1. Introduction
Freeze-drying is an excellent way of drying thermally sensitive materials to increase their long-term stability and preserve thermally labile compounds if stored properly afterward. The technique is for that reason widely used in the pharmaceutical industry and for the stabilisation of certified reference materials [
1,
2,
3,
4,
5,
6]. In the pharmaceutical industry, different formulations are used to facilitate freeze-drying of active compounds, e.g., to act as bulking agents to provide a network for the active substances contributing to rapid and complete reconstitution before use. Other agents can be added acting as cryo-protectants, buffers, and stabilisers for the active compounds [
7,
8,
9,
10,
11]. In this study, a 4:1% (
m/
m) solution of mannitol:sucrose in water was freeze-dried as a representative matrix for a pharmaceutical bulking agent and cryo-protectant formulation [
7,
8,
9,
10,
11].
A freeze-drying cycle consists of three main steps 1, 3, and 4 as given below: Whereas annealing (step 2) is optional.
- (1)
Freezing of the water present in the matrix at ambient pressure without causing severe phase separation and too small ice-crystals.
- (2)
Annealing of ice to achieve crystals of larger and uniform size [
11].
- (3)
Sublimation (primary drying), whereby the water is evaporating from solid ice in the material and is captured on the ice-condenser under a soft vacuum.
- (4)
Secondary drying where most of the remaining water is removed under a hard vacuum and a shelf temperature of around 20 °C.
In an earlier paper, we reported on the first use of combining an infrared camera (IR) with a freeze dryer for monitoring the surface temperature when freeze-drying human serum in vials and cheese slurry on trays [
12]. In that work, the major benefits of the IR camera were outlined. The coupling resulted in superior spatial and thermal resolution of the monitored materials in contrast to the use of traditional Pt-100 probes placed directly in the materials. A limitation of the previous setup was that only the surface of the materials could be monitored [
12]. In this work, we present a solution so thermal data along depth profiles inside the material could also be recorded using the IR camera.
Several other studies have since then been published describing work using an infrared camera to monitor freeze-drying processes [
13,
14,
15,
16,
17,
18,
19]. Lietta et al. described the use of an infrared camera mounted inside a freeze dryer compared with results from thermocouples when drying a sucrose formulation in glass vials. In that work, lateral observation of the glass vials was possible [
15]. Contactless techniques are preferred in PAT (Process Analytical Technologies) but Song et al. published a paper to elucidate the spatial temperature distribution inside a material based on physical probes [
13,
20]. In their work, twenty-four measurement points were systematically placed every 2 × 4 mm inside skimmed milk subjected to freeze-drying. In the current paper, an IR camera coupled to a freeze dryer was exploited further by developing custom-made vials, i.e., 10-mL glass cuvettes with one side made of germanium (Ge). The choice of germanium for one window was based on the fact that this material is almost fully transparent in LWIR 7–14 µm. The remaining walls of the cuvette to be studied were made of modified soda-lime glass, which is similar to normal glass. The size of these recipients was chosen so that they can be representative of drying behaviour in glass vials filled with about 8 mL liquid. By using cuvettes with a Ge-window it was possible to record the temperature vertically along a depth profile inside the cuvette albeit at the material-surface adjacent to the Ge-window. By placing the side made of germanium next to a 45° IR mirror, it was possible to record the temperatures prevailing at the surface as well as deeper down in the material simultaneously. This set-up allowed monitoring of the gradual change of temperature in the cuvette from the top to bottom as a function of time through a full freeze-drying cycle based on 2700 thermograms. The depth profile reached about 24 mm deep inside the cuvette corresponding to the fill height of the pharmaceutical formulation. Two main categories of results are presented: (i) temperatures along one depth profile as a function of time during the complete freeze-drying cycle are shown for freezing, annealing, sublimation, and secondary drying; (ii) contour plots of temperatures are shown for the side (depth) of the cuvette covering 14 × 24 mm of the side at specific time intervals. The spatial contour plots are based on 84 contact-free data points (IR pixels).
