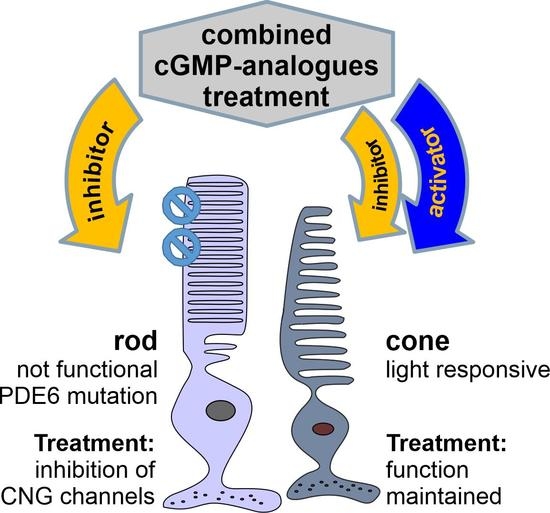
cGMP Analogues with Opposing Actions on CNG Channels Selectively Modulate Rod or Cone Photoreceptor Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Molecular Biology and Heterologous Expression of Photoreceptor CNG Channels in Xenopus laevis Oocytes
2.3. Electrophysiology
2.4. cGMP Analogues
2.5. Steady-State Concentration–Activation Relationships
2.6. Tissue Preparation
2.7. Retinal Recordings
2.8. Data Analysis & Statistics
3. Results
3.1. Rp-8-Br-PET-cGMPS Reduces Rod and Cone CNG-Channel Activity
3.2. 8-pCPT-cGMP Shows a Concentration-Dependent Selectivity for Cone over Rod CNG Channels
3.3. Rp-Modified cGMP Analogues Are Not Selective for Rod or Cone CNG Channels
3.4. Combination of 8-pCPT-cGMP and Rp-8-Br-PET-cGMPS Preserves Rod and Cone CNG-Channel Function under RP-Like Conditions
3.5. Measuring the Effects of cGMP Analogues on Rod and Cone Light Responses
3.6. Rp-8-Br-PET-cGMPS Selectively Silences Rod Photoreceptors
3.7. 8-pCPT-cGMP Counteracts the Inhibitor Effects in Cones but Not in Rods
3.8. cGMP Analogues Modulate the Kinetics of Photoreceptor Responses
4. Discussion
4.1. Targeting Rod Photoreceptors
4.2. The Combined cGMP-Analogues Treatment Modulates Kinetics of Photoreceptor Responses
4.3. Future Therapy Developments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohyama, T.; Hackos, D.H.; Frings, S.; Hagen, V.; Kaupp, U.B.; Korenbrot, J.I. Fraction of the dark current carried by Ca2+ through cGMP-gated ion channels of intact rod and cone photoreceptors. J. Gen. Physiol. 2000, 116, 735–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korenbrot, J.I. Speed, sensitivity, and stability of the light response in rod and cone photoreceptors: Facts and models. Prog. Retin. Eye Res. 2012, 31, 442–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugh, E.N., Jr.; Lamb, T.D. Amplification and kinetics of the activation steps in phototransduction. Biochim. Biophys. Acta 1993, 1141, 111–149. [Google Scholar] [CrossRef]
- Molday, R.S.; Moritz, O.L. Photoreceptors at a glance. J. Cell Sci. 2015, 128, 4039–4045. [Google Scholar] [CrossRef] [Green Version]
- Kaupp, U.B.; Seifert, R. Cyclic nucleotide-gated ion channels. Physiol. Rev. 2002, 82, 769–824. [Google Scholar] [CrossRef] [Green Version]
- Weitz, D.; Ficek, N.; Kremmer, E.; Bauer, P.J.; Kaupp, U.B. Subunit stoichiometry of the CNG channel of rod photoreceptors. Neuron 2002, 36, 881–889. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Rich, E.D.; Varnum, M.D. Subunit configuration of heteromeric cone cyclic nucleotide-gated channels. Neuron 2004, 42, 401–410. [Google Scholar] [CrossRef] [Green Version]
- Zhong, H.; Molday, L.L.; Molday, R.S.; Yau, K.W. The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature 2002, 420, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Trudeau, M.C.; Zagotta, W.N. Dynamics of Ca2+-calmodulin-dependent inhibition of rod cyclic nucleotide-gated channels measured by patch-clamp fluorometry. J. Gen. Physiol. 2004, 124, 211–223. [Google Scholar] [CrossRef] [Green Version]
