Direct and Reverse Pluronic Micelles: Design and Characterization of Promising Drug Delivery Nanosystems
Abstract
:1. Introduction
2. Physicochemical Properties of Pluronics and Self-Assembly Behavior

3. Factors Affecting the Micellization Process
3.1. Pluronic Length, Concentration and PEO:PPO Ratio

| Pluronic | MW (Da) | Molecular Formula | CMT (K) | CP (K) | Ref. |
|---|---|---|---|---|---|
| P84 | 4200 | EO19PO43EO19 | 297 a | 346 a | [31] |
| F87 | 7700 | EO61PO40EO61 | 301 a | 375 a | [31] |
| F88 | 11,400 | EO103PO39EO103 | 303 a | 375 a | [31] |
| L64 | 2900 | EO13PO30EO13 | 305 b | - | [32] |
| P103 | 4950 | EO16PO56EO16 | 356 b | - | [32] |
| P123 | 5750 | EO20PO70EO20 | 292 b | - | [32] |
| F127 | 12,600 | EO106PO70EO106 | 346 b | - | [32] |
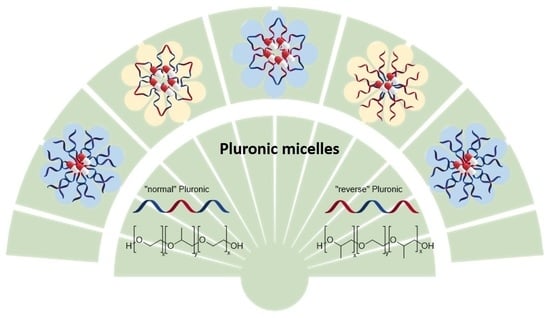
3.2. Normal versus Reverse Pluronics
3.3. Solvents
3.4. Temperature
3.5. pH and Ionic Strength
3.6. Nature of the Encapsulated Cargo
4. Techniques to Characterize the Micellization Process
4.1. Scattering Methods
| Size (nm) | PdI | ||
|---|---|---|---|
| <0.50 | 0.50–0.70 | >0.70 | |
| <2 | No aggregates. | No aggregates. | No aggregates. |
| 2–10 | Small monodisperse aggregates. Not compatible with micelles | Small polydisperse aggregates. Not compatible with micelles. | Very polydisperse aggregates. Not compatible with micelles. |
| 10–100 | Monodisperse aggregates. Compatible with micelles. | Polydisperse aggregates. Compatible with micelles. | Very polydisperse aggregates. Not compatible with micelles. |
| >100 | Big monodisperse aggregates. Not compatible with micelles | Big polydisperse aggregates. Not compatible with micelles. | Very polydisperse aggregates. Not compatible with micelles. |
4.2. Spectroscopic Methods
4.3. Rheological Methods
4.4. Microscopy-Based Methods
4.5. Calorimetric Methods
4.6. Other Methods
| Parameter | Technique | Example |
|---|---|---|
| Morphology and size (mean, distribution profile, hydrodynamic radius, PdI, hydration) | TEM CryoTEM FFEM DLS/SLS SAXS/SANS AFM IR/Raman Fluorescence spectroscopy | Doxorubicin-loaded DMs [79] Pristine DMs [81], Gallate-loaded DMs [51] DMs aggregates [82]. Pristine DMs [63] Lamotrigine-load DMs [44] DMs [57], RMs [58], oil-loaded DMs [50] Curcumin-loaded DMs [84] DMs [64,65], RMs [66] Ibuprofen and aspirin DMs [49]. |
| Aggregation number, cloud point | ITC SANS | Lavender-oil loaded mixed micelles [86] Lamotrigine-loaded DMs [44] |
| CMC | DLS Surface tension NMR UV-Vis spectroscopy Fluorescence spectr. Cyclic voltammetry Rheology (viscoelasticity) Electrical conductivity ITC | Curcumin-loaded DMs [62] DMs [91] Flurbiprofen-loaded DMs [53,70] Curcumin-loaded DMs [62] Mixed micelles [76] DMs [75,92] DMs [77,78], RMs [39] Neutral-ionic mixed DMs [93] Gemcitabine, cytarabine-loaded DMs [85], Pristine DMs and RMs [15] |
| CMT | ITC DSC | Pristine DMs and RMs [15] Heparin-DMs [89], curcumin-DMs |
| Thermodynamics (enthalpy and entropy of micellization) | ITC | DMs [18] |
| Integrity and stability in solvents or relevant media | ZP FRET | Camptothecin-loaded DM [90] Cholesterol-induced DM-noisome transition [52] |
| Cargo loading - Capacity - Locus - Efficiency - Partition coefficient, free energy of solubilization - Interactions with cargo | UV-Vis spectroscopy NMR/ITC Equation (1) Equations (2) and (3) NMR IR | Oxcarbazepine DMs [72] Flurbiprofen-loaded DMs [53,70] Curcumin-loaded DMs [94] Ciprofloxacin-loaded mixed micelles [95] Gallate-DMs [51], Ibuprofen-DMs [69] RMs [66] |
| Cargo release (in solvents or relevant media) | Dialysis | Oxcarbazepine DMs [72] |
5. The Lights and Shadows of Pluronic Micelles in Drug Delivery
5.1. Approaches towards an Increased Stability of the Micelles

5.2. Approaches towards an Improved Drug Loading in the Micelles
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Qiu, H.; Yin, S.; Wang, H.; Li, Y. Polymeric drug delivery system based on Pluronics for cancer treatment. Molecules 2021, 26, 3610. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Rohiwal, S.S. Chapter 2—Synthesis and bioconjugation of hybrid nanostructures for biomedical applications. In Hybrid Nanostructures for Cancer Theranostics; Ashok Bohara, R., Thorat, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 17–41. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, E.; Yang, J.; Cao, Z. Strategies to improve micelle stability for drug delivery. Nano Res. 2018, 11, 4985–4998. [Google Scholar] [CrossRef] [PubMed]
