Recent Applications of Contact Lenses for Bacterial Corneal Keratitis Therapeutics: A Review
Abstract
:1. Introduction
2. Diagnosis of Bacterial Keratitis
2.1. Traditional Colony Culture Investigation
2.2. Corneal Imaging
2.3. Molecular Detection
2.4. Signs and Symptoms
2.5. Traditional Treatments
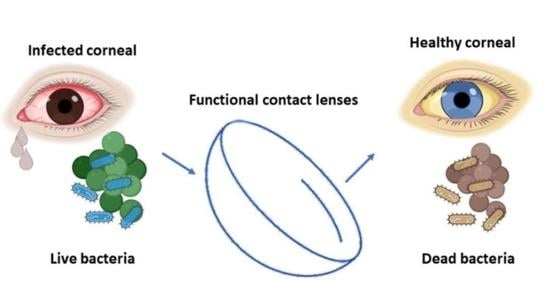
3. Contact Lenses for Corneal Keratitis
3.1. CL Hydrogel Types
3.2. Antibacterial CLs
3.2.1. Applying Antibacterial Agents in CLs
Embedded Nanomaterials in CLs
- Silver nanoparticles
- Gold nanoparticles
- Zinc oxide nanoparticles
Integration of Antibacterial Peptides with CLs
ROS Produce Reagents for Bacterial Killing
3.2.2. Prevention of Bacterial Adherence to CLs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marquart, M.E.; O’Callaghan, R.J. Infectious Keratitis: Secreted Bacterial Proteins That Mediate Corneal Damage. J. Ophthalmol. 2013, 2013, 13. [Google Scholar] [CrossRef] [Green Version]
- Park, W.; Nguyen, V.P.; Jeon, Y.; Kim, B.; Li, Y.; Yi, J.; Kim, H.; Leem, J.W.; Kim, Y.L.; Kim, D.R.; et al. Biodegradable silicon nanoneedles for ocular drug delivery. Sci. Adv. 2022, 8, eabn1772. [Google Scholar] [CrossRef]
- Li, S.; Zhu, Y.; Haghniaz, R.; Kawakita, S.; Guan, S.; Chen, J.; Li, Z.; Mandal, K.; Bahari, J.; Shah, S.; et al. A Microchambers Containing Contact Lens for the Noninvasive Detection of Tear Exosomes. Adv. Funct. Mater. 2022, 32, 2206620. [Google Scholar] [CrossRef]
- Yang, C.; Wu, Q.; Liu, J.; Mo, J.; Li, X.; Yang, C.; Liu, Z.; Yang, J.; Jiang, L.; Chen, W.; et al. Intelligent wireless theranostic contact lens for electrical sensing and regulation of intraocular pressure. Nat. Commun. 2022, 13, 2556. [Google Scholar] [CrossRef]
- Liu, Z.; Chauhan, A. Gold nanoparticles-loaded contact lenses for laser protection and Meibomian Gland Dysfunction (MGD) dry eye treatment. Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 128053. [Google Scholar] [CrossRef]
- Alam, F.; Salih, A.E.; Elsherif, M.; Yetisen, A.K.; Butt, H. 3D printed contact lenses for the management of color blindness. Addit. Manuf. 2021, 49, 102464. [Google Scholar] [CrossRef]
- Ting, D.S.; Gopal, B.P.; Deshmukh, R.; Seitzman, G.D.; Said, D.G.; Dua, H.S. Diagnostic armamentarium of infectious keratitis: A comprehensive review. Ocul. Surf. 2022, 23, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Nageeb, M.M.; Eldeek, H.E.M.; Attia, R.A.H.; Sakla, A.A.; Alkhalil, S.S.; Farrag, H.M.M. Isolation and morphological and molecular characterization of waterborne free-living amoebae: Evidence of potentially pathogenic Acanthamoeba and Vahlkampfiidae in Assiut, Upper Egypt. PLoS ONE 2022, 17, e0267591. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Ma, Y.; Zhou, W.; Liao, Y.; Jiang, Z.; Lin, J.; He, Q.; Wu, H.; Wei, W.; Wang, X.; et al. In-cytoplasm mitochondrial transplantation for mesenchymal stem cells engineering and tissue regeneration. Bioeng. Transl. Med. 2021, 7, e10250. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, R.N. Molecular Diagnostics for Ocular Infectious Diseases: 78th Edward Jackson Memorial Lecture, American Academy of Ophthalmology. Am. J. Ophthalmol. 2022, 235, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Rafat, M.; Jabbarvand, M.; Sharma, N.; Xeroudaki, M.; Tabe, S.; Omrani, R.; Thangavelu, M.; Mukwaya, A.; Fagerholm, P.; Lennikov, A.; et al. Bioengineered corneal tissue for minimally invasive vision restoration in advanced keratoconus in two clinical cohorts. Nat. Biotechnol. 2022, 89, 101031. [Google Scholar]