- Trudeau, M.C.; Zagotta, W.N. An intersubunit interaction regulates trafficking of rod cyclic nucleotide-gated channels and is disrupted in an inherited form of blindness. Neuron 2002, 34, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Nache, V.; Zimmer, T.; Wongsamitkul, N.; Schmauder, R.; Kusch, J.; Reinhardt, L.; Bonigk, W.; Seifert, R.; Biskup, C.; Schwede, F.; et al. Differential regulation by cyclic nucleotides of the CNGA4 and CNGB1b subunits in olfactory cyclic nucleotide-gated channels. Sci. Signal 2012, 5, ra48. [Google Scholar] [CrossRef] [PubMed]
- Nache, V.; Wongsamitkul, N.; Kusch, J.; Zimmer, T.; Schwede, F.; Benndorf, K. Deciphering the function of the CNGB1b subunit in olfactory CNG channels. Sci. Rep. 2016, 6, 29378. [Google Scholar] [CrossRef]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Kennan, A.; Aherne, A.; Humphries, P. Light in retinitis pigmentosa. Trends Genet. 2005, 21, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.A.; Bartoli, K.M.; Fandino, R.A.; Ngatchou, A.N.; Woch, G.; Carey, J.; Tanaka, J.C. Transmembrane S1 mutations in CNGA3 from achromatopsia 2 patients cause loss of function and impaired cellular trafficking of the cone CNG channel. Investig. Ophthalmol. Vis. Sci. 2005, 46, 2282–2290. [Google Scholar] [CrossRef] [Green Version]
- Molday, R.S.; Garces, F.A.; Scortecci, J.F.; Molday, L.L. Structure and function of ABCA4 and its role in the visual cycle and Stargardt macular degeneration. Prog. Retin. Eye Res. 2022, 89, 101036. [Google Scholar] [CrossRef] [PubMed]
- Power, M.; Das, S.; Schutze, K.; Marigo, V.; Ekstrom, P.; Paquet-Durand, F. Cellular mechanisms of hereditary photoreceptor degeneration—Focus on cGMP. Prog. Retin. Eye Res. 2020, 74, 100772. [Google Scholar] [CrossRef]
- Jones, B.W.; Pfeiffer, R.L.; Ferrell, W.D.; Watt, C.B.; Marmor, M.; Marc, R.E. Retinal remodeling in human retinitis pigmentosa. Exp. Eye Res. 2016, 150, 149–165. [Google Scholar] [CrossRef] [Green Version]
- Donato, L.; Scimone, C.; Alibrandi, S.; Abdalla, E.M.; Nabil, K.M.; D’Angelo, R.; Sidoti, A. New Omics-Derived Perspectives on Retinal Dystrophies: Could Ion Channels-Encoding or Related Genes Act as Modifier of Pathological Phenotype? Int. J. Mol. Sci. 2020, 22, 70. [Google Scholar] [CrossRef]
- Daiger, S.P.; Sullivan, L.S.; Bowne, S.J. Genes and mutations causing retinitis pigmentosa. Clin. Genet. 2013, 84, 132–141. [Google Scholar] [CrossRef]
- Farber, D.B.; Lolley, R.N. Cyclic guanosine monophosphate: Elevation in degenerating photoreceptor cells of the C3H mouse retina. Science 1974, 186, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Lolley, R.N.; Farber, D.B.; Rayborn, M.E.; Hollyfield, J.G. Cyclic GMP accumulation causes degeneration of photoreceptor cells: Simulation of an inherited disease. Science 1977, 196, 664–666. [Google Scholar] [CrossRef]
- Paquet-Durand, F.; Marigo, V.; Ekstrom, P. RD Genes Associated with High Photoreceptor cGMP-Levels (Mini-Review). Adv. Exp. Med. Biol. 2019, 1185, 245–249. [Google Scholar] [PubMed]
