Novel pH-Responsive Cubosome and Hexosome Lipid Nanocarriers of SN-38 Are Prospective for Cancer Therapy
Abstract
:Highlights
- 1)
- What are the main findings?
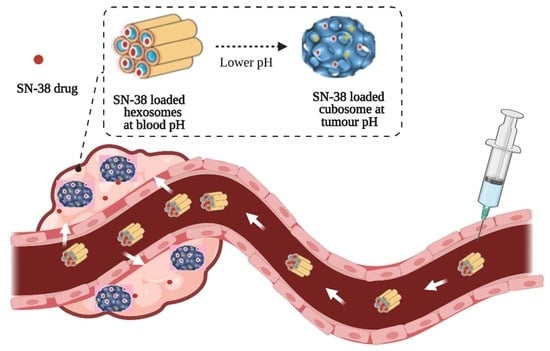
- Lipid nanoparticles containing novel synthetic aminolipids were formulated. Their internal nanostructures are sensitive to pH, being an inverse hexagonal phase at pH 7.4 and a bicontinuous cubic phase at pH 4.0–pH 7.0.
- Poorly soluble and highly potent anticancer drug SN-38 was encapsulated successfully in the nanoparticles with a ~100-fold increase in solubility.
- SN-38 was released faster at a tumour-relevant acidic pH compared to neutral pH.
- 2)
- What is the implication of the main finding?
- Aminolipids can be incorporated into self assembled lipid nanoparticles to achieve desirable pH responsiveness.
- The SN-38-loaded pH-sensitive lipid nanoparticles represent a promising candidate for delivering the potent drug SN-38 to tumour sites.
- The pH-sensitive lipid nanoparticles in this study can be a viable alternative to the currently used irinotecan.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Ionisable Aminolipids
2.3. Lipid Nanoparticle Fabrication
2.4. Particle Size Distribution of LNP
2.5. Synchrotron SAXS Characterisation of LNP
2.6. Drug Loading and Encapsulation Efficiency (EE%)
2.7. Cryogenic Transmission Electron Microscopy (Cryo-TEM)
2.8. Drug Release Study
3. Results and Discussion
3.1. Synthesis of Ionisable Aminolipids
3.2. Lipid Nanoparticle Fabrication
3.3. Synchrotron SAXS Analysis
3.3.1. Effect of Lipid Composition on Mesophase Structure of Aminolipid Doped MO-LNPs
3.3.2. Effect of pH on Mesophase Structure of Aminolipid-Doped MO LNPs
3.3.3. Effect of Temperature on Mesophase Structure of Aminolipid-Doped MO-LNPs
3.4. Drug Loading
3.4.1. Physiochemical Properties and Partial Phase Diagram for SN-38-Loaded LNPs
3.4.2. Determining Encapsulation Efficiency
3.4.3. SN-38 Release Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pfizer Injectables. U.S. Physician Prescribing Information for CAMPTOSAR (Irinotecan HCl Injection). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020571s048lbl.pdf (accessed on 1 September 2022).
- Innocenti, F.; Kroetz, D.L.; Schuetz, E.; Dolan, M.E.; Ramírez, J.; Relling, M.; Chen, P.; Das, S.; Rosner, G.L.; Ratain, M.J. Comprehensive pharmacogenetic analysis of irinotecan neutropenia and pharmacokinetics. J. Clin. Oncol. 2009, 27, 2604–2614. [Google Scholar] [CrossRef] [Green Version]
- Pommier, Y. DNA topoisomerase I Inhibitors: Chemistry, biology, and interfacial inhibition. Chem. Rev. 2009, 109, 2894–2902. [Google Scholar] [CrossRef] [Green Version]
- Mosallaei, N.; Mahmoudi, A.; Ghandehari, H.; Yellepeddi, V.K.; Jaafari, M.R.; Malaekeh-Nikouei, B. Solid lipid nanoparticles containing 7-ethyl-10-hydroxycamptothecin (SN38): Preparation, characterization, in vitro, and in vivo evaluations. Eur. J. Pharm. Biopharm. 2016, 104, 42–50. [Google Scholar] [CrossRef]
- Li, K.; Wang, S. Preparation, Pharmacokinetic Profile, and Tissue Distribution Studies of a Liposome-Based Formulation of SN-38 Using an UPLC–MS/MS Method. AAPS PharmSciTech 2016, 17, 1450–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, M.-H.; Peng, C.-L.; Yao, C.-J.; Shieh, M.-J. Enhanced efficacy of chemotherapeutic drugs against colorectal cancer using ligand-decorated self-breakable agents. RSC Adv. 2015, 5, 92361–92370. [Google Scholar] [CrossRef]