- Figueiras, A.; Domingues, C.; Jarak, I.; Santos, A.I.; Parra, A.; Pais, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Kabanov, A.; Cabral, H.; et al. New Advances in Biomedical Application of Polymeric Micelles. Pharmaceutics 2022, 14, 1700. [Google Scholar] [CrossRef]
- Ju, Y.; Guo, H.; Edman, M.; Hamm-Alvarez, S.F. Application of advances in endocytosis and membrane trafficking to drug delivery. Adv. Drug Deliv. Rev. 2020, 157, 118–141. [Google Scholar] [CrossRef]
- Chowdhury, P.; Nagesh, P.K.B.; Kumar, S.; Jaggi, M.; Chauhan, S.C.; Yallapu, M.M. Pluronic nanotechnology for overcoming drug resistance. In Bioactivity of Engineered Nanoparticles; Yan, B., Zhou, H., Gardea-Torresdey, J.L., Eds.; Springer: Singapore, 2017; pp. 207–237. [Google Scholar] [CrossRef]
- Singla, P.; Garg, S.; McClements, J.; Jamieson, O.; Peeters, M.; Mahajan, R.K. Advances in the therapeutic delivery and applications of functionalized Pluronics: A critical review. Adv. Colloid Interface Sci. 2022, 299, 102563. [Google Scholar] [CrossRef]
- Domínguez-Delgado, C.; Fuentes-Prado, E.; Escobar-Chávez, J.; Vidal-Romero, G.; Rodríguez Cruz, I.; Díaz-Torres, R. Chitosan and Pluronic® F-127: Pharmaceutical Applications. In Encyclopedia of Biomedical Polymers and Polymeric Biomaterials; Mishra, M., Ed.; CRC Press: Boca Raton, FL, USA, 2016; Volume 11, pp. 1513–1535. [Google Scholar]
- Singh-Joy, S.D.; McLain, V.C. Safety assessment of poloxamers 101, 105, 108, 122, 123, 124, 181, 182, 183, 184, 185, 188, 212, 215, 217, 231, 234, 235, 237, 238, 282, 284, 288, 331, 333, 334, 335, 338, 401, 402, 403, and 407, poloxamer 105 benzoate, and poloxamer 182 dibenzoate as used in cosmetics. Int. J. Toxicol. 2008, 27, 93–128. [Google Scholar] [CrossRef]
- Jarak, I.; Varela, C.L.; Tavares da Silva, E.; Roleira, F.F.M.; Veiga, F.; Figueiras, A. Pluronic-based nanovehicles: Recent advances in anticancer therapeutic applications. Eur. J. Med. Chem. 2020, 206, 112526. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of Poloxamers for drug delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Alexandridis, P.; Alan Hatton, T. Poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modeling. Colloids Surf. A Physicochem. Eng. Asp. 1995, 96, 1–46. [Google Scholar] [CrossRef]
- de Araújo, D.R.; Oshiro, A.; da Silva, D.C.; Akkari, A.C.S.; de Mello, J.C.; Rodrigues, T. Poloxamers as drug-delivery systems: Physicochemical, pharmaceutical, and toxicological aspects. In Nanotoxicology: Materials, Methodologies, and Assessments; Durán, N., Guterres, S.S., Alves, O.L., Eds.; Springer: New York, NY, USA, 2014; pp. 281–298. [Google Scholar] [CrossRef]
- Naskar, B.; Ghosh, S.; Moulik, S.P. Solution behavior of normal and reverse triblock copolymers (Pluronic L44 and 10R5) individually and in binary mixture. Langmuir 2012, 28, 7134–7146. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, L. Molecular interactions between PEO–PPO–PEO and PPO–PEO–PPO triblock copolymers in aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 485–497. [Google Scholar] [CrossRef]
- Noolandi, J.; Shi, A.-C.; Linse, P. Theory of phase behavior of poly(oxyethylene)−poly(oxypropylene)−poly(oxyethylene) triblock copolymers in aqueous solutions. Macromolecules 1996, 29, 5907–5919. [Google Scholar] [CrossRef]
- He, Z.; Alexandridis, P. Micellization thermodynamics of Pluronic P123 (EO20PO70EO20) amphiphilic block copolymer in aqueous ethylammonium nitrate (EAN) solutions. Polymers 2018, 10, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moroi, Y. Micelles: Theoretical and Applied Aspects; Springer: New York, NY, USA, 1992; p. 252. [Google Scholar] [CrossRef]
- Yeung, P.S.W.; Eskici, G.; Axelsen, P.H. Infrared spectroscopy of proteins in reverse micelles. Biochim. Biophys. Acta (BBA)–Biomembr. 2013, 1828, 2314–2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulthe, S.S.; Choudhari, Y.M.; Inamdar, N.N.; Mourya, V. Polymeric micelles: Authoritative aspects for drug delivery. Des. Monomers Polym. 2012, 15, 465–521. [Google Scholar] [CrossRef]
- Alexandridis, P.; Olsson, U.; Lindman, B. A record nine different phases (four cubic, two hexagonal, and one lamellar lyotropic liquid crystalline and two micellar solutions) in a ternary isothermal system of an amphiphilic block copolymer and selective solvents (water and oil). Langmuir 1998, 14, 2627–2638. [Google Scholar] [CrossRef]
