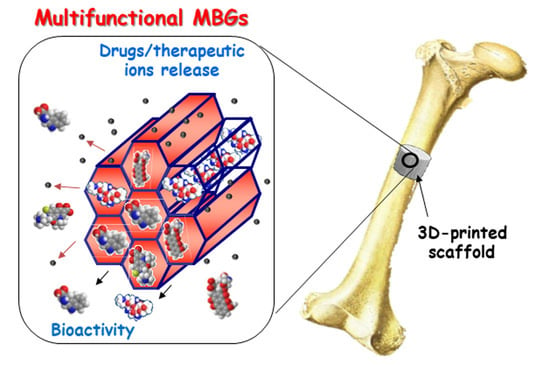
Achievements in Mesoporous Bioactive Glasses for Biomedical Applications
Abstract
:1. Introduction
2. Mesoporous Bioactive Glasses in the Context of Bone Tissue Regeneration
3. Design of Multifunctional Mesoporous Bioactive Glasses
3.1. Doping of Mesoporous Bioactive Glasses with Therapeutic Ions
3.2. Zwitterionization of Mesoporous Bioactive Glasses
4. Mesoporous Bioactive Glasses for Local Drug Delivery
4.1. Osteogenic and Antiosteoporotic Agents
| Carrier | MBGs Nominal Composition | Drug | Loading Capacity | Release Behavior | Biological Assays | Ref. |
|---|---|---|---|---|---|---|
| MBG scaffolds | (80 − 2x)SiO2-15CaO-2.5P2O5-xB2O3 (x = 0, 2.5 and 5 mol%) | Dexamethasone (DEX) | 275 µg/g (x = 0) 325 µg/g (x = 2.5) 300 µg/g (x = 5) | Sustained release independent of B-content up to ~100% after 350 h in PBS. | In vitro with human osteoblasts. | [92] |
| Biopolymer fibrous scaffolds incorporating MBG NPs | 75SiO2-25CaO (mol%) | DEX | 63% wt. | Initial burst release of ~30% within 24 h, followed by an almost linear release after 28 days of test in water. | In vitro with rat PDLSCs. In vivo in a rat calvarium defect model | [161] |
| Hollow core–shell MBG NPs | 79.4SiO2-18.1CaO-2.5P2O5(mol%) | Ipriflavone (IPF) | 13% wt. | Initial burst release of 18% within 10 h, followed by a slower release up to 25%. No further release was observed during 7 days in isopropanol: water medium. | In vitro with cocultures of osteoblasts (human Saos-2) and osteoclasts (differentiated RAW-264.7 macrophages) | [166] |
| Beta- cyclodextrin (β-CD)-modified-MBG NPs/silk fibroin (SF) mesh nanofibers | 80SiO2-15CaO-5P2O5 (mol%) | Estradiol (E2) | 37.99 µg/mg | Sustained release, reaching ca. 76% after 3 weeks in PBS. | In vitro with MC3T3-E1 and pre-osteoclasts RAW 264.7. | [170] |
| Amino-functionalized MBG scaffolds | 80SiO2-15CaO-5P2O5 (mol%) | Alendronate (ALE) | 17.1% wt. | Initial burst release of ca. 20% during 24 h followed by sustained release, reaching ~60% release after 280 h in SBF. | In vitro with rBMSCs-OVX. In vivo in an osteoporotic rat model. | [172] |
| MBG NPs | 80SiO2-16CaO-4P2O5 (mol%) | Fingolimod (FTY720) | 9.33 µg/ mg * | Initial burst release of ~35% followed by a sustained release, reaching ~95% after 300 h in NaCl 0.9%, pH = 7.4. | In vitro with mBMSCs and RAW 264.7. In vivo in rat femoral condyles defect model. | [173] |
| MBG submicronic microspheres | 80SiO2-(16-x)CaO-4P2O5-xSrO(x = 0, 5 and 10 mol%) | Icariin (ICA) | 6.89 %wt. (x = 10) * 5.53 %wt. (x = 5) * 4.21 %wt. (x = 0) * | Initial fast release during 24 h followed by sustained release up to 21 d in SBF. Faster release rate and greater maximum drug release as the Sr-content increases due to the greater mesopore size. | In vitro with BMMSCs isolated from adult rats with and without osteoporosis. | [176] |
| Silk fibroin (SF)/MBG NPs scaffolds | 80SiO2-16CaO-4P2O5 (mol%) | ICA | N.A | Sustained and slow release up to ~100% after 24 days in PBS. | In vitro with BMSCs. | [177] |
| MBG NPs | 85SiO2-10CaO-5SrO (mol%) | Phenamil (PHE) | 29% wt. | Initial burst release of 22% at 1h, followed by sustained release of 53% after 5 h, ~80% after 30 h and ~90% after 10 days in a Tris-HCl buffered solution, pH = 7.4. | In vitro with human MSCs from dental pulp. In vivo in rat model involving mal-calcification conditions. | [132] |
| MBGs in bulk compacted into disks | (80-x)SiO2-15CaO-5P2O5-xZnO (x = 0, 4 and 7 mol%) | Osteostatin (OST) | 0.8, 0.9 and 0.5 µg/g for x = 0, 4 and 7, respectively | OST release profiles were independent of the Zn-content. Fast release of ~75% after 24 h, reaching total release after 48 h in PBS. | In vitro with mouse pre-osteoblasts MC3T3-E1. | [183] |
| GA-Gel coated MBGs scaffolds | (80 − x)SiO2-15CaO-5P2O5-xZnO (x = 0, 4 and 5 mol%) | OST | 0.52, 0.71 and 0.62 µg/g for x = 0, 4 and 5, respectively | OST release profiles were independent of the Zn-content. Fast release of ~60% after 1 h, ~90% at 24 h and ~100% at 96 h | In vitro with hMSCs. | [112] |
| MBG microspheres | 85SiO2-15CaO (mol%) | BMP-2 | 66.7 µg/mg | Prolonged and sustained low-dose BMP-2 release without an initial burst up to 14 days in either PBS or tris-HCl buffer, pH = 7.4. | In vitro with primary hBMSCs. In vivo in a femoral osteotomy model of compromised healing in female rats. | [186] |
| MBG NPs | 85SiO2-15CaO (mol%) | BMP-2-plasmid DNA (BMP-2-pDNA) | 3.5 % wt. | Sustained BMP-2-pDNA release up to 2 weeks in PBS. | In vitro with rat BMSCs. In vivo in critical-sized calvarial defects in rats. | [187] |
| MBG nanotubular scaffold | 80SiO2-15CaO-5P2O5 (mol%) | rhBMP-2 | 24.7 ng/mg (MBG-NT100) and 184.3 ng/mg (MBG-NT100) for rhBMP-2 initial concentration of 100 ng/mL and 500 ng/mL, respectively. | Initial rhBMP-2 burst release of ~63% (MBG-NT100) and ~34% (MBG-NT500) for 3 days, followed by sustained release ≥80% up to 28 days in PBS. | In vitro with hBMSCs. | [189] |
| GelMA/MBG NPs-rhBMP-2 disk-shaped membrane | 80SiO2-16CaO-4P2O5 (mol%) | rhBMP-2 | 34.5 ng per disk | Long-term sustained release with an initial rhBMP-2 release of 38% after 2 days and ~69% after 28 days in PBS. | In vitro in BMSCs cultures. In vivo in critical bone defect model of the rat skull. | [190] |
4.2. Angiogenic Agents
4.3. Antibacterial Agents
4.4. Anti-Inflammatory Drugs
4.5. Antitumor Drugs
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vallet-Regí, M. Evolution of Bioceramics within the Field of Biomaterials. Comptes Rendus Chim. 2010, 13, 174–185. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Shimizu, T.; Kuroda, K.; Kato, C. The Preparation of Alkyltrimethylammonium-Kanemite Complexes and Their Conversion to Microporous Materials. Bull. Chem. Soc. Jpn. 1990, 63, 988–992. [Google Scholar] [CrossRef] [Green Version]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered Mesoporous Molecular Sieves Synthesized by a Liquid-Crystal Template Mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Ying, J.Y.; Mehnert, C.P.; Wong, M.S. Synthesis and Applications of Supramolecular-Templated Mesoporous Materials. Angew. Chemie-Int. Ed. 1999, 38, 56–77. [Google Scholar] [CrossRef]
- Corma, A. From Microporous to Mesoporous Molecular Sieve Materials and Their Use in Catalysis. Chem. Rev. 1997, 97, 2373–2419. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Schüth, F. Ordered Mesoporous Materials in Catalysis. Microporous Mesoporous Mater. 2005, 77, 1–45. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A New Family of Mesoporous Molecular Sieves Prepared with Liquid Crystal Templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-Based Mesoporous Organic-Inorganic Hybrid Materials. Angew. Chemie-Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef]
- Yang, P.; Gai, S.; Lin, J. Functionalized Mesoporous Silica Materials for Controlled Drug Delivery. Chem. Soc. Rev. 2012, 41, 3679–3698. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Rámila, A.; Del Real, R.P.; Pérez-Pariente, J. A New Property of MCM-41: Drug Delivery System. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Schüth, F.; Lozano, D.; Colilla, M.; Manzano, M. Engineering Mesoporous Silica Nanoparticles for Drug Delivery: Where Are We after Two Decades? Chem. Soc. Rev. 2022, 51, 5365–5451. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Our Contributions to Applications of Mesoporous Silica Nanoparticles. Acta Biomater. 2022, 137, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.R.; Lozano, D.; González, B.; Manzano, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Advances in Mesoporous Silica Nanoparticles for Targeted Stimuli-Responsive Drug Delivery: An Update. Expert Opin. Drug Deliv. 2019, 16, 415–439. [Google Scholar] [CrossRef]