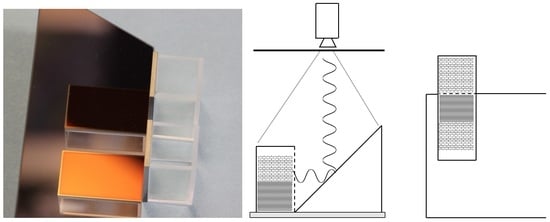
To our knowledge, it is the first time germanium vials (monitored by infrared thermography) have been used inside a freeze dryer. Thanks to this, it was possible to show minute differences in spatial and temporal temperature distribution inside these vials during a complete freeze-drying cycle also along depth profiles. When such detailed information is available, further optimisation of the freezing and annealing steps becomes straightforward. This should lead to increased efficiency of the subsequent sublimation step, which ultimately provides more uniformly dried materials of better quality.
2. Materials and Methods
An infrared camera (VarioCAM, Jenoptik, InfraTec GmbH, Dresden, Germany) was mounted vertically, with the lens facing downwards (looking through a germanium window) and placed in the vacuum chamber wall of a freeze dryer (Epsilon 2-100D, Martin Christ, Osterode, Germany) [
12]. The top shelf that was monitored by the IR camera was coated with masking tape using 50 mm wide strips that had been neatly placed next to each other. The shelf temperature could therefore be directly monitored using the IR camera since the tape layer is drastically increasing the emissivity as previously described [
12]. The masking tape was of 140 µm thickness consisting of a slightly creped paper with a rubber adhesive from Tesa type 4316 (Brussels, Belgium). The IR camera recorded images of the material in the Ge-cuvettes as well as the shelf of the freeze dryer with a high spatial and thermal resolution (2 × 2 mm, spatial) and (50 mK, thermal) by measuring long-wavelength infrared radiation (LWIR) from 7 to 14 µm. The outside dimensions of the cuvettes were 38 mm high and 22 mm wide and made of soda-lime B270 optical glass of 2 mm thickness (Hellma, Müllheim, Germany). The cuvettes were supplied with one side missing, onto which an IR transparent germanium window was glued. The germanium windows of 2.2 mm thickness measuring 38 × 22 mm were obtained from Umicore Electro-Optic Materials (Dundee, UK) having an average IR transmission >97% from 7 to 14 µm. The germanium windows were coated with a HEAR/HEAR coating (high-efficiency broad-band anti-reflective coating) of undisclosed composition that resulted in a maximum reflectance of 0.7% from 7 to 14 µm. The Ge-windows were glued onto the glass cuvettes using Fix ALL Crystal based on a modified silane polymer (Soudal, Turnhout, Belgium). This glue is compatible with temperatures from −40 °C to 90 °C. The prism (IR mirror) with a 45° angle, (Umicore Electro-Optic Materials) was placed next to the cuvettes to monitor the side of the cuvettes with the IR camera looking from above. The prism was 200 mm long and 50 mm high and made from N-BK7 borosilicate glass. To achieve >99% reflectance from 7 to 14 µm it was coated with protected gold on the chamfered side. The prism was long enough to accommodate at least five cuvettes standing next to each other along the IR reflecting surface. Furthermore, D-mannitol (>98% pure) and sucrose (>99.5% pure) were obtained from Sigma-Aldrich (Steinheim, Germany) and dissolved in Type-1 water (18.2 MΩ cm, 0.053 μS cm
−1, Merck Millipore, Billerica, MA, USA) to give a 4:1% mannitol:sucrose solution (
m/
m).
Additional technical details about the coupling of the infrared camera to the freeze-dryer, data acquisition, and performance details can be found in previous work [
12]. After drying, the water content of the mannitol/sucrose formulation was measured using volumetric Karl Fischer titration using about 160 mg of dried material per replicate, (Metrohm, Herisau, Switzerland). A CombiTitrant 5 (Aquastar
® from Supelco) made by Merck KGaA, Darmstadt, DE was used as titrant where 1 mL corresponds to about 5 mg of water. Methanol Emsure, ACS ISO Reag. Ph Eur, Merck Darmstadt, DE was used to disperse the dried material. To calibrate the titre, a sodium tartrate di-hydrate CRM was used from Honeywell Fluka, Seelze, DE. This CRM was produced under ISO17034 accreditation and controlled against NIST SRM 2890 (Gaithersburg, MD, USA). Refined “Everyday” olive oil was obtained from a grocery store, Colruyt, Mol, BE.