- Yang, X.; Xue, X.; Luo, Y.; Lin, T.-y.; Zhang, H.; Lac, D.; Xiao, K.; He, Y.; Jia, B.; Lam, K.S.; et al. Sub-100nm, long tumor retention SN-38-loaded photonic micelles for tri-modal cancer therapy. J. Control. Release 2017, 261, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Monterrubio, C.; Pascual-Pasto, G.; Cano, F.; Vila-Ubach, M.; Manzanares, A.; Schaiquevich, P.; Tornero, J.A.; Sosnik, A.; Mora, J.; Carcaboso, A.M. SN-38-loaded nanofiber matrices for local control of pediatric solid tumors after subtotal resection surgery. Biomaterials 2016, 79, 69–78. [Google Scholar] [CrossRef]
- Ranneh, A.H.; Iwao, Y.; Noguchi, S.; Oka, T.; Itai, S. The use of surfactants to enhance the solubility and stability of the water-insoluble anticancer drug SN38 into liquid crystalline phase nanoparticles. Int. J. Pharm. 2016, 515, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Medik, Y.; Li, L.; Tian, X.; Fu, D.; Brouwer, K.L.R.; Wagner, K.; Sun, B.; Sendi, H.; Mi, Y.; et al. Nanoparticle Drug Delivery Can Reduce the Hepatotoxicity of Therapeutic Cargo. Small 2020, 16, 1906360. [Google Scholar] [CrossRef]
- Si, J.; Zhao, X.; Gao, S.; Huang, D.; Sui, M. Advances in delivery of Irinotecan (CPT-11) active metabolite 7-ethyl-10-hydroxycamptothecin. Int. J. Pharm. 2019, 568, 118499. [Google Scholar] [CrossRef]
- Fang, T.; Ye, Z.; Chen, X.; Wang, Y.; Wan, J.; Wang, H. Repurposing of camptothecin: An esterase-activatable prodrug delivered by a self-emulsifying formulation that improves efficacy in colorectal cancer. Int. J. Pharm. 2021, 599, 120399. [Google Scholar] [CrossRef] [PubMed]
- Alferiev, I.S.; Iyer, R.; Croucher, J.L.; Adamo, R.F.; Zhang, K.; Mangino, J.L.; Kolla, V.; Fishbein, I.; Brodeur, G.M.; Levy, R.J.; et al. Nanoparticle-mediated delivery of a rapidly activatable prodrug of SN-38 for neuroblastoma therapy. Biomaterials 2015, 51, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, F.; Alferiev, I.; Guan, P.; Guerrero, D.T.; Kolla, V.; Moorthy, G.S.; Chorny, M.; Brodeur, G.M. Enhanced Intratumoral Delivery of SN38 as a Tocopherol Oxyacetate Prodrug Using Nanoparticles in a Neuroblastoma Xenograft Model. Clin. Cancer Res. 2018, 24, 2585–2593. [Google Scholar] [CrossRef] [Green Version]
- Bala, V.; Rao, S.; Bateman, E.; Keefe, D.; Wang, S.; Prestidge, C.A. Enabling Oral SN38-Based Chemotherapy with a Combined Lipophilic Prodrug and Self-Microemulsifying Drug Delivery System. Mol. Pharm. 2016, 13, 3518–3525. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Mao, W.; Xiang, J.; Zhou, Z.; Liu, X.; Tang, J.; Shen, Y. Assemblies of Peptide-Cytotoxin Conjugates for Tumor-Homing Chemotherapy. Adv. Funct. Mater. 2019, 29, 1807446. [Google Scholar] [CrossRef]
- Fong, C.; Le, T.; Drummond, C.J. Lyotropic liquid crystal engineering–ordered nanostructured small molecule amphiphile self-assembly materials by design. Chem. Soc. Rev. 2012, 41, 1297–1322. [Google Scholar] [CrossRef]
- Seddon, J.; Templer, R. Polymorphism of lipid-water systems. Handb. Biol. Phys. 1995, 1, 97–160. [Google Scholar]