- Castillo, R.R.; Colilla, M.; Vallet-Regí, M. Advances in Mesoporous Silica-Based Nanocarriers for Co-Delivery and Combination Therapy against Cancer. Expert Opin. Drug Deliv. 2017, 14, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Vivero-Escoto, J.L.; Slowing, I.I.; Lin, V.S.Y.; Trewyn, B.G. Mesoporous Silica Nanoparticles for Intracellular Controlled Drug Delivery. Small 2010, 6, 1952–1967. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous Materials for Drug Delivery. Angew. Chem.-Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M. Ordered Mesoporous Materials in the Context of Drug Delivery Systems and Bone Tissue Engineering. Chem.-A Eur. J. 2006, 12, 5934–5943. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Colilla, M.; Vallet-Regí, M. Drug Delivery from Ordered Mesoporous Matrices. Expert Opin. Drug Deliv. 2009, 6, 1383–1400. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhong, Q.; Wang, Y.; Hu, P.; Zhong, W.; Huang, C.B.; Yu, Z.Q.; Ding, C.D.; Liu, H.; Fu, J. Chemically Engineered Mesoporous Silica Nanoparticles-Based Intelligent Delivery Systems for Theranostic Applications in Multiple Cancerous/Non-Cancerous Diseases. Coord. Chem. Rev. 2022, 452, 214309. [Google Scholar] [CrossRef]
- Tella, J.O.; Adekoya, J.A.; Ajanaku, K.O. Mesoporous Silica Nanocarriers as Drug Delivery Systems for Anti-Tubercular Agents: A Review. R. Soc. Open Sci. 2022, 9, 220013. [Google Scholar] [CrossRef]
- Álvarez, E.; González, B.; Lozano, D.; Doadrio, A.L.; Colilla, M.; Izquierdo-Barba, I. Nanoantibiotics Based in Mesoporous Silica Nanoparticles: New Formulations for Bacterial Infection Treatment. Pharmaceutics 2021, 13, 2033. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 2020, 30, 1902634. [Google Scholar] [CrossRef]
- Salis, A.; Fanti, M.; Medda, L.; Nairi, V.; Cugia, F.; Piludu, M.; Sogos, V.; Monduzzi, M. Mesoporous Silica Nanoparticles Functionalized with Hyaluronic Acid and Chitosan Biopolymers. Effect of Functionalization on Cell Internalization. ACS Biomater. Sci. Eng. 2016, 2, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Carucci, C.; Scalas, N.; Porcheddu, A.; Piludu, M.; Monduzzi, M.; Salis, A. Adsorption and Release of Sulfamethizole from Mesoporous Silica Nanoparticles Functionalised with Triethylenetetramine. Int. J. Mol. Sci. 2021, 22, 7665. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.R.; Lozano, D.; Vallet-Regí, M. Mesoporous Silica Nanoparticles as Carriers for Therapeutic Biomolecules. Pharmaceutics 2020, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Colilla, M.; Vallet-Regí, M. Targeted Stimuli-Responsive Mesoporous Silica Nanoparticles for Bacterial Infection Treatment. Int. J. Mol. Sci. 2020, 21, 8605. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Ruiz-González, L.; Doadrio, J.C.; González-Calbet, J.M.; Vallet-Regí, M. Tissue Regeneration: A New Property of Mesoporous Materials. Solid State Sci. 2005, 7, 983–989. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions Able to Reproduce in Vivo Surface-structure Changes in Bioactive Glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Izquierdo-Barba, I.; Rámila, A.; Pérez-Pariente, J.; Babonneau, F.; González-Calbet, J.M. Phosphorous-Doped MCM-41 as Bioactive Material. Solid State Sci. 2005, 7, 233–237. [Google Scholar] [CrossRef]
- Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Phosphorus-Containing SBA-15 Materials as Bisphosphonate Carriers for Osteoporosis Treatment. Microporous Mesoporous Mater. 2010, 135, 51–59. [Google Scholar] [CrossRef]
- Horcajada, P.; Rámila, A.; Boulahya, K.; González-Calbet, J.; Vallet-Regí, M. Bioactivity in Ordered Mesoporous Materials. Solid State Sci. 2004, 6, 1295–1300. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Ruiz-González, L.; Izquierdo-Barba, I.; González-Calbet, J.M. Revisiting Silica Based Ordered Mesoporous Materials: Medical Applications. J. Mater. Chem. 2006, 16, 26–31. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Nanostructured Mesoporous Silica Matrices in Nanomedicine. J. Intern. Med. 2010, 267, 22–43. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Barba, I.; Vallet-Regí, M. Fascinating Properties of Bioactive Templated Glasses: A New Generation of Nanostructured Bioceramics. Solid State Sci. 2011, 13, 773–783. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Izquierdo-Barba, I.; Colilla, M. Structure and Functionalization of Mesoporous Bioceramics for Bone Tissue Regeneration and Local Drug Delivery. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012, 370, 1400–1421. [Google Scholar] [CrossRef] [PubMed]
- Salinas, A.J.; Esbrit, P. Mesoporous Bioglasses Enriched with Bioactive Agents for Bone Repair, with a Special Highlight of María Vallet-Regí’s Contribution. Pharmaceutics 2022, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Barba, I.; Vallet-Regí, M. Mesoporous Bioactive Glasses: Relevance of Their Porous Structure Compared to That of Classical Bioglasses. Biomed. Glas. 2015, 1, 140–150. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-Containing Mesoporous Bioactive Glass Scaffolds with Multifunctional Properties of Angiogenesis Capacity, Osteostimulation and Antibacterial Activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Arcos, D.; Vallet-Regí, M. Sol–Gel Silica-Based Biomaterials and Bone Tissue Regeneration. Acta Biomater. 2010, 6, 2874–2888. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Barba, I.; Arcos, D.; Sakamoto, Y.; Terasaki, O.; López-Noriega, A.; Vallet-Regí, M. High-Performance Mesoporous Bioceramics Mimicking Bone Mineralization. Chem. Mater. 2008, 20, 3191–3198. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Salinas, A.J. Mesoporous Bioactive Glasses for Regenerative Medicine. Mater. Today Bio 2021, 11, 100121. [Google Scholar] [CrossRef] [PubMed]
- Arcos, D.; Izquierdo-Barba, I.; Vallet-Regí, M. Promising Trends of Bioceramics in the Biomaterials Field. J. Mater. Sci. Mater. Med. 2009, 20, 447–455. [Google Scholar] [CrossRef]
- Yan, X.; Yu, C.; Zhou, X.; Tang, J.; Zhao, D. Highly Ordered Mesoporous Bioactive Glasses with Superior in Vitro Bone-Forming Bioactivities. Angew. Chemie-Int. Ed. 2004, 43, 5980–5984. [Google Scholar] [CrossRef] [PubMed]
- López-Noriega, A.; Arcos, D.; Izquierdo-Barba, I.; Sakamoto, Y.; Terasaki, O.; Vallet-Regí, M. Ordered Mesoporous Bioactive Glasses for Bone Tissue Regeneration. Chem. Mater. 2006, 18, 3137–3144. [Google Scholar] [CrossRef]
- Brinker, C.J.; Lu, Y.; Sellinger, A.; Fan, H. Evaporation-Induced Self-Assembly: Nanostructures Made Easy. Adv. Mater. 1999, 11, 579–585. [Google Scholar] [CrossRef]
- Yan, X.X.; Deng, H.X.; Huang, X.H.; Lu, G.Q.; Qiao, S.Z.; Zhao, D.Y.; Yu, C.Z. Mesoporous Bioactive Glasses. I. Synthesis and Structural Characterization. J. Non. Cryst. Solids 2005, 351, 3209–3217. [Google Scholar] [CrossRef]
- Yan, X.; Huang, X.; Yu, C.; Deng, H.; Wang, Y.; Zhang, Z.; Qiao, S.; Lu, G.; Zhao, D. The In-Vitro Bioactivity of Mesoporous Bioactive Glasses. Biomaterials 2006, 27, 3396–3403. [Google Scholar] [CrossRef] [PubMed]
- Elgayar, I.; Aliev, A.E.; Boccaccini, A.R.; Hill, R.G. Structural Analysis of Bioactive Glasses. J. Non. Cryst. Solids 2005, 351, 173–183. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Salinas, A.J.; Ramírez-Castellanos, J.; González-Calbet, J.M. Nanostructure of Bioactive Sol-Gel Glasses and Organic-Inorganic Hybrids. Chem. Mater. 2005, 17, 1874–1879. [Google Scholar] [CrossRef]
- Tilocca, A. Structural Models of Bioactive Glasses from Molecular Dynamics Simulations. Proc. R. Soc. A Math. Phys. Eng. Sci. 2009, 465, 1003–1027. [Google Scholar] [CrossRef]
- Leonova, E.; Izquierdo-Barba, I.; Arcos, D.; López-Noriega, A.; Hedin, N.; Vallet-Regí, M.; Edén, M. Multinuclear Solid-State NMR Studies of Ordered Mesoporous Bioactive Glasses. J. Phys. Chem. C 2008, 112, 5552–5562. [Google Scholar] [CrossRef]