The freeze-drying cycle for the formulation was both controlled and monitored using machine readings of shelf temperatures, product temperatures, and pressure. Product temperature and resistivity readouts (Lyo RX) were obtained from Pt100 probes or Lycontrol probes immersed in the product and connected by wire to the freeze-dryer. In addition, 2700 thermograms were recorded for 45 h. The thermograms were processed afterward and corrected for emissivity in the dedicated IRBIS 3 software from Jenoptik. Temperature data for profiles and other measurement definitions was exported as ASCI files (readable with a text editor), imported into Excel, and then further manipulated in Sigmaplot (v. 14.5) to generate spatial contour plots.
3. Results and Discussion
First, the ability of the IR camera to record the temperature accurately through the Ge-windows was verified. Two cuvettes were placed next to the prism at ambient temperature outside the freeze-dryer. One cuvette was placed so that the Ge-window was facing the prism and for the other cuvette, the glass window was facing the prism. The IR camera was placed above the setup looking downwards at cuvettes and prism using a tripod. The cuvettes were filled with 7.5 mL of cold water (3 °C) and immediately afterward, 2.5 mL of warm olive oil was added (50 °C) on top. As soon as the olive oil was added, a three-minute measuring sequence was started by recording one thermogram every ten seconds. From the start, the temperature of the cold water immediately started to increase and the temperature of the oil was rapidly decreasing as a function of time. As can be seen in
Figure 1a, the thermogram recorded through the Ge-window shows a large difference between the warm top layer of oil and the colder water at the bottom. The temperatures measured on the glass window (soda-lime B270 optical glass) show a much smaller temperature gradient and it was not possible to record the temperature prevailing inside the cuvette accurately. The glass window is opaque for IR radiation since this type of glass has little transmission in the LWIR range. If glass cuvettes would face the IR mirror in subsequent freeze-drying experiments, it would result in a measurement of the surface temperature of the glass. The temperature of the glass surface is caused by the heating or cooling of the glass from the material inside the cuvette. Such an approach results in a loss of spatial and thermal resolution concerning the actual temperature prevailing
inside the cuvette. In contrast to this, the flat Ge-windows facilitate accurate temperature measurements at the surface of the material
inside the custom-made cuvettes. In an additional initial test, the soda-lime glass window and the germanium window were placed over two recipients with cold and warm water, respectively. In this case, there was a gap of air between the water and the windows. The thermogram is shown in
Figure 1b. As can be seen, without contact with the water in the recipients, temperature measurements on the soda-lime glass window show the ambient temperature of the glass only. Hence, it is shown that this type of glass has no transmission in LWIR. On the other hand, no significant difference between the temperature read-outs through the germanium window and the water surfaces could be established at
p 0.05 by doing a
t-test of the average temperatures. As can be seen in
Table 1, there is a large difference in thermal conductivity and IR transmission efficiency between soda-lime glass and germanium in the LWIR range. In fact, only special chalcogen glasses and special ceramics exhibit transmission in LWIR [
21,
22].
One may think that the difference in thermal conductivity is too large between the two materials so that the observations from the drying process recorded with Ge-windows cannot be extrapolated to vials made from glass, as this material is a thermal insulator. In this freeze-drying process, the energy input to push water out of the materials comes from step-wise increments of the shelf temperature. As the Ge-cuvettes have their bottom made of glass as well as three other sides, they are similar to any glass vial in that respect. The energy transfer from the shelf to the material through the bottom of the vial is also similar to a regular glass cuvette/vial. In addition, as shown in
Figure 1c, the actual temperatures plotted along the depth profiles of the Ge-cuvette and glass cuvette after 0 and 180 s, show the limitations of monitoring a glass surface with an infrared camera. Nevertheless, it must be also noted as shown in
Figure 1c, that the temperature reading of the glass and germanium window is almost identical after 180 s from 24 mm towards the bottom in the cuvette. Van Bockstal et al. reported on the use of an IR camera to monitor rotating glass vials [
14]. In another publication, freezing behaviour inside glass vials was also studied using an infrared camera [
19]. Correct temperature readings were obtained as also shown here, but without high thermal and spatial resolution to reveal more subtle differences [
14,
19]. In
Figure 2a–d, spatial contour plots of the germanium and glass windows are shown for the oil–water experiment after 0 and 180 s, respectively. The colour coded temperature scale in those figures is the same to facilitate direct comparison.