- Zhai, J.; Luwor, R.B.; Ahmed, N.; Escalona, R.; Tan, F.H.; Fong, C.; Ratcliffe, J.; Scoble, J.A.; Drummond, C.J.; Tran, N. Paclitaxel-Loaded Self-Assembled Lipid Nanoparticles as Targeted Drug Delivery Systems for the Treatment of Aggressive Ovarian Cancer. ACS Appl. Mater. Interfaces 2018, 10, 25174–25185. [Google Scholar] [CrossRef]
- Tran, N.; Hocquet, M.; Eon, B.; Sangwan, P.; Ratcliffe, J.; Hinton, T.M.; White, J.; Ozcelik, B.; Reynolds, N.P.; Muir, B.W. Non-Lamellar Lyotropic Liquid Crystalline Nanoparticles Enhance the Antibacterial Effects of Rifampicin against Staphylococcus Aureus. J. Colloid. Interface Sci. 2018, 519, 107–118. [Google Scholar] [CrossRef] [Green Version]
- Zhai, J.; Fong, C.; Tran, N.; Drummond, C.J. Non-Lamellar Lyotropic Liquid Crystalline Lipid Nanoparticles for the Next Generation of Nanomedicine. ACS Nano 2019, 13, 6178–6206. [Google Scholar] [CrossRef]
- Mulet, X.; Boyd, B.J.; Drummond, C.J. Advances in drug delivery and medical imaging using colloidal lyotropic liquid crystalline dispersions. J. Colloid Interface Sci. 2013, 393, 1–20. [Google Scholar] [CrossRef]
- Drummond, C.J.; Fong, C. Surfactant self-assembly objects as novel drug delivery vehicles. Curr. Opin. Colloid Interface Sci. 1999, 4, 449–456. [Google Scholar] [CrossRef]
- Tran, N.; Mulet, X.; Hawley, A.M.; Hinton, T.M.; Mudie, S.T.; Muir, B.W.; Giakoumatos, E.C.; Waddington, L.J.; Kirbyb, N.M.; Drummond, C.J. Nanostructure and cytotoxicity of self-assembled monoolein–capric acid lyotropic liquid crystalline nanoparticles. R. Soc. Chem. Adv. 2015, 5, 26785–26795. [Google Scholar] [CrossRef]
- Assenza, S.; Mezzenga, R. Curvature and Bottlenecks Control Molecular Transport in Inverse Bicontinuous Cubic Phases. J. Chem. Phys. 2018, 148, 54902–54909. [Google Scholar] [CrossRef]
- Nazaruk, E.; Miszta, P.; Filipek, S.; Górecka, E.; Landau, E.M.; Bilewicz, R. Lyotropic cubic phases for drug delivery: Diffusion and sustained release from the mesophase evaluated by electrochemical methods. Langmuir 2015, 31, 12753–12761. [Google Scholar] [CrossRef]
- Nazaruka, E.; Pilip, A.M.; Godlewska, M.; Salamończyka, M.; Gawel, D. Electrochemical and biological characterization of lyotropic liquid crystalline phases—Retardation of drug release from hexagonal mesophases. J. Electroanal. Chem. 2018, 813, 208–215. [Google Scholar] [CrossRef]
- Negrini, R.; Fong, W.-K.; Boyd, B.J.; Mezzenga, R. pH-responsive lyotropic liquid crystals and their potential therapeutic role in cancer treatment. Chem. Commun. 2015, 51, 6671–6674. [Google Scholar] [CrossRef]
- Rajesh, S.; Zhai, J.; Drummond, C.J.; Tran, N. Synthetic ionizable aminolipids induce a pH dependent inverse hexagonal to bicontinuous cubic lyotropic liquid crystalline phase transition in monoolein nanoparticles. J. Colloid Interface Sci. 2020, 589, 85–95. [Google Scholar] [CrossRef]
- Zhai, J.; Hinton, T.M.; Waddington, L.J.; Fong, C.; Tran, N.; Mulet, X.; Drummond, C.J.; Muir, B.W. Lipid-PEG conjugates sterically stabilize and reduce the toxicity of phytantriol-based lyotropic liquid crystalline nanoparticles. Langmuir 2015, 31, 10871–10880. [Google Scholar] [CrossRef]
- Tran, N.; Hawley, A.M.; Zhai, J.; Muir, B.W.; Fong, C.; Drummond, C.J.; Mulet, X. High-Throughput Screening of Saturated Fatty Acid Influence on Nanostructure of Lyotropic Liquid Crystalline Lipid Nanoparticles. Langmuir 2016, 32, 4509–4520. [Google Scholar] [CrossRef]