- Mathew, R.; Turdean-Ionescu, C.; Yu, Y.; Stevensson, B.; Izquierdo-Barba, I.; García, A.; Arcos, D.; Vallet-Regí, M.; Edén, M. Proton Environments in Biomimetic Calcium Phosphates Formed from Mesoporous Bioactive CaO-SiO2-P2O5 Glasses in Vitro: Insights from Solid-State NMR. J. Phys. Chem. C 2017, 121, 13223–13238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, R.; Turdean-Ionescu, C.; Stevensson, B.; Izquierdo-Barba, I.; García, A.; Arcos, D.; Vallet-Regí, M.; Edén, M. Direct Probing of the Phosphate-Ion Distribution in Bioactive Silicate Glasses by Solid-State NMR: Evidence for Transitions between Random/Clustered Scenarios. Chem. Mater. 2013, 25, 1877–1885. [Google Scholar] [CrossRef]
- Gunawidjaja, P.N.; Mathew, R.; Lo, A.Y.H.; Izquierdo-Barba, I.; García, A.; Arcos, D.; Vallet-Regí, M.; Edén, M. Local Structures of Mesoporous Bioactive Glasses and Their Surface Alterations in Vitro: Inferences from Solid-State Nuclear Magnetic Resonance. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012, 370, 1376–1399. [Google Scholar] [CrossRef] [Green Version]
- García, A.; Cicuéndez, M.; Izquierdo-Barba, I.; Arcos, D.; Vallet-Regí, M. Essential Role of Calcium Phosphate Heterogeneities in 2D-Hexagonal and 3D-Cubic SiO2-CaO-P2O5 Mesoporous Bioactive Glasses. Chem. Mater. 2009, 21, 5474–5484. [Google Scholar] [CrossRef]
- Misra, S.K.; Mohn, D.; Brunner, T.J.; Stark, W.J.; Philip, S.E.; Roy, I.; Salih, V.; Knowles, J.C.; Boccaccini, A.R. Comparison of Nanoscale and Microscale Bioactive Glass on the Properties of P(3HB)/Bioglass® Composites. Biomaterials 2008, 29, 1750–1761. [Google Scholar] [CrossRef]
- Liang, Q.; Hu, Q.; Miao, G.; Yuan, B.; Chen, X. A Facile Synthesis of Novel Mesoporous Bioactive Glass Nanoparticles with Various Morphologies and Tunable Mesostructure by Sacrificial Liquid Template Method. Mater. Lett. 2015, 148, 45–49. [Google Scholar] [CrossRef]
- Hong, B.-J.; Hsiao, C.-W.; Bakare, F.; Sun, J.-T.; Shih, S.-J. Effect of Acetic Acid Concentration on Pore Structure for Mesoporous Bioactive Glass during Spray Pyrolysis. Materials 2018, 11, 963. [Google Scholar] [CrossRef] [Green Version]
- Peng, T.-Y.; Tsai, P.-Y.; Chen, M.-S.; Mine, Y.; Wu, S.-H.; Chen, C.-Y.; Lin, D.-J.; Lin, C.-K. Mesoporous Properties of Bioactive Glass Synthesized by Spray Pyrolysis with Various Polyethylene Glycol and Acid Additions. Polymers 2021, 13, 618. [Google Scholar] [CrossRef]
- Shi, Q.; Wang, J.; Zhang, J.; Fan, J.; Stucky, G.D. Rapid-Setting, Mesoporous, Bioactive Glass Cements That Induce Accelerated in Vitro Apatite Formation. Adv. Mater. 2006, 18, 1038–1042. [Google Scholar] [CrossRef]
- De Cremer, K.; Braem, A.; Gerits, E.; De Brucker, K.; Vandamme, K.; Martens, J.A.; Michiels, J.; Vleugels, J.; Cammue, B.P.A.; Thevissen, K. Controlled Release of Chlorhexidine from a Mesoporous Silica-Containing Macroporous Titanium Dental Implant Prevents Microbial Biofilm Formation. Eur. Cells Mater. 2017, 33, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Fiorilli, S.; Vitale-Brovarone, C. Composite Biomaterials Based on Sol-Gel Mesoporous Silicate Glasses: A Review. Bioengineering 2017, 4, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baino, F.; Fiume, E. 3D Printing of Hierarchical Scaffolds Based on Mesoporous Bioactive Glasses (MBGs)—Fundamentals and Applications. Materials 2020, 13, 1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Majumdar, S.; Krishnamurthy, S. Bioactive Glass: A Multifunctional Delivery System. J. Control. Release 2021, 335, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.H.; Steffi, C.; Shi, Z.; Wang, W. Development of Mesoporous Bioactive Glass Nanoparticles and Its Use in Bone Tissue Engineering. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2018, 106, 2878–2887. [Google Scholar] [CrossRef] [PubMed]
- Lalzawmliana, V.; Anand, A.; Roy, M.; Kundu, B.; Nandi, S.K. Mesoporous Bioactive Glasses for Bone Healing and Biomolecules Delivery. Mater. Sci. Eng. C 2020, 106, 110180. [Google Scholar] [CrossRef]
- Zheng, K.; Kang, J.; Rutkowski, B.; Gawȩda, M.; Zhang, J.; Wang, Y.; Founier, N.; Sitarz, M.; Taccardi, N.; Boccaccini, A.R. Toward Highly Dispersed Mesoporous Bioactive Glass Nanoparticles with High Cu Concentration Using Cu/Ascorbic Acid Complex as Precursor. Front. Chem. 2019, 7, 497. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Holguín, J.; Sánchez-Salcedo, S.; Cicuéndez, M.; Vallet-Regí, M.; Salinas, A.J. Cu-Doped Hollow Bioactive Glass Nanoparticles for Bone Infection Treatment. Pharmaceutics 2022, 14, 845. [Google Scholar] [CrossRef]
- Wu, C.; Fan, W.; Chang, J. Functional Mesoporous Bioactive Glass Nanospheres: Synthesis, High Loading Efficiency, Controllable Delivery of Doxorubicin and Inhibitory Effect on Bone Cancer Cells. J. Mater. Chem. B 2013, 1, 2710–2718. [Google Scholar] [CrossRef]
- Dey, N.; Santhiya, D.; Das, A. Bio-Inspired Synthesis of Hollow Mesoporous Bioactive Glass Nanoparticles Using Calcium Carbonate as Solid Template. ChemistrySelect 2022, 7, e202200392. [Google Scholar] [CrossRef]
- Lin, H.-N.; Peng, T.-Y.; Kung, Y.-R.; Chiou, Y.-J.; Chang, W.-M.; Wu, S.-H.; Mine, Y.; Chen, C.-Y.; Lin, C.-K. Effects of the Methyl Methacrylate Addition, Polymerization Temperature and Time on the MBG@PMMA Core-Shell Structure and Its Application as Addition in Electrospun Composite Fiber Bioscaffold. Ceram. Int. 2022. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. Mesoporous Bioactive Glasses: Structure Characteristics, Drug/Growth Factor Delivery and Bone Regeneration Application. Interface Focus 2012, 2, 292–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salinas, A.J.; Shruti, S.; Malavasi, G.; Menabue, L.; Vallet-Regí, M. Substitutions of Cerium, Gallium and Zinc in Ordered Mesoporous Bioactive Glasses. Acta Biomater. 2011, 7, 3452–3458. [Google Scholar] [CrossRef] [PubMed]
- Shruti, S.; Salinas, A.J.; Lusvardi, G.; Malavasi, G.; Menabue, L.; Vallet-Regí, M. Mesoporous Bioactive Scaffolds Prepared with Cerium-, Gallium- and Zinc-Containing Glasses. Acta Biomater. 2013, 9, 4836–4844. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Kim, H.W. Iron Ions-Releasing Mesoporous Bioactive Glass Ultrasmall Nanoparticles Designed as Ferroptosis-Based Bone Cancer Nanotherapeutics: Ultrasonic-Coupled Sol–Gel Synthesis, Properties and Iron Ions Release. Mater. Lett. 2021, 294, 129759. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. Multifunctional Mesoporous Bioactive Glasses for Effective Delivery of Therapeutic Ions and Drug/Growth Factors. J. Control. Release 2014, 193, 282–295. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A Review of the Biological Response to Ionic Dissolution Products from Bioactive Glasses and Glass-Ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Zhu, H.; Zheng, K.; Boccaccini, A.R. Multi-Functional Silica-Based Mesoporous Materials for Simultaneous Delivery of Biologically Active Ions and Therapeutic Biomolecules. Acta Biomater. 2021, 129, 1–17. [Google Scholar] [CrossRef]
- Sharifi, E.; Bigham, A.; Yousefiasl, S.; Trovato, M.; Ghomi, M.; Esmaeili, Y.; Samadi, P.; Zarrabi, A.; Ashrafizadeh, M.; Sharifi, S.; et al. Mesoporous Bioactive Glasses in Cancer Diagnosis and Therapy: Stimuli-Responsive, Toxicity, Immunogenicity, and Clinical Translation. Adv. Sci. 2022, 9, 2102678. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Lozano, D.; González, B.; Izquierdo-Barba, I. Biomaterials against Bone Infection. Adv. Healthc. Mater. 2020, 9, 2000310. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Colilla, M.; Vallet-Regí, M. Zwitterionic Ceramics for Biomedical Applications. Acta Biomater. 2016, 40, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D Bioactive Composite Scaffolds for Bone Tissue Engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollister, S.J. Scaffold Design and Manufacturing: From Concept to Clinic. Adv. Mater. 2009, 21, 3330–3342. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng.-Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salinas, A.J.; Esbrit, P.; Vallet-Regí, M. A Tissue Engineering Approach Based on the Use of Bioceramics for Bone Repair. Biomater. Sci. 2013, 1, 40–51. [Google Scholar] [CrossRef]
