capacity (WU) is directly related to hygroscopicity. Blends containing higher amounts of GE had slightly lower WU. The lowest WU was observed at GE/i-Car at ratio 85:15, lower than that of 100% GE. WU between 8–12% was lower than WU of GJE at 32% after 100 h, showing how the freeze dried matrices can reduce the hygroscopicity of GJE. | [74] |
| Aspartame | Double emulsion complex coacervation Freeze drying | Gelatin/Gum Arabic (GE/GA) | Hygroscopicity were in the range of 10.73–13.43 g/100 g for all formulations, with no significant difference from free aspartame at 10.86 g/100 g. | [75] |
| Anthocyanin | Double emulsion complex coacervation Freeze drying | Gelatin/Gum Arabic (GE/GA) | Hygroscopicity ranged from 36–49 g/100 g, which was significantly less than anthocyanin at 85.22 g/100 g. | [76] |
| Anthocyanin | Double emulsion complex coacervation Freeze drying | Gelatin/Gum Arabic (GE/GA) | Hygroscopicity ranged from 37.05–49.05 g/100 g, which was significantly less than anthocyanin at 94.60 g/100 g. | [77] |
| Anthocyanin and tea extracts | Double emulsion complex coacervation Freeze dried extracts (FDE) Or Spray dried extracts (SDE) | Gelatin/Acacia Gum (GE/AG) Chitosan/Carboxymethylcellulose (CS/CMC) | Moisture content of SDE microcapsules at 2.39% and 3.23% were significantly lower than that of FDE at 4.88% and 4.91%, for GE/AG and CS/CMC-encapsulated capsules, respectively. GE/AG capsules had lower moisture content than CS/CMC capsules as CS/CMC layer was thicker, but their hygroscopicity difference was not large. SDE capsules had lower hygroscopicity at 21.4% and 21.8% than FDE microcapsules at 45.2% and 43.5%, for GE/AG and CS/CMC-encapsulated capsules, respectively. | [78] |
| Casein Hydrolysate | Double emulsion complex coacervation Freeze drying | Soybean Protein Isolate/Pectin (SPI/Pectin) | Hygroscopicity of free hydrolysate at 53 g/100 g was almost two times higher than the encapsulated samples at 20.08–24.38 g/100 g. The lowest value was obtained at the lowest hydrolysate content (50%). The more the content, the more hygroscopic the sample. | [72] |
| Mildronate | Double emulsion complex coacervation Dried at ambient temperature | Biodegradable: Poly(lactic acid) (PLA) Or Non-biodegrable: Polysterene (PS) | The polymer coatings decreased mildronate’s hygroscopicity in the long run (75%RH at 168 h) by more than two times, from 66.28%, to 26.18% in PLA and 22.04% in PS. | [79] |
| Probiotic Lactobacilli | Emulsification and external gelation/crosslinking Freeze drying | Alginate (ALG) Shellac (LAC) Whey Protein Isolate (WPI) All formulations have sucrose: ALG ALG/LAC ALG/WPI ALG/WPI/LAC | The order of hygroscopicity is as follows ALG/WPI > ALG > ALG/WPI/LAC > ALG/LAC based on moisture absorption isotherms and vapor absorption rates of the microcapsules. LAC addition had significantly reduced hygroscopicity, which can be seen in the storage stability afforded by the encapsulation, in the otherwise quick inactivation of probiotics. | [80] |
| Drug/ Nutraceutical | Technique | Excipients | Key Findings | Ref. |
|---|---|---|---|---|
| Nitrofurantoin anhydrate | Wet granulation | Amorphous: Low-substituted Hydroxypropyl cellulose (HPC) Pregelatinized starch Partially amorphous: Silicified Microcrystalline cellulose (MCC) Crystalline: α-lactose monohydrate | Only the amorphous excipients impeded hydrate formation of the drug at high water content. The hygroscopic partially amorphous excipient hindered hydrate formation of drug at low water contents. The crystalline excipient was unable to control hydrate formation of drug. | [82] |
| Ginsenoside from Red Ginseng Extract | Dissolution and freeze drying Mortar | Silicon Dioxide (SiO2) | The water sorption rate of the extract was at <20% from 30%RH to 70%RH, compared to solid dispersion with SiO2 at <12%. Visual observation of the solid dispersion yielded no observable differences before and after storage in various humid conditions, from 30% to 70%RH for 25 days at 30 °C. The desorption isotherms show that the dispersion had negligible hysteresis, which showed the reversible change in water content and the non-hygroscopic nature of the powder. | [83] |
| Aspirin (Acetylsalicylic Acid) | Physical mixture | Highest to lowest crystallinity: Cladophora cellulose Microcrystalline cellulose (MCC-SLM) Agglomerated micronized cellulose (AMC) Low crystallinity cellulose (LCC) | The lowest degradation rates were found in formulations consisted of the lowest crystallinity LCC despite its highest moisture. | [84] |