- Rajesh, S.; Leiske, M.N.; Leitch, V.; Zhai, J.; Drummond, C.J.; Kempe, K.; Tran, N. Lipidic poly(2-oxazoline)s as PEG replacement steric stabilisers for cubosomes. J. Colloid Interface Sci. 2022, 623, 1142–1150. [Google Scholar] [CrossRef]
- Zambito, Y.; Pedreschi, E.; Di Colo, G. Is dialysis a reliable method for studying drug release from nanoparticulate systems? A case study. Int. J. Pharm. 2012, 434, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yuan, W.; Li, D.; Schwendeman, A.; Schwendeman, S.P. Predicting drug release kinetics from nanocarriers inside dialysis bags. J. Control. Release 2019, 315, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Wan, X.; Beaudoin, J.J.; Vinod, N.; Min, Y.; Makita, N.; Bludau, H.; Jordan, R.; Wang, A.; Sokolsky, M.; Kabanov, A.V. Co-delivery of paclitaxel and cisplatin in poly(2-oxazoline) polymeric micelles: Implications for drug loading, release, pharmacokinetics and outcome of ovarian and breast cancer treatments. Biomaterials 2019, 192, 1–14. [Google Scholar] [CrossRef]
- Sethi, M.; Sukumar, R.; Karve, S.; Werner, M.E.; Wang, E.C.; Moore, D.T.; Kowalczyk, S.R.; Zhang, L.; Wang, A.Z. Effect of drug release kinetics on nanoparticle therapeutic efficacy and toxicity. Nanoscale 2014, 6, 2321–2327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Israelachvili, J.N.; Findenegg, G.H. Intermolecular and Surface Forces (With Applications to Colloidal and Biological Systems). Ber. Der Bunsenges. Für Phys. Chem. 1986, 90, 1241–1242. [Google Scholar] [CrossRef]
- Hyde, S.; Blum, Z.; Landh, T.; Lidin, S.; Ninham, B.W.; Andersson, S.; Larsson, K. The language of shape: The role of curvature in condensed matter physics, chemistry and biology. Nature 1997, 387, 249. [Google Scholar]
- Barauskas, J.; Johnsson, M.; Tiberg, F. Self-assembled lipid superstructures: Beyond vesicles and liposomes. Nano Lett. 2005, 5, 1615–1619. [Google Scholar] [CrossRef]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The Next Generation of Smart Lipid Nanoparticles? Angew. Chem. Int. Ed. 2019, 58, 2958–2978. [Google Scholar] [CrossRef] [Green Version]
- Barriga, H.M.G.; Tyler, A.I.I.; McCarthy, N.L.C.; Parsons, E.S.; Ces, O.; Law, R.V.; Seddon, J.M.; Brooks, N.J. Temperature and pressure tuneable swollen bicontinuous cubic phases approaching nature’s length scales. Soft Matter. 2015, 11, 600–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, S.; Tran, N.; Rashid, M.H.; Le, T.C.; Yarovsky, I.; Conn, C.E.; Drummond, C.J. Toward Cell Membrane Biomimetic Lipidic Cubic Phases: A High-Throughput Exploration of Lipid Compositional Space. ACS Appl. Bio Mater. 2018, 2, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Laggner, P.; Almgren, M.; Rappolt, M. Self-assembly in monoelaidin aqueous dispersions: Direct vesicles to cubosomes transition. PLoS ONE 2008, 3, e3747. [Google Scholar] [CrossRef]
- Lyubartsev, A.P.; Jacobsson, S.P.; Sundholm, G.; Laaksonen, A. Solubility of Organic Compounds in Water/Octanol Systems. A Expanded Ensemble Molecular Dynamics Simulation Study of log P Parameters. J. Phys. Chem. B 2001, 105, 7775–7782. [Google Scholar] [CrossRef]
- Gontsarik, M.; Buhmann, M.T.; Yaghmur, A.; Ren, Q.; Maniura-Weber, K.; Salentinig, S. Antimicrobial peptide-driven colloidal transformations in liquid-crystalline nanocarriers. J. Phys. Chem. Lett. 2016, 7, 3482–3486. [Google Scholar] [CrossRef]