- García, A.; Cabañas, M.V.; Peña, J.; Sánchez-Salcedo, S. Design of 3D Scaffolds for Hard Tissue Engineering: From Apatites to Silicon Mesoporous Materials. Pharmaceutics 2021, 13, 1981. [Google Scholar] [CrossRef]
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired Structural Materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef]
- Koester, K.J.; Ager, J.W.; Ritchie, R.O. The True Toughness of Human Cortical Bone Measured with Realistically Short Cracks. Nat. Mater. 2008, 7, 672–677. [Google Scholar] [CrossRef]
- Yun, H.S.; Kim, S.E.; Hyun, Y.T.; Heo, S.J.; Shin, J.W. Hierarchically Mesoporous-Macroporous Bioactive Glasses Scaffolds for Bone Tissue Regeneration. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2008, 87, 374–380. [Google Scholar] [CrossRef]
- Lian, R.; Xie, P.; Xiao, L.; Iqbal, Z.; Zhang, S.; Kohn, J.; Qu, X.; Liu, C.; Li, Y. Rational Design and Fabrication of Biomimetic Hierarchical Scaffolds With Bone-Matchable Strength for Bone Regeneration. Front. Mater. 2021, 7, 622669. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent Advances in Biomaterials for 3D Scaffolds: A Review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Miron, R.; Sculean, A.; Kaskel, S.; Doert, T.; Schulze, R.; Zhang, Y. Proliferation, Differentiation and Gene Expression of Osteoblasts in Boron-Containing Associated with Dexamethasone Deliver from Mesoporous Bioactive Glass Scaffolds. Biomaterials 2011, 32, 7068–7078. [Google Scholar] [CrossRef]
- Tang, W.; Lin, D.; Yu, Y.; Niu, H.; Guo, H.; Yuan, Y.; Liu, C. Bioinspired Trimodal Macro/Micro/Nano-Porous Scaffolds Loading RhBMP-2 for Complete Regeneration of Critical Size Bone Defect. Acta Biomater. 2016, 32, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Ciraldo, F.E.; Arango-Ospina, M.; Goldmann, W.H.; Beltrán, A.M.; Detsch, R.; Gruenewald, A.; Roether, J.A.; Boccaccini, A.R. Fabrication and Characterization of Ag- and Ga-Doped Mesoporous Glass-Coated Scaffolds Based on Natural Marine Sponges with Improved Mechanical Properties. J. Biomed. Mater. Res.-Part A 2021, 109, 1309–1327. [Google Scholar] [CrossRef]
- Wang, X.; Ruan, J.M.; Chen, Q.Y. Effects of Surfactants on the Microstructure of Porous Ceramic Scaffolds Fabricated by Foaming for Bone Tissue Engineering. Mater. Res. Bull. 2009, 44, 1275–1279. [Google Scholar] [CrossRef]
- Zadpoor, A.A.; Malda, J. Additive Manufacturing of Biomaterials, Tissues, and Organs. Ann. Biomed. Eng. 2017, 45, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Liaw, C.Y.; Guvendiren, M. Current and Emerging Applications of 3D Printing in Medicine. Biofabrication 2017, 9, 24102. [Google Scholar] [CrossRef]
- Mirkhalaf, M.; Men, Y.; Wang, R.; No, Y.; Zreiqat, H. Personalized 3D Printed Bone Scaffolds: A Review. Acta Biomater. 2022. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-Dimensional (3D) Printed Scaffold and Material Selection for Bone Repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef]
- García, A.; Izquierdo-Barba, I.; Colilla, M.; De Laorden, C.L.; Vallet-Regí, M. Preparation of 3-D Scaffolds in the SiO2-P2O5 System with Tailored Hierarchical Meso-Macroporosity. Acta Biomater. 2011, 7, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Luo, Y.; Cuniberti, G.; Xiao, Y.; Gelinsky, M. Three-Dimensional Printing of Hierarchical and Tough Mesoporous Bioactive Glass Scaffolds with a Controllable Pore Architecture, Excellent Mechanical Strength and Mineralization Ability. Acta Biomater. 2011, 7, 2644–2650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhao, S.; Zhu, Y.; Huang, Y.; Zhu, M.; Tao, C.; Zhang, C. Three-Dimensional Printing of Strontium-Containing Mesoporous Bioactive Glass Scaffolds for Bone Regeneration. Acta Biomater. 2014, 10, 2269–2281. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, J.; Zhu, M.; Zhang, Y.; Liu, Z.; Tao, C.; Zhu, Y.; Zhang, C. Three-Dimensional Printed Strontium-Containing Mesoporous Bioactive Glass Scaffolds for Repairing Rat Critical-Sized Calvarial Defects. Acta Biomater. 2015, 12, 270–280. [Google Scholar] [CrossRef]
- Qi, X.; Pei, P.; Zhu, M.; Du, X.; Xin, C.; Zhao, S.; Li, X.; Zhu, Y. Three Dimensional Printing of Calcium Sulfate and Mesoporous Bioactive Glass Scaffolds for Improving Bone Regeneration in Vitro and in Vivo. Sci. Rep. 2017, 7, 42556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Wu, C.; Lode, A.; Gelinsky, M. Hierarchical Mesoporous Bioactive Glass/Alginate Composite Scaffolds Fabricated by Three-Dimensional Plotting for Bone Tissue Engineering. Biofabrication 2013, 5, 15005. [Google Scholar] [CrossRef]
- Yun, H.S.; Kim, S.E.; Park, E.K. Bioactive Glass-Poly (ε-Caprolactone) Composite Scaffolds with 3 Dimensionally Hierarchical Pore Networks. Mater. Sci. Eng. C 2011, 31, 198–205. [Google Scholar] [CrossRef]
- Min, Z.; Kun, L.; Yufang, Z.; Jianhua, Z.; Xiaojian, Y. 3D-Printed Hierarchical Scaffold for Localized Isoniazid/Rifampin Drug Delivery and Osteoarticular Tuberculosis Therapy. Acta Biomater. 2015, 16, 145–155. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; García, A.; González-Jiménez, A.; Vallet-Regí, M. Antibacterial Effect of 3D Printed Mesoporous Bioactive Glass Scaffolds Doped with Metallic Silver Nanoparticles. Acta Biomater. 2022. [Google Scholar] [CrossRef] [PubMed]
- Cicuéndez, M.; Doadrio, J.C.; Hernández, A.; Portolés, M.T.; Izquierdo-Barba, I.; Vallet-Regí, M. Multifunctional PH Sensitive 3D Scaffolds for Treatment and Prevention of Bone Infection. Acta Biomater. 2018, 65, 450–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicuéndez, M.; Portolés, P.; Montes-Casado, M.; Izquierdo-Barba, I.; Vallet-Regí, M.; Portolés, M.T. Effects of 3D Nanocomposite Bioceramic Scaffolds on the Immune Response. J. Mater. Chem. B 2014, 2, 3469–3479. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lin, D.; Wang, M.; Mao, R.; Zhao, H.; Huang, X.; GF Shen, S. Seamless Route of Self-Assembly and 3D Printing to Fabricate Hierarchical Mesoporous Bioactive Glass Scaffold for Customized Bone Regeneration with Enhanced Efficacy. Chem. Eng. J. 2022, 446, 137270. [Google Scholar] [CrossRef]
- Heras, C.; Sanchez-Salcedo, S.; Lozano, D.; Peña, J.; Esbrit, P.; Vallet-Regí, M.; Salinas, A.J. Osteostatin Potentiates the Bioactivity of Mesoporous Glass Scaffolds Containing Zn2+ Ions in Human Mesenchymal Stem Cells. Acta Biomater. 2019, 89, 359–371. [Google Scholar] [CrossRef]
- Gómez-Cerezo, N.; Sánchez-Salcedo, S.; Izquierdo-Barba, I.; Arcos, D.; Vallet-Regí, M. In Vitro Colonization of Stratified Bioactive Scaffolds by Pre-Osteoblast Cells. Acta Biomater. 2016, 44, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cerezo, N.; Casarrubios, L.; Saiz-Pardo, M.; Ortega, L.; de Pablo, D.; Díaz-Güemes, I.; Fernández-Tomé, B.; Enciso, S.; Sánchez-Margallo, F.M.; Portolés, M.T.; et al. Mesoporous Bioactive Glass/ε-Polycaprolactone Scaffolds Promote Bone Regeneration in Osteoporotic Sheep. Acta Biomater. 2019, 90, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cerezo, M.N.; Peña, J.; Ivanovski, S.; Arcos, D.; Vallet-Regí, M.; Vaquette, C. Multiscale Porosity in Mesoporous Bioglass 3D-Printed Scaffolds for Bone Regeneration. Mater. Sci. Eng. C 2021, 120, 111706. [Google Scholar] [CrossRef]