- Wang, T.; Tsang, S.H.; Chen, J. Two pathways of rod photoreceptor cell death induced by elevated cGMP. Hum. Mol. Genet. 2017, 26, 2299–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, D.A.; Poblenz, A.T.; He, L.H. Calcium overload triggers rod photoreceptor apoptotic cell death in chemical-induced and inherited retinal degenerations. Ann. N. Y. Acad. Sci. 1999, 893, 282–285. [Google Scholar] [CrossRef]
- Paquet-Durand, F.; Beck, S.; Michalakis, S.; Goldmann, T.; Huber, G.; Muhlfriedel, R.; Trifunovic, D.; Fischer, M.D.; Fahl, E.; Duetsch, G.; et al. A key role for cyclic nucleotide gated (CNG) channels in cGMP-related retinitis pigmentosa. Hum. Mol. Genet. 2011, 20, 941–947. [Google Scholar] [CrossRef] [Green Version]
- Canzoniero, L.M.; Adornetto, A.; Secondo, A.; Magi, S.; Dell’Aversano, C.; Scorziello, A.; Amoroso, S.; Di Renzo, G. vInvolvement of the nitric oxide/protein kinase G pathway in polychlorinated biphenyl-induced cell death in SH-SY 5Y neuroblastoma cells. J. Neurosci. Res. 2006, 84, 692–697. [Google Scholar] [CrossRef]
- Arango-Gonzalez, B.; Trifunovic, D.; Sahaboglu, A.; Kranz, K.; Michalakis, S.; Farinelli, P.; Koch, S.; Koch, F.; Cottet, S.; Janssen-Bienhold, U.; et al. Identification of a Common Non-Apoptotic Cell Death Mechanism in Hereditary Retinal Degeneration. PLoS ONE 2014, 9, e112142. [Google Scholar] [CrossRef]
- Paquet-Durand, F.; Hauck, S.M.; van Veen, T.; Ueffing, M.; Ekstrom, P. PKG activity causes photoreceptor cell death in two retinitis pigmentosa models. J. Neurochem. 2009, 108, 796–810. [Google Scholar] [CrossRef]
- Vighi, E.; Trifunovic, D.; Veiga-Crespo, P.; Rentsch, A.; Hoffmann, D.; Sahaboglu, A.; Strasser, T.; Kulkarni, M.; Bertolotti, E.; van den Heuvel, A.; et al. Combination of cGMP analogue and drug delivery system provides functional protection in hereditary retinal degeneration. Proc. Natl. Acad. Sci. USA 2018, 115, E2997–E3006. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.F.; Joo, K.; Kemp, J.A.; Fialho, S.L.; de Silva Cunha, A., Jr.; Woo, S.J.; Kwon, Y.J. Molecular genetics and emerging therapies for retinitis pigmentosa: Basic research and clinical perspectives. Prog. Retin. Eye Res. 2018, 63, 107–131. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Popp, V.; Power, M.; Groenveld, K.; Yan, J.; Melle, C.; Rogerson, L.; Achury, M.; Schwede, F.; Strawsser, T.; et al. Redefining the role of Ca(2+)-permeable channels in photoreceptor degeneration using diltiazem. Cell Death Dis. 2022, 13, 47. [Google Scholar] [CrossRef]
- Butt, E.; Eigenthaler, M.; Genieser, H.G. (Rp)-8-pCPT-cGMPS, a novel cGMP-dependent protein kinase inhibitor. Eur. J. Pharmacol. 1994, 269, 265–268. [Google Scholar] [CrossRef]
- Wei, J.Y.; Cohen, E.D.; Yan, Y.Y.; Genieser, H.G.; Barnstable, C.J. Identification of competitive antagonists of the rod photoreceptor cGMP-gated cation channel: Beta-phenyl-1,N2-etheno-substituted cGMP analogues as probes of the cGMP-binding site. Biochemistry 1996, 35, 16815–16823. [Google Scholar] [CrossRef] [PubMed]
- Liman, E.R.; Tytgat, J.; Hess, P. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 1992, 9, 861–871. [Google Scholar] [CrossRef]