- Abdellah, N.; Desoky, S.M.M.E. Novel detection of stem cell niche within the stroma of limbus in the rabbit during postnatal development. Sci. Rep. 2022, 12, 13711. [Google Scholar] [CrossRef] [PubMed]
- Tuft, S.; Somerville, T.F.; Li, J.-P.O.; Neal, T.; De, S.; Horsburgh, M.J.; Fothergill, J.L.; Foulkes, D.; Kaye, S. Bacterial keratitis: Identifying the areas of clinical uncertainty. Prog. Retin. Eye Res. 2022, 89, 101031. [Google Scholar] [CrossRef] [PubMed]
- Palmer, M.L.; Hyndiuk, R.A. Contact lens-related infectious keratitis. Int. Ophthalmol. Clin. 1993, 33, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.; Lietman, T.; Rose-Nussbaumer, J. Update on the Management of Infectious Keratitis. Ophthalmology 2017, 124, 678–1689. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, S.; Li, J.; Falcone, N.; Cui, Q.; Shah, S.; Hartel, M.C.; Yu, N.; Young, P.; de Barros, N.R.; et al. Lab-on-a-Contact Lens: Recent Advances and Future Opportunities in Diagnostics and Therapeutics. Adv. Mater. 2022, 34, 2108389. [Google Scholar] [CrossRef]
- Ma, X.; Ahadian, S.; Liu, S.; Zhang, J.; Liu, S.; Cao, T.; Lin, W.; Wu, D.; de Barros, N.R.; Zare, M.R.; et al. Smart contact lenses for biosensing applications. Adv. Intell. Syst. 2021, 3, 2000263. [Google Scholar] [CrossRef]
- Wolf, M.P. GB Salieb-Beugelaar i P. Hunziker, PDMS z funkcjami projektanta-Właściwości, strategie modyfikacji i aplikacje. Prog. Polym. Sci. 2018, 83, 97–134. [Google Scholar] [CrossRef]
- Kihara, S.; Yamazaki, K.; Litwak, K.N.; Litwak, P.; Kameneva, M.V.; Ushiyama, H.; Tokuno, T.; Borzelleca, D.C.; Umezu, M.; Tomioka, J.; et al. In Vivo Evaluation of a MPC Polymer Coated Continuous Flow Left Ventricular Assist System. Artif. Organs 2003, 27, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K. Revolutionary advances in 2-methacryloyloxyethyl phosphorylcholine polymers as biomaterials. J. Biomed. Mater. Res. Part A 2019, 107, 933–943. [Google Scholar] [CrossRef]
- Ishihara, K.; Mu, M.; Konno, T.; Inoue, Y.; Fukazawa, K. The unique hydration state of poly(2-methacryloyloxyethyl phosphorylcholine). J. Biomater. Sci. Polym. Ed. 2017, 28, 884–899. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, P.E.; Huff, T.J.; Zuniga, J.M. The potential role of bioengineering and three-dimensional printing in curing global corneal blindness. J. Tissue Eng. 2018, 9, 2041731418769863. [Google Scholar] [CrossRef] [Green Version]
- Sarvari, R.; Agbolaghi, S.; Beygi-Khosrowshahi, Y.; Massoumi, B.; Bahadori, A. 3D Scaffold Designing based on Conductive/Degradable Tetrapolymeric Nanofibers of PHEMA-co-PNIPAAm-co-PCL/PANI for Bone Tissue Engineering. J. Ultrafine Grained Nanostruct. Mater. 2018, 51, 101–114. [Google Scholar] [CrossRef]