- Lozano, D.; Gil-Albarova, J.; Heras, C.; Sánchez-Salcedo, S.; Gómez-Palacio, V.E.; Gómez-Blasco, A.; Doadrio, J.C.; Vallet-Regí, M.; Salinas, A.J. ZnO-Mesoporous Glass Scaffolds Loaded with Osteostatin and Mesenchymal Cells Improve Bone Healing in a Rabbit Bone Defect. J. Mater. Sci. Mater. Med. 2020, 31, 100. [Google Scholar] [CrossRef] [PubMed]
- García-Alvarez, R.; Izquierdo-Barba, I.; Vallet-Regí, M. 3D Scaffold with Effective Multidrug Sequential Release against Bacteria Biofilm. Acta Biomater. 2017, 49, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Holguín, J.; López-Hidalgo, A.; Sánchez-Salcedo, S.; Peña, J.; Vallet-Regí, M.; Salinas, A.J. Strontium-Modified Scaffolds Based on Mesoporous Bioactive Glasses/Polyvinyl Alcohol Composites for Bone Regeneration. Materials 2020, 13, 5526. [Google Scholar] [CrossRef]
- Saberi, A.; Behnamghader, A.; Aghabarari, B.; Yousefi, A.; Majda, D.; Huerta, M.V.M.; Mozafari, M. 3D Direct Printing of Composite Bone Scaffolds Containing Polylactic Acid and Spray Dried Mesoporous Bioactive Glass-Ceramic Microparticles. Int. J. Biol. Macromol. 2022, 207, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Du, X.; Zhu, M.; Tian, Z.; Wei, D.; Zhu, Y. 3D Printing of Layered Mesoporous Bioactive Glass/Sodium Alginatesodium Alginate Scaffolds with Controllable Dual-Drug Release Behaviors. Biomed. Mater. 2019, 14, 65011. [Google Scholar] [CrossRef]
- Du, X.; Wei, D.; Huang, L.; Zhu, M.; Zhang, Y.; Zhu, Y. 3D Printing of Mesoporous Bioactive Glass/Silk Fibroin Composite Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. C 2019, 103, 109731. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for Bone Tissue Regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Montalbano, G.; Borciani, G.; Cerqueni, G.; Licini, C.; Banche-Niclot, F.; Janner, D.; Sola, S.; Fiorilli, S.; Mattioli-Belmonte, M.; Ciapetti, G.; et al. Collagen Hybrid Formulations for the 3d Printing of Nanostructured Bone Scaffolds: An Optimized Genipin-Crosslinking Strategy. Nanomaterials 2020, 10, 1681. [Google Scholar] [CrossRef]
- Gaihre, B.; Uswatta, S.; Jayasuriya, A. Reconstruction of Craniomaxillofacial Bone Defects Using Tissue-Engineering Strategies with Injectable and Non-Injectable Scaffolds. J. Funct. Biomater. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreifke, M.B.; Ebraheim, N.A.; Jayasuriya, A.C. Investigation of Potential Injectable Polymeric Biomaterials for Bone Regeneration. J. Biomed. Mater. Res.-Part A 2013, 101A, 2436–2447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimatteo, R.; Darling, N.J.; Segura, T. In Situ Forming Injectable Hydrogels for Drug Delivery and Wound Repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef]
- Giannini, G.; Mauro, V.; Agostino, T.; Gianfranco, B. Use of Autologous Fibrin-Platelet Glue and Bone Fragments in Maxillofacial Surgery. Transfus. Apher. Sci. 2004, 30, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A Review of Fibrin and Fibrin Composites for Bone Tissue Engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Fan, L.; Zhang, F.M.; Jiang, Y.; Cai, M.; Dai, C.; Luo, Y.A.; Tu, L.J.; Zhou, Z.N.; Li, X.J.; et al. Hybrid Gelatin/Oxidized Chondroitin Sulfate Hydrogels Incorporating Bioactive Glass Nanoparticles with Enhanced Mechanical Properties, Mineralization, and Osteogenic Differentiation. Bioact. Mater. 2021, 6, 890–904. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, X.; Jin, A.; Wang, M.; Wang, Z.; Huang, X.; Dai, J.; Wang, X.; Lin, D.; Shen, S.G. Reducing Relapse and Accelerating Osteogenesis in Rapid Maxillary Expansion Using an Injectable Mesoporous Bioactive Glass/Fibrin Glue Composite Hydrogel. Bioact. Mater. 2022, 18, 507–525. [Google Scholar] [CrossRef] [PubMed]
- Fiorilli, S.; Molino, G.; Pontremoli, C.; Iviglia, G.; Torre, E.; Cassinelli, C.; Morra, M.; Vitale-Brovarone, C. The Incorporation of Strontium to Improve Bone-Regeneration Ability of Mesoporous Bioactive Glasses. Materials 2018, 11, 678. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Mandakhbayar, N.; El-Fiqi, A.; Kim, H.W. Intracellular Co-Delivery of Sr Ion and Phenamil Drug through Mesoporous Bioglass Nanocarriers Synergizes BMP Signaling and Tissue Mineralization. Acta Biomater. 2017, 60, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Bari, A.; Bloise, N.; Fiorilli, S.; Novajra, G.; Vallet-Regí, M.; Bruni, G.; Torres-Pardo, A.; González-Calbet, J.M.; Visai, L.; Vitale-Brovarone, C. Copper-Containing Mesoporous Bioactive Glass Nanoparticles as Multifunctional Agent for Bone Regeneration. Acta Biomater. 2017, 55, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Paterson, T.E.; Bari, A.; Bullock, A.J.; Turner, R.; Montalbano, G.; Fiorilli, S.; Vitale-Brovarone, C.; MacNeil, S.; Shepherd, J. Multifunctional Copper-Containing Mesoporous Glass Nanoparticles as Antibacterial and Proangiogenic Agents for Chronic Wounds. Front. Bioeng. Biotechnol. 2020, 8, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Fiqi, A.; Mandakhbayar, N.; Jo, S.B.; Knowles, J.C.; Lee, J.H.; Kim, H.W. Nanotherapeutics for Regeneration of Degenerated Tissue Infected by Bacteria through the Multiple Delivery of Bioactive Ions and Growth Factor with Antibacterial/Angiogenic and Osteogenic/Odontogenic Capacity. Bioact. Mater. 2021, 6, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Balasubramanian, P.; Paterson, T.E.; Stein, R.; MacNeil, S.; Fiorilli, S.; Vitale-Brovarone, C.; Shepherd, J.; Boccaccini, A.R. Ag Modified Mesoporous Bioactive Glass Nanoparticles for Enhanced Antibacterial Activity in 3D Infected Skin Model. Mater. Sci. Eng. C 2019, 103, 109764. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Torre, E.; Bari, A.; Taccardi, N.; Cassinelli, C.; Morra, M.; Fiorilli, S.; Vitale-Brovarone, C.; Iviglia, G.; Boccaccini, A.R. Antioxidant Mesoporous Ce-Doped Bioactive Glass Nanoparticles with Anti-Inflammatory and pro-Osteogenic Activities. Mater. Today Bio 2020, 5, 100041. [Google Scholar] [CrossRef]
- Romero-Sánchez, L.B.; Marí-Beffa, M.; Carrillo, P.; Medina, M.Á.; Díaz-Cuenca, A. Copper-Containing Mesoporous Bioactive Glass Promotes Angiogenesis in an in Vivo Zebrafish Model. Acta Biomater. 2018, 68, 272–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wei, L.; Chang, J.; Miron, R.J.; Shi, B.; Yi, S.; Wu, C. Strontium-Incorporated Mesoporous Bioactive Glass Scaffolds Stimulating in Vitro Proliferation and Differentiation of Bone Marrow Stromal Cells and in Vivo Regeneration of Osteoporotic Bone Defects. J. Mater. Chem. B 2013, 1, 5711–5722. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, X.; Zhang, Y.; Wang, A.; Zhu, W.; Xu, M.; Zhuang, S. Rapid Hemostasis and High Bioactivity Cerium-Containing Mesoporous Bioglass for Hemostatic Materials. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2022, 110, 1255–1264. [Google Scholar] [CrossRef]
- Neščáková, Z.; Zheng, K.; Liverani, L.; Nawaz, Q.; Galusková, D.; Kaňková, H.; Michálek, M.; Galusek, D.; Boccaccini, A.R. Multifunctional Zinc Ion Doped Sol-Gel Derived Mesoporous Bioactive Glass Nanoparticles for Biomedical Applications. Bioact. Mater. 2019, 4, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Heras, C.; Jiménez-Holguín, J.; Doadrio, A.L.; Vallet-Regí, M.; Sánchez-Salcedo, S.; Salinas, A.J. Multifunctional Antibiotic- and Zinc-Containing Mesoporous Bioactive Glass Scaffolds to Fight Bone Infection. Acta Biomater. 2020, 114, 395–406. [Google Scholar] [CrossRef]
- Lee, J.H.; El-Fiqi, A.; Mandakhbayar, N.; Lee, H.H.; Kim, H.W. Drug/Ion Co-Delivery Multi-Functional Nanocarrier to Regenerate Infected Tissue Defect. Biomaterials 2017, 142, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A Review of the Biomaterials Technologies for Infection-Resistant Surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef] [PubMed]
- Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. The Role of Zwitterionic Materials in the Fight against Proteins and Bacteria. Medicines 2018, 5, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, G.; Zhang, Z.; Chen, S.; Bryers, J.D.; Jiang, S. Inhibition of Bacterial Adhesion and Biofilm Formation on Zwitterionic Surfaces. Biomaterials 2007, 28, 4192–4199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.; Cao, Z. Ultralow-Fouling, Functionalizable, and Hydrolyzable Zwitterionic Materials and Their Derivatives for Biological Applications. Adv. Mater. 2010, 22, 920–932. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface Hydration: Principles and Applications toward Low-Fouling/Nonfouling Biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef] [Green Version]
- Rosen, J.E.; Gu, F.X. Surface Functionalization of Silica Nanoparticles with Cysteine: A Low-Fouling Zwitterionic Surface. Langmuir 2011, 27, 10507–10513. [Google Scholar] [CrossRef]
- Villegas, M.F.; Garcia-Uriostegui, L.; Rodríguez, O.; Izquierdo-Barba, I.; Salinas, A.J.; Toriz, G.; Vallet-Regí, M.; Delgado, E. Lysine-Grafted MCM-41 Silica as an Antibacterial Biomaterial. Bioengineering 2017, 4, 80. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Barba, I.; Sánchez-Salcedo, S.; Colilla, M.; Feito, M.J.; Ramírez-Santillán, C.; Portolés, M.T.; Vallet-Regí, M. Inhibition of Bacterial Adhesion on Biocompatible Zwitterionic SBA-15 Mesoporous Materials. Acta Biomater. 2011, 7, 2977–2985. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Ruan, J.; Li, Y.; Terasaki, O.; Che, S. Synthesis and Characterization of the Amphoteric Amino Acid Bifunctional Mesoporous Silica. Chem. Mater. 2007, 19, 2860–2867. [Google Scholar] [CrossRef]
- Colilla, M.; Izquierdo-Barba, I.; Sánchez-Salcedo, S.; Fierro, J.L.G.; Hueso, J.L.; Vallet-Regí, M. Synthesis and Characterization of Zwitterionic SBA-15 Nanostructured Materials. Chem. Mater. 2010, 22, 6459–6466. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Design and Preparation of Biocompatible Zwitterionic Hydroxyapatite. J. Mater. Chem. B 2013, 1, 1595–1606. [Google Scholar] [CrossRef]
- Rodríguez-Palomo, A.; Monopoli, D.; Afonso, H.; Izquierdo-Barba, I.; Vallet-Regí, M. Surface Zwitterionization of Customized 3D Ti6Al4V Scaffolds: A Promising Alternative to Eradicate Bone Infection. J. Mater. Chem. B 2016, 4, 4356–4365. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Preventing Bacterial Adhesion on Scaffolds for Bone Tissue Engineering. Int. J. Bioprinting 2016, 2, 20–34. [Google Scholar] [CrossRef]
- Encinas, N.; Angulo, M.; Astorga, C.; Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Mixed-Charge Pseudo-Zwitterionic Mesoporous Silica Nanoparticles with Low-Fouling and Reduced Cell Uptake Properties. Acta Biomater. 2019, 84, 317–327. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; García, A.; Vallet-Regí, M. Prevention of Bacterial Adhesion to Zwitterionic Biocompatible Mesoporous Glasses. Acta Biomater. 2017, 57, 472–486. [Google Scholar] [CrossRef]
- Pontremoli, C.; Izquierdo-Barba, I.; Montalbano, G.; Vallet-Regí, M.; Vitale-Brovarone, C.; Fiorilli, S. Strontium-Releasing Mesoporous Bioactive Glasses with Anti-Adhesive Zwitterionic Surface as Advanced Biomaterials for Bone Tissue Regeneration. J. Colloid Interface Sci. 2020, 563, 92–103. [Google Scholar] [CrossRef]
- Naruphontjirakul, P.; Tsigkou, O.; Li, S.; Porter, A.E.; Jones, J.R. Human Mesenchymal Stem Cells Differentiate into an Osteogenic Lineage in Presence of Strontium Containing Bioactive Glass Nanoparticles. Acta Biomater. 2019, 90, 373–392. [Google Scholar] [CrossRef] [PubMed]
- El-Fiqi, A.; Kim, J.H.; Kim, H.W. Osteoinductive Fibrous Scaffolds of Biopolymer/Mesoporous Bioactive Glass Nanocarriers with Excellent Bioactivity and Long-Term Delivery of Osteogenic Drug. ACS Appl. Mater. Interfaces 2015, 7, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Reginster, J.Y.L. Ipriflavone: Pharmacological Properties and Usefulness in Postmenopausal Osteoporosis. Bone Miner. 1993, 23, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, M.G. Pharmacokinetics of Ipriflavone, an Isoflavone Derivative, after Intravenous and Oral Administration to Rats: Hepatic and Intestinal First-Pass Effects. Life Sci. 2002, 70, 1299–1315. [Google Scholar] [CrossRef]
- O’Connell, M.B. Pharmacokinetic and Pharmacologic Variation Between Different Estrogen Products. J. Clin. Pharmacol. 1995, 35, 18S–24S. [Google Scholar] [CrossRef] [PubMed]
- López-Noriega, A.; Arcos, D.; Vallet-Regí, M. Functionalizing Mesoporous Bioglasses for Long-Term Anti-Osteoporotic Drug Delivery. Chem.-Eur. J. 2010, 16, 10879–10886. [Google Scholar] [CrossRef]
- Casarrubios, L.; Gómez-Cerezo, N.; Feito, M.J.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Incorporation and Effects of Mesoporous SiO2-CaO Nanospheres Loaded with Ipriflavone on Osteoblast/Osteoclast Cocultures. Eur. J. Pharm. Biopharm. 2018, 133, 258–268. [Google Scholar] [CrossRef] [Green Version]
- Mathew, R.; Gunawidjaja, P.N.; Izquierdo-Barba, I.; Jansson, K.; García, A.; Arcos, D.; Vallet-Regí, M.; Edén, M. Solid-State 31P and 1H NMR Investigations of Amorphous and Crystalline Calcium Phosphates Grown Biomimetically from a Mesoporous Bioactive Glass. J. Phys. Chem. C 2011, 115, 20572–20582. [Google Scholar] [CrossRef]
- Gómez-Cerezo, N.; Izquierdo-Barba, I.; Arcos, D.; Vallet-Regí, M. Tailoring the Biological Response of Mesoporous Bioactive Materials. J. Mater. Chem. B 2015, 3, 3810–3819. [Google Scholar] [CrossRef]
- Casarrubios, L.; Polo-Montalvo, A.; Serrano, M.C.; Feito, M.J.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Effects of Ipriflavone-Loaded Mesoporous Nanospheres on the Differentiation of Endothelial Progenitor Cells and Their Modulation by Macrophages. Nanomaterials 2021, 11, 1102. [Google Scholar] [CrossRef]
- Wang, D.; Steffi, C.; Wang, Z.; Kong, C.H.; Lim, P.N.; Shi, Z.; Thian, E.S.; Wang, W. Beta-Cyclodextrin Modified Mesoporous Bioactive Glass Nanoparticles/Silk Fibroin Hybrid Nanofibers as an Implantable Estradiol Delivery System for the Potential Treatment of Osteoporosis. Nanoscale 2018, 10, 18341–18353. [Google Scholar] [CrossRef]
- Ezra, A.; Golomb, G. Administration Routes and Delivery Systems of Bisphosphonates for the Treatment of Bone Resorption. Adv. Drug Deliv. Rev. 2000, 42, 175–195. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, D.; Weng, W.; Huang, Q.; Zhang, X.; Wen, J.; Wu, J.; Jiang, X. Alendronate Delivery on Amino Modified Mesoporous Bioactive Glass Scaffolds to Enhance Bone Regeneration in Osteoporosis Rats. Artif. Cells Nanomed. Biotechnol. 2018, 46, 171–181. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhao, F.; Chen, X.; Luo, M.; Yang, Z.; Cao, X.; Miao, G.; Chen, D.; Chen, X. Local Delivery of FTY720 in Mesoporous Bioactive Glass Improves Bone Regeneration by Synergistically Immunomodulating Osteogenesis and Osteoclastogenesis. J. Mater. Chem. B 2020, 8, 6148–6158. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Nie, Y.; Cao, D.P.; Xue, Y.Y.; Wang, J.S.; Zhao, L.; Rahman, K.; Zhang, Q.Y.; Qin, L.P. Potential Antiosteoporotic Agents from Plants: A Comprehensive Review. Evid.-Based Complement. Altern. Med. 2012, 2012, 364604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Wang, D.; Yang, D.; Zhen, W.; Zhang, J.; Peng, S. The Effect of Icariin on Bone Metabolism and Its Potential Clinical Application. Osteoporos. Int. 2018, 29, 535–544. [Google Scholar] [CrossRef]
- Mosqueira, L.; Barrioni, B.R.; Martins, T.; Ocarino, N.D.M.; Serakides, R.; Pereira, M.D.M. In Vitro Effects of the Co-Release of Icariin and Strontium from Bioactive Glass Submicron Spheres on the Reduced Osteogenic Potential of Rat Osteoporotic Bone Marrow Mesenchymal Stem Cells. Biomed. Mater. 2020, 15, 055023. [Google Scholar] [CrossRef]