- Zheng, J.; Trudeau, M.C.; Zagotta, W.N. Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron 2002, 36, 891–896. [Google Scholar] [CrossRef] [Green Version]
- Shammat, I.M.; Gordon, S.E. Stoichiometry and arrangement of subunits in rod cyclic nucleotide-gated channels. Neuron 1999, 23, 809–819. [Google Scholar] [CrossRef] [Green Version]
- Jonas, P. Single-Channel Recording; Sakmann, B., Neher, E., Eds.; Plenum Press: New York, NY, USA, 1995; pp. 231–243. [Google Scholar]
- Haq, W.; Dietter, J.; Zrenner, E. Electrical activation of degenerated photoreceptors in blind mouse retina elicited network-mediated responses in different types of ganglion cells. Sci. Rep. 2018, 8, 16998. [Google Scholar] [CrossRef]
- Benchorin, G.; Calton, M.A.; Beaulieu, M.O.; Vollrath, D. Assessment of Murine Retinal Function by Electroretinography. Bio. Protoc. 2017, 7, e2218. [Google Scholar] [CrossRef] [Green Version]
- Butt, E.; Pohler, D.; Genieser, H.G.; Huggins, J.P.; Bucher, B. Inhibition of cyclic GMP-dependent protein kinase-mediated effects by (Rp)-8-bromo-PET-cyclic GMPS. Br. J. Pharmacol. 1995, 116, 3110–3116. [Google Scholar] [CrossRef] [Green Version]
- Korschen, H.G.; Illing, M.; Seifer, R.; Sesti, F.; Williams, A.; Gotzes, S.; Colville, C.; Muller, F.; Dose, A.; Godde, M.; et al. A 240 kDa protein represents the complete beta subunit of the cyclic nucleotide-gated channel from rod photoreceptor. Neuron 1995, 15, 627–636. [Google Scholar] [CrossRef] [Green Version]
- Shuart, N.G.; Haitin, Y.; Camp, S.S.; Black, K.D.; Zagotta, W.N. Molecular mechanism for 3:1 subunit stoichiometry of rod cyclic nucleotide-gated ion channels. Nat. Commun. 2011, 2, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, R.H.; Tibbs, G.R. Antagonists of cyclic nucleotide-gated channels and molecular mapping of their site of action. J. Neurosci. 1996, 16, 1285–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, M.; Trifunovic, D.; Schubert, T.; Euler, T.; Paquet-Durand, F. Calcium dynamics change in degenerating cone photoreceptors. Hum. Mol. Genet. 2016, 25, 3729–3740. [Google Scholar] [CrossRef]
- Stett, A.; Egert, U.; Guenther, E.; Hofmann, F.; Meyer, T.; Nisch, W.; Haemmerle, H. Biological application of microelectrode arrays in drug discovery and basic research. Anal. Bioanal. Chem. 2003, 377, 486–495. [Google Scholar] [CrossRef]
- Carter-Dawson, L.D.; LaVail, M.M. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J. Comp. Neurol. 1979, 188, 245–262. [Google Scholar] [CrossRef]
- Butt, E.; van Bemmelen, M.; Fischer, L.; Walter, U.; Jastorff, B. Inhibition of cGMP-dependent protein kinase by (Rp)-guanosine 3′,5′-monophosphorothioates. FEBS Lett. 1990, 263, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Breton, M.E.; Schueller, A.W.; Lamb, T.D.; Pugh, E.N., Jr. Analysis of ERG a-wave amplification and kinetics in terms of the G-protein cascade of phototransduction. Investig. Ophthalmol. Vis. Sci. 1994, 35, 295–309. [Google Scholar]
- Biskup, C.; Kusch, J.; Schulz, E.; Nache, V.; Schwede, F.; Lehmann, F.; Hagen, V.; Benndorf, L. Relating ligand binding to activation gating in CNGA2 channels. Nature 2007, 446, 440–443. [Google Scholar] [CrossRef]