| Aspirin (Acetylsalicylic Acid) | Physical mixture | High crystallinity cellulose (HCC) Microcrystalline cellulose (MCC) Low crystallinity cellulose (LCC) Lactose | Drug degradation increased with high amounts of crystallinity cellulose HCC and MCC. It was lowest and decreased with higher amount of LCC despite its higher moisture content. | [85] |
| Traditional Chinese Medicines (TCM) | Powder: Wet granulation Oven drying Tablets formation: Compression | Porous calcium silicate (Florite RE, FLR) | Wet granulation was a success with the addition of FLR. It is suitable for use in wet granulation of very hygroscopic powders as water retained in FLR will be transferred to the added hygroscopic material very slowly, resulting in granules. | [86] |
| Limaprost | Dissolution and freeze drying | Dextran40 Dextrin Pullulan | Although water content after storage was extremely high at >10%, the drug was stabilized. | [87] |
| Herbs: Radix ophiopogonis Rhizoma polygonati | Physical mixture Oven drying | Dextrans T10, T40, T70 | The moisture sorption was depressed by increasing mass of dextrans. Dextran also increased the Tg of the extracts at all RH, reducing their tackiness and ability to absorb water. | [88] |
| Physalis peruviana fruit extract | Wet granulation Oven drying Or Fluidized bed drying | Combination of corn starch and Microcrystalline cellulose (MCC) | The formulation improved the hygroscopicity of powder slightly, changing it from class III (moderately hygroscopic) to class II (slightly hygroscopic) powder. It also helped to avoid deliquescence of the extract. | [89] |
| Raw date | Physical mixture Oven drying Hammer mill | Maltodextrin (MD) | MD increased Tg The higher the MD content, the lower the hygroscopicity. Hygroscopicity value was 6.2% at MD to date ratio of 35:65, and 4.0% at 50:50. MD:date at 50:50 remained free flowing even after a year of storage. | [90] |
| Betahistine dihydrochloride | Direct compression | Quick Tab™ (mix of tricalcium phosphate, microcrystalline cellulose, povidone, cross povidone) | After 3 months in 25 °C/60%RH accelerated stability testing conditions, the formulation appearance was found to be satisfactory with no change. Its weight variation was also insignificant. | [91] |
| Betahistine (BTH) | Kneading and oven drying Or Dissolution and freeze drying | Inclusion complex: β-cyclodextrins (β-CD) | Compared with BTH which liquefied completely after 100 min, the solids obtained from both kneading and freeze drying were not completely liquefied even after a month. At 300 h in 75%RH, BTH’s weight varied by 70–80%, compared with that of the solids obtained by both methods which increased by <10%. At 240 min in 33%RH, BTH completely liquefied, compared with the weight variation in the solids obtained by both methods which increased by <10%. | [92] |
| Drug/ Nutraceutical | Technique | Co-Former | Key Findings | Ref. |
|---|---|---|---|---|
| Phloroglucinol (SPF) | Solvent evaporation | Progesterone (Prog) | In dynamic vapor sorption, SPF absorbed 25% water to convert to dihydrate at 40%RH. Prog-SPF and Prog-SPF-HH (hemihydrate) had remarkable hygroscopic advantage over SPF, as they absorbed 0.5% and 0.3% water, respectively, at 80%RH. Furthermore, Prog-SPF absorbed 0.3% and 0.4% water at 60% and 80%RH in 2 weeks, showing their non-hygroscopic nature. | [94] |
| Berberine Chloride (BCl) | Solvent evaporation | Succinic acid (SUA) Glutaric acid (GLA) Adipic acid (ADA) Pimelic acid (PIA) | In dynamic vapor sorption, BCl absorbed 8.1–10% water to convert to dihydrate from 10% to 70%RH, and absorbed 11– 16% water to convert to tetrahydrate from 80% to 90%RH. All cocrystals adsorbed negligible amounts of moisture of <1.2% up to 70%RH. Hygroscopicity follows the ascending order of BCI-SUA < BCl-GLA < BCl-ADA < BCl-PIA < BCl.2H2O. BCl-SUA and BCI-GLA were less hygroscopic than the other 2 cocrystals as they did not form hydrates when exposed to high RH. | [95] |
| Isosorbide (ISO) | Solvent evaporation | Piperazine (PZ) Hydrochlorothiazide (HCT) 3,5-dihydroxybenzoic acid (35DHBA) Gallic acid (GaA) | The critical RH of cocrystals at 56–85%RH were higher than that of of ISO at 48%RH. At 95%RH, ISO and ISO-PZ deliquesced, whereas ISO-HCT, ISO-35DHBA and ISO-GA remained solid. | [96] |