- Larrañeta, E.; Isasi, J.R. Phase behavior of reverse Poloxamers and Poloxamines in water. Langmuir 2013, 29, 1045–1053. [Google Scholar] [CrossRef] [Green Version]
- Kralova, I.; Sjöblom, J. Surfactants Used in Food Industry: A Review. J. Disp. Sci. Technol. 2009, 30, 1363–1383. [Google Scholar] [CrossRef]
- Hezaveh, S.; Samanta, S.; De Nicola, A.; Milano, G.; Roccatano, D. Understanding the interaction of block copolymers with DMPC lipid bilayer using coarse-grained molecular dynamics simulations. J. Phys. Chem. B 2012, 116, 14333–14345. [Google Scholar] [CrossRef]
- Verma, P.; Nath, S.; Singh, P.K.; Kumbhakar, M.; Pal, H. Effects of block size of pluronic polymers on the water structure in the corona region and its effect on the electron transfer reactions. J. Phys. Chem. B 2008, 112, 6363–6372. [Google Scholar] [CrossRef] [PubMed]
- De Neve, L.; York, M.; Dickens, J.; Leys, J.; Meurs, G.; Sinnaeve, D.; Van der Meeren, P. Molecular structure and ionic strength both affect the micellization and solubilization behavior of PEO-PPO-PEO surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 172–179. [Google Scholar] [CrossRef]
- Bohorquez, M.; Koch, C.; Trygstad, T.; Pandit, N. A study of the temperature-dependent micellization of Pluronic F127. J. Colloid Interface Sci. 1999, 216, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Pitto-Barry, A.; Barry, N.P.E. Pluronic® block-copolymers in medicine: From chemical and biological versatility to rationalisation and clinical advances. Polym. Chem. 2014, 5, 3291–3297. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Lorenzo, C.; Sosnik, A.; Concheiro, A. PEO-PPO block copolymers for passive micellar targeting and overcoming multidrug resistance in cancer therapy. Curr. Drug Targets 2011, 12, 1112–1130. [Google Scholar] [CrossRef]
- Khimani, M.; Rao, U.; Bahadur, P.; Bahadur, P. Calorimetric and Scattering Studies on Micellization of Pluronics in Aqueous Solutions: Effect of the Size of Hydrophilic PEO End Blocks, Temperature, and Added Salt. J. Disp. Sci. Technol. 2014, 35, 1599–1610. [Google Scholar] [CrossRef]
- Shvartzman-Cohen, R.; Ren, C.-l.; Szleifer, I.; Yerushalmi-Rozen, R. An isotopic effect in self-assembly of amphiphilic block copolymers: The role of hydrogen bonds. Soft Matter 2009, 5, 5003–5011. [Google Scholar] [CrossRef]
- Hassanzadeh, S.; Feng, Z.; Pettersson, T.; Hakkarainen, M. A proof-of-concept for folate-conjugated and quercetin-anchored pluronic mixed micelles as molecularly modulated polymeric carriers for doxorubicin. Polymer 2015, 74, 193–204. [Google Scholar] [CrossRef]
- Oh, K.T.; Bronich, T.K.; Kabanov, A.V. Micellar formulations for drug delivery based on mixtures of hydrophobic and hydrophilic Pluronic block copolymers. J. Control. Release 2004, 94, 411–422. [Google Scholar] [CrossRef]
- Garg, S.; Peeters, M.; Mahajan, R.K.; Singla, P. Loading of hydrophobic drug silymarin in pluronic and reverse pluronic mixed micelles. J. Drug Deliv. Sci. Technol. 2022, 75, 103699. [Google Scholar] [CrossRef]
- Bharatiya, B.; Guo, C.; Ma, J.H.; Hassan, P.A.; Bahadur, P. Aggregation and clouding behavior of aqueous solution of EO–PO block copolymer in presence of n-alkanols. Eur. Polym. J. 2007, 43, 1883–1891. [Google Scholar] [CrossRef]
- Svensson, B.; Alexandridis, P.; Olsson, U. Self-assembly of a poly(ethylene oxide)/poly(propylene oxide) block copolymer (Pluronic P104, (EO)27(PO)61(EO)27) in the presence of water and xylene. J. Phys. Chem. B 1998, 102, 7541–7548. [Google Scholar] [CrossRef]
- Alexandridis, P.; Olsson, U.; Lindman, B. Self-assembly of amphiphilic block copolymers: The (EO)13(PO)30(EO)13-water-p-xylene system. Macromolecules 1995, 28, 7700–7710. [Google Scholar] [CrossRef]
- Ben Henda, M.; Ghaouar, N.; Gharbi, A. Rheological properties and reverse micelles conditions of PEO-PPO-PEO Pluronic F68: Effects of temperature and solvent mixtures. J. Polymers 2013, 2013, 768653. [Google Scholar] [CrossRef] [Green Version]
- Vasilescu, M.; Caragheorgheopol, A.; Caldararu, H.; Bandula, R.; Lemmetyinen, H.; Joela, H. Micropolarity and order in the reverse micelles of L62 and L64 Pluronic copolymers, as studied by molecular probe techniques. J. Phys. Chem. B 1998, 102, 7740–7751. [Google Scholar] [CrossRef]