- García-Millán, E.; Koprivnik, S.; Otero-Espinar, F.J. Drug loading optimization and extended drug delivery of corticoids from pHEMA based soft contact lenses hydrogels via chemical and microstructural modifications. Int. J. Pharm. 2015, 487, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Motealleh, A.; Dorri, P.; Kehr, N.S. Injectable polymer/nanomaterial composites for the fabrication of three-dimensional biomaterial scaffolds. Biomed. Mater. 2020, 15, 045021. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Yeh, Y.-H.; Lin, W.-C.; Yang, M.-C. Novel silicone hydrogel based on PDMS and PEGMA for contact lens application. Colloids Surf. B Biointerfaces 2014, 123, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Keum, D.H.; Kim, S.K.; Koo, J.; Lee, G.H.; Jeon, C.; Mok, J.W.; Lee, K.J.; Kamrani, E.; Joo, C.; Shin, S.; et al. Wireless smart contact lens for diabetic diagnosis and therapy. Sci. Adv. 2020, 6, eaba3252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A Mini Review of Antibacterial Properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [Green Version]
- Malik, A.N.; Gilbert, C. Cochrane corner: Interventions for preventing. Eye 2022, 36, 356–357. [Google Scholar] [CrossRef]
- Leal, S.M., Jr.; Rodino, K.G.; Fowler, W.C.; Gilligan, P.H. Practical guidance for clinical microbiology laboratories: Diagnosis of ocular infections. Clin. Microbiol. Rev. 2021, 34, e00070-19. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.; Dhand, C.; Leung, C.M.; Beuerman, R.W.; Ramakrishna, S.; Lakshminarayanan, R. Strategies to design antimicrobial contact lenses and contact lens cases. J. Mater. Chem. B 2018, 6, 2171–2186. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; BarathManiKanth, S.; Pandian, S.R.K.; Deepak, V.; Gurunathan, S. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf. B Biointerfaces 2010, 79, 340–344. [Google Scholar] [CrossRef]
- Bazzaz, B.S.F.; Khameneh, B.; Jalili-Behabadi, M.M.; Malaekeh-Nikouei, B.; Mohajeri, S.A. Preparation, characterization and antimicrobial study of a hydrogel (soft contact lens) material impregnated with silver nanoparticles. Contact Lens and Anterior Eye 2014, 37, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.D.; Hume, E.B.; Vijay, A.K.; Petcavich, R. Ability of silver-impregnated contact lenses to control microbial growth and colonisation. J. Optom. 2010, 3, 143–148. [Google Scholar] [CrossRef]
- Lakkis, C.; Anastasopoulos, F.; Slater, J.; May, L. The effect of silver-infused silicone hydrogel contact lenses on the ocular biota during daily wear. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6477. [Google Scholar] [CrossRef]
- Huang, J.-F.; Zhong, J.; Chen, G.-P.; Lin, Z.-T.; Deng, Y.; Liu, Y.-L.; Cao, P.-Y.; Wang, B.; Wei, Y.; Wu, T.; et al. A Hydrogel-Based Hybrid Theranostic Contact Lens for Fungal Keratitis. ACS Nano 2016, 10, 6464–6473. [Google Scholar] [CrossRef] [PubMed]
- Santoro, C.M.; Duchsherer, N.L.; Grainger, D.W. Minimal In Vitro Antimicrobial Efficacy and Ocular Cell Toxicity from Silver Nanoparticles. Nanobiotechnology 2007, 3, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Söderstjerna, E.; Bauer, P.; Cedervall, T.; Abdshill, H.; Johansson, F.; Johansson, U.E. Silver and gold nanoparticles exposure to in vitro cultured retina—Studies on nanoparticle internalization, apoptosis, oxidative stress, glial-and microglial activity. PLoS ONE 2014, 9, e105359. [Google Scholar] [CrossRef] [Green Version]