| Palmatine Chloride (PMTCl) | Solvent evaporation | Gallic acid (GaA) | The hygroscopic stability of PMTCl-GA salt cocrystals were significantly better than PMTCl. At 75%, 80% and 95%RH, PMTCl took more days to reach moisture equilibrium to gain 7.07%, 16.46% and 19.01% water mass at each RH, respectively, as compared to 2.83%, 4.84% and 5.78% moisture gained by PMTCl-GA. | [97] |
| Metformin (MET) | Solvent evaporation | Epalrestat (EP) | The hygroscopicity of MET and METCl (commercial) were higher than EP-MET and EP-MET monohydrate (MH). At 95%RH, the weight of MET increased 63%, EP-MET increased 0.25%, EP-MET MH increased <1%, and METCl increased 1.54%. | [98] |
| Sodium Valproate (VAL) | Solvent evaporation &and Liquid-assisted grinding | Carbamazepine (CBM) Tromethamine (TMA) | TMA-VAL absorbed almost no water at <1% up to 75%RH after 1 week, whereas sodium VAL absorbed 70.02%. CBM-VAL absorbed <10% at 100%RH after 1 week, whereas sodium VAL absorbed 144.69%. | [99] |
| Amorphous vemurafenib (VEM) | Liquid-assisted grinding and Solvent evaporation | D-camphorsulfonic acid (D-CSA) L-camphorsulfonic acid (L-CSA) DL-camphorsulfonic acid (DL-CSA) | Improved hygroscopicity was observed as amorphous VEM absorbed 2.45% water at 95%RH, and cocrystals VEM-D-CSA, VEM-L-CSA, and VEM-DL-CSA absorbed 1.05%, 1.10% and 1.17%, respectively. | [100] |
| Berberine Chloride (BCl) | Liquid-assisted grinding | L-Lactic acid (LA) | In dynamic vapor sorption, BCl rapidly absorbed water at 8.8% to convert to dihydrate from 0% to 10%RH, remained stable up to 70%RH, and absorbed significant amount of water from 70% to 90%RH to convert to tetrahydrate. BCl-LA gained insignificant moisture at 0.3% from 0 to 10%RH, remained stable up to 70%RH, and absorbed 2 water molecules to become BCl-LA.H2O from 70% to 95%RH. The cocrystal exhibited lower hygroscopicity than BCl. | [101] |
| Berberine Chloride (BCl) | Liquid-assisted grinding | Citric acid (CA) | In dynamic vapor sorption, BCl rapidly absorbed water to convert to dihydrate from 0% to 10%RH, gained weight gradually via surface water adsorption to 70%RH, and absorbed significant amount of water to become tetrahydrate from 70% to 90%RH. BCl-CA gained insignificant moisture at < 2% from 0 to 70%RH, gained moisture gradually at 8% from 70% to 95%RH. The lack of significant hysteresis and step weight gain from 70% to 95%RH showed that BCl-CA did not transform to a hydrate at 95%RH, and that it exhibited greater physical stability over BCl. | [102] |
| Levofloxacin (LVFX) | Neat grinding | Metacetamol (AMAP) | In dynamic vapor sorption, LVFX absorbed water at 2.2% to become a hydrate when RH increased from 0% to 10%. LVFX-AMAP absorbed water at 0.3% at 95%RH, indicating its non-hygroscopic properties. | [103] |
| L-Lactic acid (LA) | Melt crystallization | D-tryptophan (D-T) 3-nitrobenzamide (3-N) | In dynamic vapor sorption, LA deliquesced and had a net increase of 1.3157 g/g sample between 0% to 90%RH, compared to the little mass increase in cocrystals at 0.0017 g/g sample of LA-D-T and 0.0299 g/g sample for LA-3-N. Visual observation at RH96% confirmed the deliquescence of LA, and how both cocrystals remained the same. | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, L.H.; Ling, J.K.U.; Hadinoto, K. Formulation Strategies to Improve the Stability and Handling of Oral Solid Dosage Forms of Highly Hygroscopic Pharmaceuticals and Nutraceuticals. Pharmaceutics 2022, 14, 2015. https://doi.org/10.3390/pharmaceutics14102015
Ng LH, Ling JKU, Hadinoto K. Formulation Strategies to Improve the Stability and Handling of Oral Solid Dosage Forms of Highly Hygroscopic Pharmaceuticals and Nutraceuticals. Pharmaceutics. 2022; 14(10):2015. https://doi.org/10.3390/pharmaceutics14102015
Chicago/Turabian StyleNg, Liu Han, Jordy Kim Ung Ling, and Kunn Hadinoto. 2022. "Formulation Strategies to Improve the Stability and Handling of Oral Solid Dosage Forms of Highly Hygroscopic Pharmaceuticals and Nutraceuticals" Pharmaceutics 14, no. 10: 2015. https://doi.org/10.3390/pharmaceutics14102015
APA StyleNg, L. H., Ling, J. K. U., & Hadinoto, K. (2022). Formulation Strategies to Improve the Stability and Handling of Oral Solid Dosage Forms of Highly Hygroscopic Pharmaceuticals and Nutraceuticals. Pharmaceutics, 14(10), 2015. https://doi.org/10.3390/pharmaceutics14102015