- Sarkar, B.; Ravi, V.; Alexandridis, P. Micellization of amphiphilic block copolymers in binary and ternary solvent mixtures. J. Colloid Int. Sci. 2013, 390, 137–146. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, S.M.; Li, B.; De Nicola, A.; Yu, N.S.; Dong, B. Micellization of Pluronic P123 in water/ethanol/turpentine oil mixed solvents: Hybrid particle-field molecular dynamic simulation. Polymers 2019, 11, 1806. [Google Scholar] [CrossRef] [Green Version]
- Ur-Rehman, T.; Tavelin, S.; Gröbner, G. Effect of DMSO on micellization, gelation and drug release profile of Poloxamer 407. Int. J. Pharm. 2010, 394, 92–98. [Google Scholar] [CrossRef]
- Singla, P.; Singh, O.; Sharma, S.; Betlem, K.; Aswal, V.K.; Peeters, M.; Mahajan, R.K. Temperature-dependent solubilization of the hydrophobic antiepileptic drug lamotrigine in different Pluronic micelles—A spectroscopic, heat transfer method, small-angle neutron scattering, dynamic light scattering, and in vitro release study. ACS Omega 2019, 4, 11251–11262. [Google Scholar] [CrossRef] [Green Version]
- Tsui, H.-W.; Wang, J.-H.; Hsu, Y.-H.; Chen, L.-J. Study of heat of micellization and phase separation for Pluronic aqueous solutions by using a high sensitivity differential scanning calorimetry. Colloid Polym. Sci. 2010, 288, 1687–1696. [Google Scholar] [CrossRef]
- Bahadur, P.; Pandya, K.; Almgren, M.; Li, P.; Stilbs, P. Effect of inorganic salts on the micellar behaviour of ethylene oxide-propylene oxide block copolymers in aqueous solution. Colloid Polym. Sci 1993, 271, 657–667. [Google Scholar] [CrossRef]
- Varade, D.; Sharma, R.; Aswal, V.K.; Goyal, P.S.; Bahadur, P. Effect of hydrotropes on the solution behavior of PEO/PPO/PEO block copolymer L62 in aqueous solutions. Eur. Polym. J. 2004, 40, 2457–2464. [Google Scholar] [CrossRef]
- Nguyen, T.T.C.; Nguyen, C.K.; Nguyen, T.H.; Tran, N.Q. Highly lipophilic pluronics-conjugated polyamidoamine dendrimer nanocarriers as potential delivery system for hydrophobic drugs. Mater. Sci. Eng. C 2017, 70, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Basak, R.; Bandyopadhyay, R. Encapsulation of hydrophobic drugs in Pluronic F127 micelles: Effects of drug hydrophobicity, solution temperature, and pH. Langmuir 2013, 29, 4350–4356. [Google Scholar] [CrossRef] [Green Version]
- Grillo, I.; Morfin, I.; Prévost, S. Structural characterization of Pluronic micelles swollen with perfume molecules. Langmuir 2018, 34, 13395–13408. [Google Scholar] [CrossRef]
- Luo, H.; Jiang, K.; Liang, X.; Hua, C.; Li, Y.; Liu, H. Insights into morphological transition of Pluronic P123 micelles as a function of gallate. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 221–229. [Google Scholar] [CrossRef]
- Roy, A.; Kundu, N.; Banik, D.; Sarkar, N. Comparative fluorescence resonance energy-transfer study in Pluronic triblock copolymer micelle and niosome composed of biological component cholesterol: An investigation of effect of cholesterol and sucrose on the FRET parameters. J. Phys. Chem. B 2016, 120, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Alexander, S.; de Vos, W.M.; Castle, T.C.; Cosgrove, T.; Prescott, S.W. Growth and shrinkage of Pluronic micelles by uptake and release of flurbiprofen: Variation of pH. Langmuir 2012, 28, 6539–6545. [Google Scholar] [CrossRef]
- Akiba, I.; Sakurai, K. Characterizing block-copolymer micelles used in nanomedicines via solution static scattering techniques. Polym. J. 2021, 53, 951–973. [Google Scholar] [CrossRef]
- Sanjeeva Murthy, N. Scattering techniques for structural analysis of biomaterials. In Characterization of Biomaterials; Jaffe, M., Hammond, W., Tolias, P., Arinzeh, T., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 34–72. [Google Scholar] [CrossRef]
- Ree, B.J.; Lee, J.; Satoh, Y.; Kwon, K.; Isono, T.; Satoh, T.; Ree, M. A comparative study of dynamic light and X-ray scatterings on micelles of topological polymer amphiphiles. Polymers 2018, 10, 1347. [Google Scholar] [CrossRef] [Green Version]
- Manet, S.; Lecchi, A.; Impéror-Clerc, M.; Zholobenko, V.; Durand, D.; Oliveira, C.L.; Pedersen, J.S.; Grillo, I.; Meneau, F.; Rochas, C. Structure of micelles of a nonionic block copolymer determined by SANS and SAXS. J. Phys. Chem. B 2011, 115, 11318–11329. [Google Scholar] [CrossRef] [PubMed]
- Svensson, B.; Olsson, U.; Alexandridis, P.; Mortensen, K. A SANS investigation of reverse (water-in-oil) micelles of amphiphilic block copolymers. Macromolecules 1999, 32, 6725–6733. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, S.; Ma, X.; Zhang, T.; Gao, Y.-E.; Wang, Y.; Gao, Y.; Xu, Z. Chapter 14. Polymeric micelles as delivery systems. In Nanoengineered Biomaterials for Advanced Drug Delivery; Mozafari, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 261–278. [Google Scholar] [CrossRef]