Figure 3 shows the temperatures at the surface and top of the side of the cuvette (1–2 mm below the surface). Admittedly, the temperature changes in the oil–water experiment are generally faster than in a freeze-drying process. At the same time, frozen or drying materials do not necessarily have equally good contact with the cuvette material surface as liquids have (as in this oil–water experiment). This could also be detrimental to the correct readout of the temperature of the glass surface as good material contact is necessary for an accurate temperature reading of the glass surface.
In
Figure 4a, the instrument setup is shown to give an overview of how the cuvettes were monitored inside the freeze dryer using the IR camera. The resulting reflection on the IR mirror and direct reading of the surface temperatures were recorded from above as already mentioned. In
Figure 4b three cuvettes are shown with their respective measurement definitions, e.g., areas. Additionally, in one cuvette, a depth profile was evaluated through the complete freeze-drying cycle (labelled “profile” and placed centrally in the cuvette to the right in
Figure 4b). The full freeze-drying cycle of 47 h is displayed in
Figure 5a whereof the first 45 h were monitored using the infrared camera. The machine readings from the freeze dyer of shelf temperature, pressure, product temperatures, resistivity, and ice condenser temperature are shown in
Figure 5a. The emissivity correction for all thermograms of 0.90 was deduced by matching the readout of the average shelf temperature for the large rectangle shown on top in
Figure 4b (SHELF) in comparison with the actual shelf temperature readout from the freeze-dryer (separately checked with calibrated temperature sensors) shown in
Figure 5a. That correction factor is identical to the one used in previous work [
12]. The temperature recordings through the complete freeze-drying cycle of the 3 × 3 areas displayed in
Figure 4b are shown in
Figure 5b. That data can be directly compared with the temperature read-outs from the product temperature sensors (Pt100) shown in
Figure 5a. Data obtained from the depth profile, the line shown in
Figure 4b, are displayed in
Figure 6 and
Figure 7e for specific time windows corresponding to freezing, annealing, sublimation, and secondary drying. Spatial information was extracted from seven depth profiles placed next to each other as shown in
Figure 4c resulting in 84 pixels (data points). Based on those 84 pixels, spatial contour plots could be made and are shown in
Figure 8a–i. The specific time points for the spatial contour plots are indicated with vertical arrows in
Figure 5a and in
Figure 7a–e.
5. Conclusions
Infrared thermography provides superior spatial and thermal resolution when monitoring a freeze-drying cycle in contrast to traditional means such as Pt-100 probes. In this work, this feature was further exploited by using cuvettes with one side made of germanium. The correct choice of material in the vial/cuvette wall is important to achieve sufficient thermal and spatial resolution to record very small temperature differences inside the material that is drying. In this work, the full depth profiles of temperature could also be recorded thanks to this development. The isotherms show different rates of temperature change per mm of material as a function of time during freezing, sublimation, and secondary drying. These slopes also differ depending on the depth in the material. These effects are derived from different dynamics in different parts of the freeze-drying process. The dynamics of freezing and annealing have an impact on the subsequent sublimation phase. There was also some degree of asymmetry in the temperature distribution in the annealing step that could be observed throughout the sublimation step. Thanks to the high thermal resolution of this set-up and the geometry of the measurement capability, it was possible to detect relatively cooler upper layers in the material seen at the end of the secondary drying. This effect could be related to evaporative cooling.
Newer IR cameras come with an increased number of pixels of their micro-bolometers, which should make studies that are even more detailed possible with higher resolution, especially in combination with appropriate lenses. Data transfer and temperature control of the germanium lens in the IR camera and its microbolometer is easier if the IR camera is kept outside the freeze-drying chamber. No detailed study was made to compare cuvettes equipped with a Pt-100 probe with cuvettes that had no Pt-100 probe. No striking differences between the thermograms recorded for these two categories of cuvettes were observed. This suggests that the effect on the temperature distribution in the presence of probes is relatively small but it could now be investigated further thanks to the high thermal and spatial resolution shown here. This new development consequently provides a much sharper tool for these kinds of studies.