- Salih, A.E.; Elsherif, M.; Alam, F.; Yetisen, A.K.; Butt, H. Gold Nanocomposite Contact Lenses for Color Blindness Management. ACS Nano 2021, 15, 4870–4880. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, Y.; Wang, Y.-H.; Jiang, H.; Wang, X. Advances and challenges in metallic nanomaterial synthesis and antibacterial applications. J. Mater. Chem. B 2020, 8, 4764–4777. [Google Scholar] [CrossRef] [PubMed]
- Mobed, A.; Hasanzadeh, M.; Seidi, F. Anti-bacterial activity of gold nanocomposites as a new nanomaterial weapon to combat photogenic agents: Recent advances and challenges. RSC Adv. 2021, 11, 34688–34698. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, X. Multiple strategies to activate gold nanoparticles as antibiotics. Nanoscale 2013, 5, 8340–8350. [Google Scholar] [CrossRef] [PubMed]
- Maulvi, F.A.; Patil, R.J.; Desai, A.R.; Shukla, M.R.; Vaidya, R.J.; Ranch, K.M.; Vyas, B.A.; Shah, S.A.; Shah, D.O. Effect of gold nanoparticles on timolol uptake and its release kinetics from contact lenses: In vitro and in vivo evaluation. Acta Biomater. 2019, 86, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Nune, S.K.; Chanda, N.; Katti, K.; Mekapothula, S.; Kulkarni, R.R.; Welshons, W.V.; Kannan, R.; Katti, K.V. Soybeans as a phytochemical reservoir for the production and stabilization of biocompatible gold nanoparticles. Small 2008, 4, 1425–1436. [Google Scholar] [CrossRef]
- Priya, M.R.K.; Iyer, P.R. Antiproliferative effects on tumor cells of the synthesized gold nanoparticles against Hep2 liver cancer cell line. Egypt. Liver J. 2020, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Lim, Z.-Z.J.; Li, J.-E.J.; Ng, C.-T.; Yung, L.-Y.L.; Bay, B.-H. Gold nanoparticles in cancer therapy. Acta Pharmacol. Sin. 2011, 32, 983–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarwal, H.; Menon, S.; Kumar, S.V.; Rajeshkumar, S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadiyala, U.; Turali-Emre, E.S.; Bahng, J.H.; Kotov, N.A.; VanEpps, J.S. Unexpected insights into antibacterial activity of zinc oxide nanoparticles against methicillin resistant Staphylococcus aureus (MRSA). Nanoscale 2018, 10, 4927–4939. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.; Somasundaran, P.; Fred Klaessig, V.C.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef]
- Khan, S.A.; Shahid, S.; Mahmood, T.; Lee, C.S. Contact lenses coated with hybrid multifunctional ternary nano-coatings (Phytomolecule-coated ZnO nanoparticles: Gallic Acid: Tobramycin) for the treatment of bacterial and fungal keratitis. Acta Biomater. 2021, 128, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Sung, A.-Y.; Kim, T.-H. Physical Properties of Ophthalmic Hydrogel Polymer Containing Zinc Oxide Nanoparticles. J. Chosun Nat. Sci. 2013, 6, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Salvagni, E.; García, C.; Müller-Sánchez, C.; Reina, M.; Rodríguez-Abreu, C.; García-Celma, M.J.; Esquena, J. Short and ultrashort antimicrobial peptides anchored onto soft commercial contact lenses inhibit bacterial adhesion. Colloids Surf. B Biointerfaces 2020, 196, 111283. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Jin, L.; Yu, F.; Wang, F.; Weng, Z.; Liu, J.; Han, Z.; Wang, X. ZnO/CPAN Modified Contact Lens with Antibacterial and Harmful Light Reduction Capabilities. Adv. Health Mater. 2021, 10, e2100259. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.H.; Meneguetti, B.T.; Costa, B.O.; Buccini, D.F.; Oshiro, K.G.N.; Preza, S.L.E.; Carvalho, C.M.E.; Migliolo, L.; Franco, O.L. Non-Lytic Antibacterial Peptides That Translocate Through Bacterial Membranes to Act on Intracellular Targets. Int. J. Mol. Sci. 2019, 20, 4877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo, R.L.; Kim, K.J.; Bernfield, M.; Kozak, C.A.; Zanetti, M.; Merluzzi, L.; Gennaro, R. Identification of CRAMP, a Cathelin-related Antimicrobial Peptide Expressed in the Embryonic and Adult Mouse. J. Biol. Chem. 1997, 272, 13088–13093. [Google Scholar] [CrossRef] [Green Version]
- Kazemzadeh-Narbat, M.; Cheng, H.; Chabok, R.; Alvarez, M.M.; De La Fuente-Nunez, C.; Phillips, K.S.; Khademhosseini, A. Strategies for antimicrobial peptide coatings on medical devices: A review and regulatory science perspective. Crit. Rev. Biotechnol. 2020, 41, 94–120. [Google Scholar] [CrossRef] [PubMed]
- Morgera, F.; Antcheva, N.; Pacor, S.; Quaroni, L.; Berti, F.; Vaccari, L.; Tossi, A. Structuring and interactions of human β-defensins 2 and 3 with model membranes. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2008, 14, 518–523. [Google Scholar] [CrossRef]
- Dutta, D.; Kumar, N.; Willcox, M. Antimicrobial activity of four cationic peptides immobilised to poly-hydroxyethylmethacrylate. Biofouling 2016, 32, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Dutta, D.; Willcox, M.D.P. Comparative mode of action of the antimicrobial peptide melimine and its derivative Mel4 against Pseudomonas aeruginosa. Sci. Rep. 2019, 9, 7063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, B.M.; Shirtliff, M.E.; Jabra-Rizk, M.A. Antimicrobial Peptides: Primeval Molecules or Future Drugs? PLoS Pathog. 2010, 6, e1001067. [Google Scholar] [CrossRef] [PubMed]
- Schomburg, L. The other view: The trace element selenium as a micronutrient in thyroid disease, diabetes, and beyond. Hormones 2020, 19, 15–24. [Google Scholar] [CrossRef]
- Al-hakimi, A.N.; Asnag, G.M.; Alminderej, F.; Alhagri, I.A.; Al-Hazmy, S.M.; Abdallah, E.M. Enhanced structural, optical, electrical properties and antibacterial activity of selenium nanoparticles loaded PVA/CMC blend for electro-chemical batteries and food packaging applications. Polym. Test. 2022, 116, 107794. [Google Scholar] [CrossRef]
- Tran, P.; Kopel, J.; Ristic, B.; Marsh, H.; Fralick, J.; Reid, T. Antimicrobial seleno-organic coatings and compounds acting primarily on the plasma membrane: A review. Adv. Redox Res. 2022, 4, 100031. [Google Scholar] [CrossRef]
- Jeong, H.; Park, J.-H.; Shin, J.H.; Yoo, J.-C.; Park, C.Y.; Hong, J. Prolonged Release Period of Nitric Oxide Gas for Treatment of Bacterial Keratitis by Amine-Rich Polymer Decoration of Nanoparticles. Chem. Mater. 2018, 30, 8528–8537. [Google Scholar] [CrossRef]