- Topel, Ö.; Çakır, B.A.; Budama, L.; Hoda, N. Determination of critical micelle concentration of polybutadiene-block-poly(ethyleneoxide) diblock copolymer by fluorescence spectroscopy and dynamic light scattering. J. Mol. Liq. 2013, 177, 40–43. [Google Scholar] [CrossRef]
- Thapa, R.K.; Cazzador, F.; Grønlien, K.G.; Tønnesen, H.H. Effect of curcumin and cosolvents on the micellization of Pluronic F127 in aqueous solution. Colloids Surf. B 2020, 195, 111250. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Chu, B. Light-scattering study on the association behavior of triblock polymers of ethylene oxide and propylene oxide in aqueous solution. J. Colloid. Interface Sci. 1988, 126, 171–180. [Google Scholar] [CrossRef]
- Šturcová, A.; Schmidt, P.; Dybal, J. Role of hydration and water coordination in micellization of Pluronic block copolymers. J. Colloid Interface Sci. 2010, 352, 415–423. [Google Scholar] [CrossRef]
- Su, Y.-L.; Liu, H.-Z.; Guo, C.; Wang, J. Association behavior of PEO–PPO–PEO block copolymers in water or organic solvent observed by FTIR spectroscopy. Mol. Simul. 2003, 29, 803–808. [Google Scholar] [CrossRef]
- Guo, C.; Liu, H.-Z.; Chen, J.-Y. A Fourier transform infrared study on water-induced reverse micelle formation of block copoly(oxyethylene–oxypropylene–oxyethylene) in organic solvent. Colloids Surf. A Physicochem. Eng. Asp. 2000, 175, 193–202. [Google Scholar] [CrossRef]
- Furó, I. NMR spectroscopy of micelles and related systems. J. Mol. Liq. 2005, 117, 117–137. [Google Scholar] [CrossRef]
- Ma, J.-H.; Guo, C.; Tang, Y.-L.; Chen, L.; Bahadur, P.; Liu, H.-Z. Interaction of urea with Pluronic block copolymers by 1H NMR spectroscopy. J. Phys. Chem. B 2007, 111, 5155–5161. [Google Scholar] [CrossRef]
- Foster, B.; Cosgrove, T.; Espidel, Y. PFGSE-NMR study of pH-triggered behavior in Pluronic−ibuprofen solutions. Langmuir 2009, 25, 6767–6771. [Google Scholar] [CrossRef]
- Alexander, S.; Cosgrove, T.; Prescott, S.W.; Castle, T.C. Flurbiprofen encapsulation using Pluronic triblock copolymers. Langmuir 2011, 27, 8054–8060. [Google Scholar] [CrossRef]
- Salem, J.K.; El-Nahhal, I.M.; Salama, S.F. Determination of the critical micelle concentration by absorbance and fluorescence techniques using fluorescein probe. Chem. Phys. Lett. 2019, 730, 445–450. [Google Scholar] [CrossRef]
- Singla, P.; Chabba, S.; Mahajan, R.K. A systematic physicochemical investigation on solubilization and in vitro release of poorly water soluble oxcarbazepine drug in pluronic micelles. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 479–488. [Google Scholar] [CrossRef]
- Kadam, Y.; Yerramilli, U.; Bahadur, A. Solubilization of poorly water-soluble drug carbamezapine in pluronic micelles: Effect of molecular characteristics, temperature and added salt on the solubilizing capacity. Colloids and surfaces. B, Biointerfaces 2009, 72, 141–147. [Google Scholar] [CrossRef]
- Agafonov, M.; Volkova, T.; Kumeev, R.; Chibunova, E.; Terekhova, I. Impact of pluronic F127 on aqueous solubility and membrane permeability of antirheumatic compounds of different structure and polarity. J. Mol. Liq. 2019, 274, 770–777. [Google Scholar] [CrossRef]
- Lee, K.; Shin, S.-C.; Oh, I. Fluorescence spectroscopy studies on micellization of poloxamer 407 solution. Arch. Pharm. Res. 2003, 26, 653–658. [Google Scholar] [CrossRef]
- Kulthe, S.S.; Inamdar, N.N.; Choudhari, Y.M.; Shirolikar, S.M.; Borde, L.C.; Mourya, V.K. Mixed micelle formation with hydrophobic and hydrophilic Pluronic block copolymers: Implications for controlled and targeted drug delivery. Colloid Surf. B 2011, 88, 691–696. [Google Scholar] [CrossRef]
- Kurumada, K.-I.; Robinson, B.H. Viscosity studies of pluronic F127 in aqueous solution. In Trends in Colloid and Interface Science XVI; Springer: Berlin/Heidelberg, Germany, 2004; pp. 12–15. [Google Scholar] [CrossRef]
- Costanzo, S.; Di Sarno, A.; D’Apuzzo, M.; Avallone, P.R.; Raccone, E.; Bellissimo, A.; Auriemma, F.; Grizzuti, N.; Pasquino, R. Rheology and morphology of Pluronic F68 in water. Phys. Fluids 2021, 33, 043113. [Google Scholar] [CrossRef]