- Shen, X.; Yu, P.; Chen, H.; Wang, J.; Lu, B.; Cai, X.; Gu, C.; Liang, G.; Hao, D.; Ma, Q.; et al. Icariin Controlled Release on a Silk Fibroin/Mesoporous Bioactive Glass Nanoparticles Scaffold for Promoting Stem Cell Osteogenic Differentiation. RSC Adv. 2020, 10, 12105–12112. [Google Scholar] [CrossRef] [PubMed]
- Park, K.W.; Waki, H.; Kim, W.-K.; Davies, B.S.J.; Young, S.G.; Parhami, F.; Tontonoz, P. The Small Molecule Phenamil Induces Osteoblast Differentiation and Mineralization. Mol. Cell. Biol. 2009, 29, 3905–3914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, K.W.H.; Jiang, T.; Gagnon, K.A.; Nelson, C.; Laurencin, C.T. Small-Molecule Based Musculoskeletal Regenerative Engineering. Trends Biotechnol. 2014, 32, 74–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrijens, K.; Lin, W.; Cui, J.; Farmer, D.; Low, J.; Pronier, E.; Zeng, F.Y.; Shelat, A.A.; Guy, K.; Taylor, M.R.; et al. Identification of Small Molecule Activators of BMP Signaling. PLoS ONE 2013, 8, e59045. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, C.J.; Saul, J.M. Biomaterials for the Delivery of Growth Factors and Other Therapeutic Agents in Tissue Engineering Approaches to Bone Regeneration. Front. Pharmacol. 2018, 9, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, D.; Manzano, M.; Doadrio, J.C.; Salinas, A.J.; Vallet-Regí, M.; Gómez-Barrena, E.; Esbrit, P. Osteostatin-Loaded Bioceramics Stimulate Osteoblastic Growth and Differentiation. Acta Biomater. 2010, 6, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.; Sanchez-Salcedo, S.; Lozano, D.; Heras, C.; Esbrit, P.; Vallet-Regí, M.; Salinas, A.J. Osteogenic Effect of ZnO-Mesoporous Glasses Loaded with Osteostatin. Nanomaterials 2018, 8, 592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, F.; Yu, D.; Wei, L.; Su, N.; Liu, Y. Preclinical Application of Recombinant Human Bone Morphogenetic Protein 2 on Bone Substitutes for Vertical Bone Augmentation: A Systematic Review and Meta-Analysis. J. Prosthet. Dent. 2019, 122, 355–363. [Google Scholar] [CrossRef]
- Kuroda, Y.; Kawai, T.; Goto, K.; Matsuda, S. Clinical Application of Injectable Growth Factor for Bone Regeneration: A Systematic Review. Inflamm. Regen. 2019, 39, 20. [Google Scholar] [CrossRef] [PubMed]
- Berkmann, J.C.; Herrera Martin, A.X.; Pontremoli, C.; Zheng, K.; Bucher, C.H.; Ellinghaus, A.; Boccaccini, A.R.; Fiorilli, S.; Brovarone, C.V.; Duda, G.N.; et al. In Vivo Validation of Spray-Dried Mesoporous Bioactive Glass Microspheres Acting as Prolonged Local Release Systems for Bmp-2 to Support Bone Regeneration. Pharmaceutics 2020, 12, 823. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Singh, R.K.; Kang, M.S.; Kim, J.H.; Kim, H.W. Gene Delivery Nanocarriers of Bioactive Glass with Unique Potential to Load BMP2 Plasmid DNA and to Internalize into Mesenchymal Stem Cells for Osteogenesis and Bone Regeneration. Nanoscale 2016, 8, 8300–8311. [Google Scholar] [CrossRef]
- Dai, C.; Guo, H.; Lu, J.; Shi, J.; Wei, J.; Liu, C. Osteogenic Evaluation of Calcium/Magnesium-Doped Mesoporous Silica Scaffold with Incorporation of RhBMP-2 by Synchrotron Radiation-Based ΜCT. Biomaterials 2011, 32, 8506–8517. [Google Scholar] [CrossRef]
- Xiao, J.; Luo, H.; Ao, H.; Huang, Y.; Yao, F.; Zhang, Q.; Wan, Y. A RhBMP-2-Loaded Three-Dimensional Mesoporous Bioactive Glass Nanotubular Scaffold Prepared from Bacterial Cellulose. Colloids Surf. A Physicochem. Eng. Asp. 2019, 581, 123838. [Google Scholar] [CrossRef]
- Xin, T.; Mao, J.; Liu, L.; Tang, J.; Wu, L.; Yu, X.; Gu, Y.; Cui, W.; Chen, L. Programmed Sustained Release of Recombinant Human Bone Morphogenetic Protein-2 and Inorganic Ion Composite Hydrogel as Artificial Periosteum. ACS Appl. Mater. Interfaces 2020, 12, 6840–6851. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Reither, L.; Thomas, J.; Kampschulte, M.; Gbureck, U.; Lode, A.; Gelinsky, M. Calcium Phosphate Bone Cement/Mesoporous Bioactive Glass Composites for Controlled Growth Factor Delivery. Biomater. Sci. 2017, 5, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Fan, W.; Chang, J.; Xiao, Y. Mesoporous Bioactive Glass Scaffolds for Efficient Delivery of Vascular Endothelial Growth Factor. J. Biomater. Appl. 2013, 28, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Shi, M.; Jones, J.R.; Chen, Z.; Chang, J.; Wu, C.; Xiao, Y. Strategies to Direct Vascularisation Using Mesoporous Bioactive Glass-Based Biomaterials for Bone Regeneration. Int. Mater. Rev. 2017, 62, 392–414. [Google Scholar] [CrossRef]
- Schumacher, M.; Habibović, P.; Van Rijt, S. Peptide-Modified Nano-Bioactive Glass for Targeted Immobilization of Native VEGF. ACS Appl. Mater. Interfaces 2022, 14, 4959–4968. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.; Cresswell, M.; Boccaccini, A.R. Mesoporous Silica-Based Bioactive Glasses for Antibiotic-Free Antibacterial Applications. Mater. Sci. Eng. C 2018, 83, 99–107. [Google Scholar] [CrossRef]
- Kargozar, S.; Montazerian, M.; Hamzehlou, S.; Kim, H.W.; Baino, F. Mesoporous Bioactive Glasses: Promising Platforms for Antibacterial Strategies. Acta Biomater. 2018, 81, 1–19. [Google Scholar] [CrossRef]
- Tabia, Z.; El Mabrouk, K.; Bricha, M.; Nouneh, K. Mesoporous Bioactive Glass Nanoparticles Doped with Magnesium: Drug Delivery and Acellular: In Vitro Bioactivity. RSC Adv. 2019, 9, 12232–12246. [Google Scholar] [CrossRef] [Green Version]
- Polo, L.; Gómez-Cerezo, N.; García-Fernández, A.; Aznar, E.; Vivancos, J.L.; Arcos, D.; Vallet-Regí, M.; Martínez-Máñez, R. Mesoporous Bioactive Glasses Equipped with Stimuli-Responsive Molecular Gates for Controlled Delivery of Levofloxacin against Bacteria. Chem.-Eur. J. 2018, 24, 18944–18951. [Google Scholar] [CrossRef] [Green Version]
- Pouroutzidou, G.K.; Liverani, L.; Theocharidou, A.; Tsamesidis, I.; Lazaridou, M.; Christodoulou, E.; Beketova, A.; Pappa, C.; Triantafyllidis, K.S.; Anastasiou, A.D.; et al. Article Synthesis and Characterization of Mesoporous Mg-and Sr-Doped Nanoparticles for Moxifloxacin Drug Delivery in Promising Tissue Engineering Applications. Int. J. Mol. Sci. 2021, 22, 577. [Google Scholar] [CrossRef] [PubMed]
- El-Kady, A.M.; Farag, M.M.; El-Rashedi, A.M.I. Bioactive Glass Nanoparticles Designed for Multiple Deliveries of Lithium Ions and Drugs: Curative and Restorative Bone Treatment. Eur. J. Pharm. Sci. 2016, 91, 243–250. [Google Scholar] [CrossRef]
- Seedher, N.; Agarwal, P. Effect of Metal Ions on Some Pharmacologically Relevant Interactions Involving Fl Uoroquinolone Antibiotics. Drug Metabol. Drug Interact. 2010, 25, 17–24. [Google Scholar] [CrossRef]
- Epsley, S.; Tadros, S.; Farid, A.; Kargilis, D.; Mehta, S.; Rajapakse, C.S. The Effect of Inflammation on Bone. Front. Physiol. 2021, 11, 511799. [Google Scholar] [CrossRef]
- Majumdar, S.; Hira, S.K.; Tripathi, H.; Kumar, A.S.; Manna, P.P.; Singh, S.P.; Krishnamurthy, S. Synthesis and Characterization of Barium-Doped Bioactive Glass with Potential Anti-Inflammatory Activity. Ceram. Int. 2021, 47, 7143–7158. [Google Scholar] [CrossRef]
- Chitra, S.; Bargavi, P.; Balasubramaniam, M.; Chandran, R.R.; Balakumar, S. Impact of Copper on In-Vitro Biomineralization, Drug Release Efficacy and Antimicrobial Properties of Bioactive Glasses. Mater. Sci. Eng. C 2020, 109, 110598. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Shi, B. Mesoporous Bioglass/Silk Fibroin Scaffolds as a Drug Delivery System: Fabrication, Drug Loading and Release in Vitro and Repair Calvarial Defects in Vivo. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2014, 29, 401–406. [Google Scholar] [CrossRef]