- Kuehlewein, L.; Zobor, D.; Stingl, K.; Kempf, M.; Nasser, F.; Bernd, A.; Biskup, S.; Cremers, F.P.M.; Khan, M.I.; Mazzola, P.; et al. Clinical Phenotype of PDE6B-Associated Retinitis Pigmentosa. Int. J. Mol. Sci. 2021, 22, 2374. [Google Scholar] [CrossRef]
- Li, M.; Zhou, X.; Wang, S.; Michailidis, I.; Gong, Y.; Su, D.; Li, H.; Li, X.; Yang, J. Structure of a eukaryotic cyclic-nucleotide-gated channel. Nature 2017, 542, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Fu, Z.; Su, D.; Zhang, Y.; Li, M.; Pan, Y.; Li, H.; Li, S.; Grassucci, R.A.; Ren, A.; et al. Mechanism of ligand activation of a eukaryotic cyclic nucleotide-gated channel. Nat. Struct. Mol. Biol. 2020, 27, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Han, Y.; Zeng, W.; Wang, Y.; Jiang, Y. Structural mechanisms of gating and selectivity of human rod CNGA1 channel. Neuron 2021, 109, P1302–P1313. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Han, Y.; Zeng, W.; Jiang, Y. Structural mechanisms of assembly, permeation, gating, and pharmacology of native human rod CNG channel. Neuron 2021, 110, 86–95.e5. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Hu, Z.; Li, H.; Yang, J. Structure of the human cone photoreceptor cyclic nucleotide-gated channel. Nat. Struct. Mol. Biol. 2022, 29, 40–46. [Google Scholar] [CrossRef]
- Himawan, E.; Ekstrom, P.; Buzgo, M.; Gaillard, P.; Stefansson, E.; Marigo, V.; Loftsson, T.; Paquet-Durand, F. Drug delivery to retinal photoreceptors. Drug Discov. Today 2019, 24, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Chawla, H.; Vohra, V. StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Hamel, C.P. Cone rod dystrophies. Orphanet J. Rare Dis. 2007, 2, 7. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wucherpfennig, S.; Haq, W.; Popp, V.; Kesh, S.; Das, S.; Melle, C.; Rentsch, A.; Schwede, F.; Paquet-Durand, F.; Nache, V. cGMP Analogues with Opposing Actions on CNG Channels Selectively Modulate Rod or Cone Photoreceptor Function. Pharmaceutics 2022, 14, 2102. https://doi.org/10.3390/pharmaceutics14102102
Wucherpfennig S, Haq W, Popp V, Kesh S, Das S, Melle C, Rentsch A, Schwede F, Paquet-Durand F, Nache V. cGMP Analogues with Opposing Actions on CNG Channels Selectively Modulate Rod or Cone Photoreceptor Function. Pharmaceutics. 2022; 14(10):2102. https://doi.org/10.3390/pharmaceutics14102102
Chicago/Turabian StyleWucherpfennig, Sophie, Wadood Haq, Valerie Popp, Sandeep Kesh, Soumyaparna Das, Christian Melle, Andreas Rentsch, Frank Schwede, François Paquet-Durand, and Vasilica Nache. 2022. "cGMP Analogues with Opposing Actions on CNG Channels Selectively Modulate Rod or Cone Photoreceptor Function" Pharmaceutics 14, no. 10: 2102. https://doi.org/10.3390/pharmaceutics14102102
APA StyleWucherpfennig, S., Haq, W., Popp, V., Kesh, S., Das, S., Melle, C., Rentsch, A., Schwede, F., Paquet-Durand, F., & Nache, V. (2022). cGMP Analogues with Opposing Actions on CNG Channels Selectively Modulate Rod or Cone Photoreceptor Function. Pharmaceutics, 14(10), 2102. https://doi.org/10.3390/pharmaceutics14102102