- Manaspon, C.; Viravaidya-Pasuwat, K.; Pimpha, N. Preparation of folate-conjugated Pluronic F127/chitosan core-shell nanoparticles encapsulating doxorubicin for breast cancer treatment. J. Nanomater. 2012, 2012, 593878. [Google Scholar] [CrossRef] [Green Version]
- Goel, H.; Saini, K.; Razdan, K.; Khurana, R.K.; Elkordy, A.A.; Singh, K.K. Chapter 3—In vitro physicochemical characterization of nanocarriers: A road to optimization. In Nanoparticle Therapeutics; Kesharwani, P., Singh, K.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 133–179. [Google Scholar] [CrossRef]
- Lam, Y.-M.; Grigorieff, N.; Goldbeck-Wood, G. Direct visualisation of micelles of Pluronic block copolymers in aqueous solution by cryo-TEM. Phys. Chem. Chem. Phys. 1999, 1, 3331–3334. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Li, W.; Feng, X.; Hou, M.; Han, B. Formation of micelles of Pluronic block copolymers in PEG 200. J. Colloid. Interface Sci. 2008, 327, 157–161. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Sahu, A.; Kasoju, N.; Goswami, P.; Bora, U. Encapsulation of curcumin in Pluronic block copolymer micelles for drug delivery applications. J. Biomater. Appl. 2011, 25, 619–639. [Google Scholar] [CrossRef]
- Prasanthan, P.; Kishore, N. Self-assemblies of pluronic micelles in partitioning of anticancer drugs and effectiveness of this system towards target protein. RSC Adv. 2021, 11, 22057–22069. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Kumar, S.; Basu, M.; Kunwar, A.; Dutta, D.; Aswal, V.K. Micellar solubilization of Lavender oil in aqueous P85/P123 systems: Investigating the associated micellar structural transitions, therapeutic properties and existence of double cloud points. J. Mol. Liq. 2021, 338, 116643. [Google Scholar] [CrossRef]
- Raudino, A.; Sarpietro, M.G.; Pannuzzo, M. 4—Differential scanning calorimetry (DSC): Theoretical fundamentals. In Drug-Biomembrane Interaction Studies; Pignatello, R., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 127–168. [Google Scholar] [CrossRef]
- Šarac, B.; Bešter-Rogač, M.; Lah, J. Thermodynamics of micellization from heat-capacity measurements. ChemPhysChem 2014, 15, 1827–1833. [Google Scholar] [CrossRef]
- Lee, S.Y.; Tae, G.; Kim, Y.H. Accelerated micellization and aggregation of pluronic micelles by interaction with heparin. J. Biomater. Sci. Polym. Ed. 2010, 21, 727–739. [Google Scholar] [CrossRef]
- Sezgin, Z.; Yüksel, N.; Baykara, T. Preparation and characterization of polymeric micelles for solubilization of poorly soluble anticancer drugs. Eur. J. Pharm. Biopharm. 2006, 64, 261–268. [Google Scholar] [CrossRef]
- Bąk, A.; Pilarek, M.; Podgórska, W.; Markowska-Radomska, A.; Hubacz, R. Surface properties of perfluorodecalin–containing liquid/liquid systems: The influence of Pluronic F-68 dissolved in the aqueous phase. J. Fluor. Chem. 2018, 215, 36–43. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Guo, R. Diffusion coefficients and structure properties in the Pluronic F127/n-C4H9OH/H2O system. J. Dispers. Sci. Technol. 2003, 24, 673–681. [Google Scholar] [CrossRef]
- Karunanithi, P.; Vigneshwari, R.; Paul Raj, E.; Rajesh, P.; Krishnamoorthy, S.; Dash, S. Pluronic based neutral-ionic binary micellar surfactant systems for solubilizing the cationic methylene blue dye. Chem. Phys. Impact 2022, 5, 100092. [Google Scholar] [CrossRef]
- Ganguly, R.; Kunwar, A.; Dutta, B.; Kumar, S.; Barick, K.C.; Ballal, A.; Aswal, V.K.; Hassan, P.A. Heat-induced solubilization of curcumin in kinetically stable pluronic P123 micelles and vesicles: An exploit of slow dynamics of the micellar restructuring processes in the aqueous pluronic system. Colloid. Surf. B 2017, 152, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Ezhilrani, V.C.; Karunanithi, P.; Sarangi, B.; Joshi, R.G.; Dash, S. Hydrophilic-hydrophilic mixed micellar system: Effect on solubilization of drug. SN Appl. Sci. 2021, 3, 371. [Google Scholar] [CrossRef]
- Kwon, G.S.; Kataoka, K. Block copolymer micelles as long-circulating drug vehicles. Adv. Drug Deliv. Rev. 1995, 16, 295–309. [Google Scholar] [CrossRef]
- Popovici, C.; Popa, M.; Sunel, V.; Atanase, L.I.; Ichim, D.L. Drug delivery systems based on Pluronic micelles with antimicrobial activity. Polymers 2022, 14, 3007. [Google Scholar] [CrossRef]
- Carvalho, S.G.; Araujo, V.H.S.; dos Santos, A.M.; Duarte, J.L.; Silvestre, A.L.P.; Fonseca-Santos, B.; Villanova, J.C.O.; Gremião, M.P.D.; Chorilli, M. Advances and challenges in nanocarriers and nanomedicines for veterinary application. Int. J. Pharm. 2020, 580, 119214. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef]