- Drummond, C.J.; Grieser, T.F.; Healy, T.W. Acid-Base Equilibria in Aqueous Micellar Solutions. J. Chem. Soc. Faraday Trans. 1 1989, 85, 521–535. [Google Scholar] [CrossRef]
- Casadó, A.; Giuffrida, M.C.; Sagristá, M.L.; Castelli, F.; Pujol, M.; Alsina, M.A.; Mora, M. Langmuir monolayers and Differential Scanning Calorimetry for the study of the interactions between camptothecin drugs and biomembrane models. Biochim. Et Biophys. Acta (BBA) Biomembr. 2016, 1858, 422–433. [Google Scholar] [CrossRef]
- Ebrahimnejad, P.; Dinarvand, R.; Sajadi, A.; Jaafari, M.R.; Nomani, A.R.; Azizi, E.; Rad-Malekshahi, M.; Atyabi, F. Preparation and in vitro evaluation of actively targetable nanoparticles for SN-38 delivery against HT-29 cell lines. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Clogston, J.; Craciun, G.; Hart, D.; Caffrey, M. Controlling release from the lipidic cubic phase by selective alkylation. J. Control. Release 2005, 102, 441–461. [Google Scholar] [CrossRef]
- Van‘t Hag, L.; Gras, S.L.; Conn, C.E.; Drummond, C.J. Lyotropic liquid crystal engineering moving beyond binary compositional space–ordered nanostructured amphiphile self-assembly materials by design. Chem. Soc. Rev. 2017, 46, 2705–2731. [Google Scholar] [CrossRef]
- Bala, V.; Rao, S.; Boyd, B.J.; Prestidge, C.A. Prodrug and nanomedicine approaches for the delivery of the camptothecin analogue SN38. J. Control. Release 2013, 172, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Casadó, A.; Sagristá, M.L.; Mora, M. A novel microfluidic liposomal formulation for the delivery of the SN-38 camptothecin: Characterization and in vitro assessment of its cytotoxic effect on two tumor cell lines. Int. J. Nanomed. 2018, 13, 5301–5320. [Google Scholar] [CrossRef] [PubMed]
- Schwarzl, R.; Du, F.; Haag, R.; Netz, R.R. General method for the quantification of drug loading and release kinetics of nanocarriers. Eur. J. Pharm. Biopharm. 2017, 116, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Abouelmagd, S.A.; Sun, B.; Chang, A.C.; Ku, Y.J.; Yeo, Y. Release Kinetics Study of Poorly Water-Soluble Drugs from Nanoparticles: Are We Doing It Right? Mol. Pharm. 2015, 12, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Roger, E.; Lagarce, F.; Benoit, J.-P. Development and characterization of a novel lipid nanocapsule formulation of Sn38 for oral administration. Eur. J. Pharm. Biopharm. 2011, 79, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Boyd, B.J.; Whittaker, D.V.; Khoo, S.-M.; Davey, G. Hexosomes formed from glycerate surfactants—Formulation as a colloidal carrier for irinotecan. Int. J. Pharm. 2006, 318, 154–162. [Google Scholar] [CrossRef]
- Boyd, B.J. Characterisation of drug release from cubosomes using the pressure ultrafiltration method. Int. J. Pharm. 2003, 260, 239–247. [Google Scholar] [CrossRef]
- Boyd, B.J.; Whittaker, D.V.; Khoo, S.-M.; Davey, G. Lyotropic liquid crystalline phases formed from glycerate surfactants as sustained release drug delivery systems. Int. J. Pharm. 2006, 309, 218–226. [Google Scholar] [CrossRef]
- Phan, S.; Fong, W.-K.; Kirby, N.; Hanley, T.; Boyd, B.J. Evaluating the link between self-assembled mesophase structure and drug release. Int. J. Pharm. 2011, 421, 176–182. [Google Scholar] [CrossRef]