- Mo, Y.; Zhao, F.; Lin, Z.; Cao, X.; Chen, D.; Chen, X. Local Delivery of Naringin in Beta-Cyclodextrin Modified Mesoporous Bioactive Glass Promotes Bone Regeneration: From Anti-Inflammatory to Synergistic Osteogenesis and Osteoclastogenesis. Biomater. Sci. 2022, 10, 1697–1712. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Zhang, Y. Research on the Biological Activity and Doxorubicin Release Behavior in Vitro of Mesoporous Bioactive SiO 2 -CaO-P 2 O 5 Glass Nanospheres. Appl. Surf. Sci. 2017, 419, 531–539. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Lin, C.; Zhong, W. Sol-Gel Derived Terbium-Containing Mesoporous Bioactive Glasses Nanospheres: In Vitro Hydroxyapatite Formation and Drug Delivery. Colloids Surf. B Biointerfaces 2017, 160, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Su, Y.; Chen, D.; Zhong, W. A Doxorubicin Delivery System: Samarium/Mesoporous Bioactive Glass/Alginate Composite Microspheres. Mater. Sci. Eng. C 2016, 67, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Ma, Y.; Chen, D.; Yang, H.; Li, M. Selenium-Containing Mesoporous Bioactive Glass Particles: Physicochemical and Drug Delivery Properties. Ceram. Int. 2016, 42, 3609–3617. [Google Scholar] [CrossRef]
- Bains, R.; Sharma, P.; Mir, R.A.; Jeet, S.; Kaur, G.; Pandey, O.P. Influence of CuO/MgO Ratio on the Gene Expression, Cytocompatibilty, and Antibacterial/Anticancerous/Analgesic Drug Loading Kinetics for (15-x) CuO-XMgO-10P2O5-60SiO2-10CaO-5ZnO (2.5 ≤ x ≤ 12.5) Mesoporous Bioactive Glasses. J. Biomed. Mater. Res. Part A 2018, 106, 2116–2130. [Google Scholar] [CrossRef]
- Shoaib, M.; Ur Rahman, M.S.; Saeed, A.; Naseer, M.M. Mesoporous Bioactive Glass-Polyurethane Nanocomposites as Reservoirs for Sustained Drug Delivery. Colloids Surf. B Biointerfaces 2018, 172, 806–811. [Google Scholar] [CrossRef]
- Shruti, S.; Salinas, A.J.; Ferrari, E.; Malavasi, G.; Lusvardi, G.; Doadrio, A.L.; Menabue, L.; Vallet-Regí, M. Curcumin Release from Cerium, Gallium and Zinc Containing Mesoporous Bioactive Glasses. Microporous Mesoporous Mater. 2013, 180, 92–101. [Google Scholar] [CrossRef]
- Garg, S.; Thakur, S.; Gupta, A.; Kaur, G.; Pandey, O.P. Antibacterial and Anticancerous Drug Loading Kinetics for (10-x)CuO-XZnO-20CaO-60SiO2-10P2O5 (2 ≤ x ≤ 8) Mesoporous Bioactive Glasses. J. Mater. Sci. Mater. Med. 2017, 28, 11. [Google Scholar] [CrossRef] [PubMed]
- Ur Rahman, M.S.; Tahir, M.A.; Noreen, S.; Yasir, M.; Khan, M.B.; Mahmood, T.; Bahadur, A.; Shoaib, M. Osteogenic Silver Oxide Doped Mesoporous Bioactive Glass for Controlled Release of Doxorubicin against Bone Cancer Cell Line (MG-63): In Vitro and in Vivo Cytotoxicity Evaluation. Ceram. Int. 2020, 46, 10765–10770. [Google Scholar] [CrossRef]
- Hu, M.; Fang, J.; Zhang, Y.; Wang, X.; Zhong, W.; Zhou, Z. Design and Evaluation a Kind of Functional Biomaterial for Bone Tissue Engineering: Selenium/Mesoporous Bioactive Glass Nanospheres. J. Colloid Interface Sci. 2020, 579, 654–666. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, M.; Wang, X.; Zhou, Z.; Liu, Y. Design and Evaluation of Europium Containing Mesoporous Bioactive Glass Nanospheres: Doxorubicin Release Kinetics and Inhibitory Effect on Osteosarcoma MG 63 Cells. Nanomaterials 2018, 8, 961. [Google Scholar] [CrossRef] [Green Version]
- Sui, B.; Liu, X.; Sun, J. Dual-Functional Dendritic Mesoporous Bioactive Glass Nanospheres for Calcium Influx-Mediated Specific Tumor Suppression and Controlled Drug Delivery in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 23548–23559. [Google Scholar] [CrossRef]
- Das, M.P.; Pandey, G.; Neppolian, B.; Das, J. Design of Poly-L-Glutamic Acid Embedded Mesoporous Bioactive Glass Nanospheres for PH-Stimulated Chemotherapeutic Drug Delivery and Antibacterial Susceptibility. Colloids Surf. B Biointerfaces 2021, 202, 111700. [Google Scholar] [CrossRef]
- Singh, R.K.; Kurian, A.G.; Patel, K.D.; Mandakhbayar, N.; Lee, N.H.; Knowles, J.C.; Lee, J.H.; Kim, H.W. Label-Free Fluorescent Mesoporous Bioglass for Drug Delivery, Optical Triple-Mode Imaging, and Photothermal/Photodynamic Synergistic Cancer Therapy. ACS Appl. Bio Mater. 2020, 3, 2218–2229. [Google Scholar] [CrossRef]
- Chen, S.Y.; Chou, P.F.; Chan, W.K.; Lin, H.M. Preparation and Characterization of Mesoporous Bioactive Glass from Agricultural Waste Rice Husk for Targeted Anticancer Drug Delivery. Ceram. Int. 2017, 43, 2239–2245. [Google Scholar] [CrossRef]
- Shoaib, M.; Saeed, A.; Rahman, M.S.U.; Naseer, M.M. Mesoporous Nano-Bioglass Designed for the Release of Imatinib and in Vitro Inhibitory Effects on Cancer Cells. Mater. Sci. Eng. C 2017, 77, 725–730. [Google Scholar] [CrossRef]
- Nawaz, Q.; Fuentes-Chandía, M.; Tharmalingam, V.; Ur Rehman, M.A.; Leal-Egaña, A.; Boccaccini, A.R. Silibinin Releasing Mesoporous Bioactive Glass Nanoparticles with Potential for Breast Cancer Therapy. Ceram. Int. 2020, 46, 29111–29119. [Google Scholar] [CrossRef]
- Rahman, M.S.U.; Tahir, M.A.; Noreen, S.; Yasir, M.; Ahmad, I.; Khan, M.B.; Ali, K.W.; Shoaib, M.; Bahadur, A.; Iqbal, S. Magnetic Mesoporous Bioactive Glass for Synergetic Use in Bone Regeneration, Hyperthermia Treatment, and Controlled Drug Delivery. RSC Adv. 2020, 10, 21413–21419. [Google Scholar] [CrossRef]








| Material | Ion | Therapeutic Action | Studies | Ref. |
|---|---|---|---|---|
| MBG NPs | Cu2+ | Antimicrobial Angiogenic | In vitro bacterial viability Biofilm disaggregation/dispersion | [133] |
| MBG NPs | Cu2+ | Angiogenic Antibacterial | Aortic Ring/CAM assay Infected 3D tissue model (S. aureus and Pseudormonas aeruginosa) Biofilm formation and disruption | [134] |
| MBGs NPs | Cu2+ | Angiogenesis (synergic effect induced by Si, Ca, and P ions) | In vivo zebrafish model, Subintestinal vessels (SIVs) | [138] |
| MBG scaffolds | Cu2+ | Angiogenesis/ Osteostimulation/Antibacterial | In vitro study with hBMSCs (VEGF expression, osteogenic differentiation); E. coli viability | [38] |
| MBG NPs | Sr2+ | Osteogenesis, Anticlastogenic Anti-inflammatory | Osteoblast-like SAOS, Mesenchymal Stem Cells In vivo studies Murine macrophage cell line | [131,132] |
| MBG scaffolds | Sr2+ | Osteogenesis Anticlastogenic | Osteoblast-like cells, MC3T3-E1 (ALP activity, osteogenic expression) In vivo studies | [102,139] |
| MBG NPs | Ce3+ | Anti-oxidant, Anti-inflammatory Osteogenic Hemostatic | Mouse fibroblast cells, Osteoblast-like Saos-2 cells In vitro hemostasis assay, platelet adhesion | [137,140] |
| MBG NPs | Zn2+ | Osteogenic | Osteoblast-like cells mouse Embryonic fibroblasts cells | [141] |
| MBG Meso-macroporous 3D scaffolds | Zn2+ | Antibacterial (also in synergy with antibiotics) | Agar disk diffusion test Planktonic growth inhibition Biofilm degradation | [142] |
| MBG NPs | Ag+ | Antibacterial (also in synergy with antibiotics) | Planktonic bacteria model 3D tissue-engineered infected tissue model In vivo infected dental pulp tissue | [136,143] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallet-Regí, M.; Colilla, M.; Izquierdo-Barba, I.; Vitale-Brovarone, C.; Fiorilli, S. Achievements in Mesoporous Bioactive Glasses for Biomedical Applications. Pharmaceutics 2022, 14, 2636. https://doi.org/10.3390/pharmaceutics14122636
Vallet-Regí M, Colilla M, Izquierdo-Barba I, Vitale-Brovarone C, Fiorilli S. Achievements in Mesoporous Bioactive Glasses for Biomedical Applications. Pharmaceutics. 2022; 14(12):2636. https://doi.org/10.3390/pharmaceutics14122636
Chicago/Turabian StyleVallet-Regí, María, Montserrat Colilla, Isabel Izquierdo-Barba, Chiara Vitale-Brovarone, and Sonia Fiorilli. 2022. "Achievements in Mesoporous Bioactive Glasses for Biomedical Applications" Pharmaceutics 14, no. 12: 2636. https://doi.org/10.3390/pharmaceutics14122636
APA StyleVallet-Regí, M., Colilla, M., Izquierdo-Barba, I., Vitale-Brovarone, C., & Fiorilli, S. (2022). Achievements in Mesoporous Bioactive Glasses for Biomedical Applications. Pharmaceutics, 14(12), 2636. https://doi.org/10.3390/pharmaceutics14122636