- Yang, T.-F.; Chen, C.-N.; Chen, M.-C.; Lai, C.-H.; Liang, H.-F.; Sung, H.-W. Shell-crosslinked Pluronic L121 micelles as a drug delivery vehicle. Biomaterials 2007, 28, 725–734. [Google Scholar] [CrossRef]
- Wakaskar, R.R.; Bathena, S.P.; Tallapaka, S.B.; Ambardekar, V.V.; Gautam, N.; Thakare, R.; Simet, S.M.; Curran, S.M.; Singh, R.K.; Dong, Y.; et al. Peripherally cross-linking the shell of core-shell polymer micelles decreases premature release of physically loaded combretastatin A4 in whole blood and increases its mean residence time and subsequent potency against primary murine breast tumors after IV administration. Pharm. Res. 2015, 32, 1028–1044. [Google Scholar] [CrossRef] [PubMed]
- Abdullah Al, N.; Lee, H.; Lee, Y.S.; Lee, K.D.; Park, S.Y. Development of disulfide core-crosslinked pluronic nanoparticles as an effective anticancer-drug-delivery system. Macromol. Biosci. 2011, 11, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Han, M.; Li, Y.; Jin, Y.; Gao, J.Q. Chemosensitization of doxorubicin in multidrug-resistant cells by unimolecular micelles via increased cellular accumulation and apoptosis. J. Pharm. Pharmacol. 2016, 68, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Tănase, M.A.; Raducan, A.; Oancea, P.; Diţu, L.M.; Stan, M.; Petcu, C.; Scomoroşcenco, C.; Ninciuleanu, C.M.; Nistor, C.L.; Cinteza, L.O. Mixed Pluronic—Cremophor Polymeric Micelles as Nanocarriers for Poorly Soluble Antibiotics—The Influence on the Antibacterial Activity. Pharmaceutics 2021, 13, 435. [Google Scholar] [CrossRef] [PubMed]
- Nambam, J.S.; Philip, J. Effects of interaction of ionic and nonionic surfactants on self-assembly of PEO–PPO–PEO triblock copolymer in aqueous solution. J. Phys. Chem. B 2012, 116, 1499–1507. [Google Scholar] [CrossRef]
- Gao, Y.; Li, L.B.; Zhai, G. Preparation and characterization of Pluronic/TPGS mixed micelles for solubilization of camptothecin. Colloid. Surf. B. Biointerfaces 2008, 64, 194–199. [Google Scholar] [CrossRef]
- Holland, P.M.; Rubingh, D.N. Mixed Surfactant Systems; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1992. [Google Scholar]
- Kaur, J.; Singla, P.; Kaur, I. Labrasol mediated enhanced solubilization of natural hydrophobic drugs in Pluronic micelles: Physicochemical and in vitro release studies. J. Mol. Liq. 2022, 361, 119596. [Google Scholar] [CrossRef]
- Kouser Qadri, H.; Shaheen, A.; Rashid, S.; Ahmad Bhat, I.; Mohammad Rather, G.; Ahmad Dar, A. Micellization and gelation characteristics of Pluronic P123 and single ester-bonded cleavable cationic gemini surfactant: A potential system for solubilization and release of ibuprofen. J. Mol. Liq. 2022, 366, 120311. [Google Scholar] [CrossRef]
- Patel, H.S.; Shaikh, S.J.; Ray, D.; Aswal, V.K.; Vaidya, F.; Pathak, C.; Varade, D.; Rahdar, A.; Sharma, R.K. Structural transitions in mixed Phosphatidylcholine/Pluronic micellar systems and their in vitro therapeutic evaluation for poorly water-soluble drug. J. Mol. Liq. 2022, 364, 120003. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Dash, S.; Vigneshwari, R.; Paulraj, E. Aceclofenac-loaded pluronic F108/L81 mixed polymeric micelles: Effect of HLB on solubilization. Des. Mon. Polym. 2022, 25, 1–11. [Google Scholar] [CrossRef]
- Patel, H.S.; Shaikh, S.J.; Ray, D.; Aswal, V.K.; Vaidya, F.; Pathak, C.; Sharma, R.K. Formulation, Solubilization, and In Vitro Characterization of Quercetin-Incorporated Mixed Micelles of PEO-PPO-PEO Block Copolymers. Appl. Biochem. Biotechnol. 2022, 194, 445–463. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.F.; Wang, W.C.; Zeng, X.Y.; Lu, Y.X.; Shih, P.J. Preparation, Structural Characterization of Anti-Cancer Drugs-Mediated Self-Assembly from the Pluronic Copolymers through Synchrotron SAXS Investigation. Materials 2022, 15, 5387. [Google Scholar] [CrossRef]
- Arsene, M.-L.; Răut, I.; Călin, M.; Jecu, M.-L.; Doni, M.; Gurban, A.-M. Versatility of reverse micelles: From biomimetic models to nano (bio)sensor design. Processes 2021, 9, 345. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Wang, Q.; Wu, J.; Fang, X. Pharmacokinetics and biodistribution of paclitaxel-loaded pluronic P105/L101 mixed polymeric micelles. J. Pharm. Soc. Jpn. 2008, 128, 941–950. [Google Scholar] [CrossRef] [Green Version]