- Aveyard, J.L.; Deller, R.C.; Lace, R.; Williams, R.L.; Kaye, S.B.; Kolegraff, K.N.; Curran, J.M.; D’Sa, R.A. Antimicrobial Nitric Oxide Releasing Contact Lens Gels for the Treatment of Microbial Keratitis. ACS Appl. Mater. Interfaces 2019, 11, 37491–37501. [Google Scholar] [CrossRef]
- Mordmuang, A.; Udomwech, L.; Karnjana, K. Influence of contact lens materials and cleaning procedures on bacterial adhesion and biofilm formation. Clin. Ophthalmol. 2021, 15, 2391. [Google Scholar] [CrossRef]
- Udomwech, L.; Karnjana, K.; Jewboonchu, J.; Rattanathamma, P.; Narkkul, U.; Juhong, J.; Mordmuang, A. Bacterial microbiota of the contact lens surface and associated care behaviours. Heliyon 2022, 8, e09038. [Google Scholar] [CrossRef] [PubMed]
- Kodjikian, L.; Casoli-Bergeron, E.; Malet, F.; Janin-Manificat, H.; Freney, J.; Burillon, C.; Burillon, C.; Colin, J.; Steghens, J.P. Bacterial adhesion to conventional hydrogel and new silicone-hydrogel contact lens materials. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Rediske, A.M.; Koenig, A.L.; Barekzi, N.; Ameen, L.C.; Slunt, J.B.; Grainger, D.W. Polyclonal human antibodies reduce bacterial attachment to soft contact lens and corneal cell surfaces. Biomaterials 2002, 23, 4565–4572. [Google Scholar] [CrossRef] [PubMed]




| Material | Molecular Formula | Advantages | Disadvantages | Reference(s) | |
|---|---|---|---|---|---|
| Hard CLs | PMMA | (C5H8O2)n | Excellent optical properties, high toughness, rigid, inexpensive | Low gas permeability, limited hydrophilic ability, inflexible | [16,17] |
| PET | (C10H8O4)n | Outstanding chemical and thermal resistance, inexpensive | Low hydrophobic ability, glass transition temperature, rigidity, and surface energy | [16] | |
| Soft CLs | MPC | C11H22NO6P | Good surface wettability, high gas permeability, low protein adsorption, and inexpensive | Low mechanical strength | [17] |
| PDMS | (C2H6OSi)n | High gas permeability, inexpensive | Limited wearability | [16,18] | |
| HEMA | (C6H10O3)n | High water content, good chemical and thermal stability, high hydrophobic ability, flexibility, gas permeability, biocompatibility, and inexpensive | Low gas permeability, protein deposition issues | [16] | |
| Silicone | (R2SiO)n | High gas permeability | Highly hydrophobic, expensive | [19] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, L.; Li, Y.; Liu, Y.; Shi, L.; Chen, H. Recent Applications of Contact Lenses for Bacterial Corneal Keratitis Therapeutics: A Review. Pharmaceutics 2022, 14, 2635. https://doi.org/10.3390/pharmaceutics14122635
Nie L, Li Y, Liu Y, Shi L, Chen H. Recent Applications of Contact Lenses for Bacterial Corneal Keratitis Therapeutics: A Review. Pharmaceutics. 2022; 14(12):2635. https://doi.org/10.3390/pharmaceutics14122635
Chicago/Turabian StyleNie, Linyan, Yuanfeng Li, Yong Liu, Linqi Shi, and Huiyun Chen. 2022. "Recent Applications of Contact Lenses for Bacterial Corneal Keratitis Therapeutics: A Review" Pharmaceutics 14, no. 12: 2635. https://doi.org/10.3390/pharmaceutics14122635
APA StyleNie, L., Li, Y., Liu, Y., Shi, L., & Chen, H. (2022). Recent Applications of Contact Lenses for Bacterial Corneal Keratitis Therapeutics: A Review. Pharmaceutics, 14(12), 2635. https://doi.org/10.3390/pharmaceutics14122635