- Laughlin, R. Correction. the aqueous phase behavior of surfactants. J. Am. Chem. Soc. 1995, 117, 10603. [Google Scholar] [CrossRef]
- Fong, W.-K.; Hanley, T.L.; Thierry, B.; Hawley, A.; Boyd, B.J.; Landersdorfer, C.B. External manipulation of nanostructure in photoresponsive lipid depot matrix to control and predict drug release in vivo. J. Control. Release 2016, 228, 67–73. [Google Scholar] [CrossRef]
- Costa-Balogh, F.O.; Sparr, E.; Sousa, J.J.S.; Pais, A.C. Drug release from lipid liquid crystalline phases: Relation with phase behavior. Drug Dev. Ind. Pharm. 2010, 36, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.Y.; Nguyen, T.-H.; Hanley, T.; Boyd, B.J. Nanostructure of liquid crystalline matrix determines in vitro sustained release and in vivo oral absorption kinetics for hydrophilic model drugs. Int. J. Pharm. 2009, 365, 190–199. [Google Scholar] [CrossRef]







| Particle Size in nm | ||||
|---|---|---|---|---|
| RAL | OAPy-4 | OAPy-2 | OAMo-1 | OAPi-1 |
| 0.05 | 217 ± 6 | 284 ± 2 | 214 ± 4 | 180 ± 3 |
| 0.1 | 224 ± 3 | 255 ± 4 | 218 ± 3 | 176 ± 4 |
| 0.15 | 259 ± 8 | 300 ± 7 | 239 ± 3 | 168 ± 4 |
| 0.2 | 271 ± 4 | 267 ± 4 | 250 ± 2 | 185 ± 6 |
| 0.25 | 250 ± 3 | 313 ± 4 | 191 ± 8 | 180 ± 2 |
| 0.3 | 246 ± 4 | 290 ± 4 | 206 ± 11 | 194 ± 5 |
| 0.4 | 244 ± 6 | 272 ± 5 | 205 ± 6 | 200 ± 5 |
| 0.5 | 265 ± 4 | 300 ± 4 | 137 ± 6 | 209 ± 5 |
| PDI | ||||
|---|---|---|---|---|
| RAL | OAPy-4 | OAPy-2 | OAMo-1 | OAPi-1 |
| 0.05 | 0.16 ± 0.03 | 0.23 ± 0.04 | 0.23 ± 0.03 | 0.25 ± 0.03 |
| 0.1 | 0.21 ± 0.02 | 0.18 ± 0.03 | 0.20 ± 0.05 | 0.20 ± 0.04 |
| 0.15 | 0.2 ± 0.05 | 0.20 ± 0.03 | 0.20 ± 0.05 | 0.23 ± 0.04 |
| 0.2 | 0.19 ± 0.03 | 0.23 ± 0.02 | 0.30 ± 0.03 | 0.22 ± 0.05 |
| 0.25 | 0.39 ± 0.03 | 0.28 ± 0.02 | 0.25 ± 0.03 | 0.19 ± 0.03 |
| 0.3 | 0.22 ± 0.02 | 0.20 ± 0.02 | 0.25 ± 0.05 | 0.23 ± 0.03 |
| 0.4 | 0.24 ± 0.03 | 0.10 ± 0.02 | 0.40 ± 0.04 | 0.15 ± 0.05 |
| 0.5 | 0.25 ± 0.04 | 0.21 ± 0.03 | 0.38 ± 0.03 | 0.18 ± 0.05 |
| Formulation | EE% | Drug Loading (µg) | DL% |
|---|---|---|---|
| SN-38 (1%) | 82 ± 13 | 172 ± 24 | 0.78 |
| SN-38 (2%) | 74 ± 11 | 297 ± 35 | 1.35 |
| SN-38 (5%) | 51 ± 18 | 516 ± 92 | 2.55 |
| SN-38 (10%) | 42 ± 14 | 844 ± 119 | 3.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajesh, S.; Zhai, J.; Drummond, C.J.; Tran, N. Novel pH-Responsive Cubosome and Hexosome Lipid Nanocarriers of SN-38 Are Prospective for Cancer Therapy. Pharmaceutics 2022, 14, 2175. https://doi.org/10.3390/pharmaceutics14102175
Rajesh S, Zhai J, Drummond CJ, Tran N. Novel pH-Responsive Cubosome and Hexosome Lipid Nanocarriers of SN-38 Are Prospective for Cancer Therapy. Pharmaceutics. 2022; 14(10):2175. https://doi.org/10.3390/pharmaceutics14102175
Chicago/Turabian StyleRajesh, Sarigama, Jiali Zhai, Calum J. Drummond, and Nhiem Tran. 2022. "Novel pH-Responsive Cubosome and Hexosome Lipid Nanocarriers of SN-38 Are Prospective for Cancer Therapy" Pharmaceutics 14, no. 10: 2175. https://doi.org/10.3390/pharmaceutics14102175
APA StyleRajesh, S., Zhai, J., Drummond, C. J., & Tran, N. (2022). Novel pH-Responsive Cubosome and Hexosome Lipid Nanocarriers of SN-38 Are Prospective for Cancer Therapy. Pharmaceutics, 14(10), 2175. https://doi.org/10.3390/pharmaceutics14102175