- Pellosi, D.S.; Tessaro, A.L.; Moret, F.; Gaio, E.; Reddi, E.; Caetano, W.; Quaglia, F.; Hioka, N. Pluronic® mixed micelles as efficient nanocarriers for benzoporphyrin derivatives applied to photodynamic therapy in cancer cells. J. Photochem. Photobiol. 2016, 314, 143–154. [Google Scholar] [CrossRef]
- Öztürk, K.; Arslan, F.B.; Öztürk, S.C.; Çalış, S. Mixed micelles formulation for carvedilol delivery: In-vitro characterization and in-vivo evaluation. Int. J. Pharm. 2022, 611, 121294. [Google Scholar] [CrossRef]
- Wang, G.; Nie, Q.; Zang, C.; Zhang, B.; Zhu, Q.; Luo, G.; Wang, S. Self-assembled thermoresponsive nanogels prepared by reverse micelle; positive micelle method for ophthalmic delivery of muscone, a poorly water-soluble drug. J. Pharm. Sci. 2016, 105, 2752–2759. [Google Scholar] [CrossRef] [Green Version]
- Jaquilin, R.P.J.; Oluwafemi, O.S.; Thomas, S.; Oyedeji, A.O. Recent advances in drug delivery nanocarriers incorporated in temperature-sensitive Pluronic F-127–A critical review. J. Drug Deliv. Sci. Technol. 2022, 72, 103390. [Google Scholar] [CrossRef]
- Gaucher, G.; Dufresne, M.-H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.-C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef]
- Zhou, W.; Li, C.; Wang, Z.; Zhang, W.; Liu, J. Factors affecting the stability of drug-loaded polymeric micelles and strategies for improvement. J. Nanopart. Res. 2016, 18, 275. [Google Scholar] [CrossRef]
- Sotoudegan, F.; Amini, M.; Faizi, M.; Aboofazeli, R. Nimodipine-loaded Pluronic® block copolymer micelles: Preparation, characterization, in-vitro and in-vivo studies. Iran J. Pharm. Res. 2016, 15, 641–661. [Google Scholar] [PubMed]
- Stammet, M.; Kwon, G.S.; Rao, D.A. Drug loading in Pluronic® micelles made by solvent casting and equilibrium methods using resveratrol as a model drug. J. Control. Release 2010, 148, e50–e51. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.; Qiao, C.; Wang, S. Study on Central Composite Design Method to Optimize the Preparation Process of Chrysophanol-Pluronic F127 Nanomicelles and Pharmacokinetics. J. Nanomater. 2022, 2022, 7428740. [Google Scholar] [CrossRef]
- Pellosi, D.S.; Paula, L.B.; de Melo, M.T.; Tedesco, A.C. Targeted and synergic glioblastoma treatment: Multifunctional nanoparticles delivering verteporfin as adjuvant therapy for temozolomide chemotherapy. Mol. Pharm. 2019, 16, 1009–1024. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Bai, H.; Shen, J.; Chen, X.; Ping, Y.; Tang, G. A cooperative dimensional strategy for enhanced nucleus-targeted delivery of anticancer drugs. Adv. Funct. Mater. 2017, 27, 1700339. [Google Scholar] [CrossRef]



| Solvent/s | Effect on Micellization | |
|---|---|---|
| Binary mixtures | Short-chain alcohols (ethanol) [28,33] | Negative |
| Medium-chain alcohols (butanol, glycerol) [28] | Positive | |
| Oils (p-xylene) [29,30] | Positive (different assemblies) | |
| Glucose [33] | Positive | |
| Propylene carbonate [33] | Positive | |
| Triacetin [33] | Positive | |
| DMSO [35] | Positive | |
| Ionic liquids (ethylammonium nitrate) [12] | Positive | |
| Ternary mixtures | Ethanol/glycerol [33] | No effect |
| Ethanol/propylene carbonate [33] | No effect | |
| Ethanol/turpentine oil [34] | Positive (different assemblies) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naharros-Molinero, A.; Caballo-González, M.Á.; de la Mata, F.J.; García-Gallego, S. Direct and Reverse Pluronic Micelles: Design and Characterization of Promising Drug Delivery Nanosystems. Pharmaceutics 2022, 14, 2628. https://doi.org/10.3390/pharmaceutics14122628
Naharros-Molinero A, Caballo-González MÁ, de la Mata FJ, García-Gallego S. Direct and Reverse Pluronic Micelles: Design and Characterization of Promising Drug Delivery Nanosystems. Pharmaceutics. 2022; 14(12):2628. https://doi.org/10.3390/pharmaceutics14122628
Chicago/Turabian StyleNaharros-Molinero, Almudena, María Ángela Caballo-González, Francisco Javier de la Mata, and Sandra García-Gallego. 2022. "Direct and Reverse Pluronic Micelles: Design and Characterization of Promising Drug Delivery Nanosystems" Pharmaceutics 14, no. 12: 2628. https://doi.org/10.3390/pharmaceutics14122628
APA StyleNaharros-Molinero, A., Caballo-González, M. Á., de la Mata, F. J., & García-Gallego, S. (2022). Direct and Reverse Pluronic Micelles: Design and Characterization of Promising Drug Delivery Nanosystems. Pharmaceutics, 14(12), 2628. https://doi.org/10.3390/pharmaceutics14122628