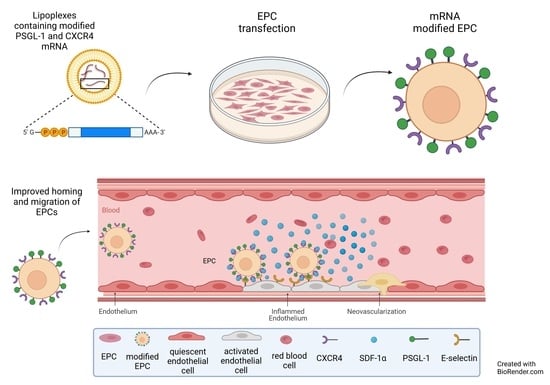
Homing of mRNA-Modified Endothelial Progenitor Cells to Inflamed Endothelium
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Synthesis of Modified mRNA
2.2. Cultivation of EPCs
2.3. Cultivation of Human Umbilical Vein Endothelial Cells (HUVECs)
2.4. Transfection of EPCs with Synthetic, Modified mRNA
2.5. Viability Assay
2.6. Analysis of CXCR4 and PSGL-1 Expression by Flow Cytometry
2.7. Analysis of E-Selectin Expression on HUVECs
2.8. Chemotactic Migration Assay
2.9. Dynamic Adhesion Assay
2.10. Single-Cell Atomic Force Microscopy (AFM)
2.11. Statistical Analysis
3. Results
3.1. Analysis of CXCR4 and PSGL-1 Expression after the Transfection of EPCs with Synthetic, Modified mRNAs Encoding CXCR4 and PSGL-1
3.2. Analysis of Cell Viability of EPCs after Transfection with Synthetic, Modified CXCR4 or PSGL-1 mRNAs
3.3. Chemotactic Migration Assay of EPCs toward Chemoattractant SDF1-α
3.4. Single-Cell AFM Analysis of the Adhesion of mRNA-Modified EPCs to TNF-α-Activated Endothelium
3.5. Adhesion of mRNA-Modified EPCs on TNF-α-Activated Endothelium in a Dynamic Flow Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wu, X.; Reboll, M.R.; Korf-Klingebiel, M.; Wollert, K.C. Angiogenesis after acute myocardial infarction. Cardiovasc. Res. 2020, 117, 1257–1273. [Google Scholar] [CrossRef]
- Avci-Adali, M.; Paul, A.; Ziemer, G.; Wendel, H.P. New strategies for in vivo tissue engineering by mimicry of homing factors for self-endothelialisation of blood contacting materials. Biomaterials 2008, 29, 3936–3945. [Google Scholar] [CrossRef]
- Inoue, T.; Sata, M.; Hikichi, Y.; Sohma, R.; Fukuda, D.; Uchida, T.; Shimizu, M.; Komoda, H.; Node, K. Mobilization of CD34-positive bone marrow-derived cells after coronary stent implantation: Impact on restenosis. Circulation 2007, 115, 553–561. [Google Scholar] [CrossRef] [Green Version]
- Massa, M.; Rosti, V.; Ferrario, M.; Campanelli, R.; Ramajoli, I.; Rosso, R.; De Ferrari, G.M.; Ferlini, M.; Goffredo, L.; Bertoletti, A.; et al. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood 2005, 105, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Sobrino, T.; Hurtado, O.; Moro, M.A.; Rodríguez-Yáñez, M.; Castellanos, M.D.M.; Brea, D.; Moldes, O.; Blanco, M.; Arenillas, J.F.; Leira, R.; et al. The Increase of Circulating Endothelial Progenitor Cells After Acute Ischemic Stroke Is Associated with Good Outcome. Stroke 2007, 38, 2759. [Google Scholar] [CrossRef] [Green Version]
- Asahara, T. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999, 18, 3964–3972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Askari, A.T.; Unzek, S.; Popovic, Z.B.; Goldman, C.K.; Forudi, F.; Kiedrowski, M.; Rovner, A.; Ellis, S.G.; Thomas, J.D.; DiCorleto, P.E.; et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 2003, 362, 697–703. [Google Scholar] [CrossRef]
- Ceradini, D.J.; Kulkarni, A.R.; Callaghan, M.J.; Tepper, O.M.; Bastidas, N.; Kleinman, E.M.; Capla, J.M.; Galiano, R.D.; Levine, J.P.; Gurtner, G.C. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat. Med. 2004, 10, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-H.; Li, Y.; Huang, D.; Yao, M. Role of Stromal Cell-Derived Factor-1 in Endothelial Progenitor Cell-Mediated Vascular Repair and Regeneration. Tissue Eng. Regen. Med. 2021, 18, 747–758. [Google Scholar] [CrossRef]
- Orlic, D.; Kajstura, J.; Chimenti, S.; Limana, F.; Jakoniuk, I.; Quaini, F.; Nadal-Ginard, B.; Bodine, D.M.; Leri, A.; Anversa, P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc. Natl. Acad. Sci. USA 2001, 98, 10344–10349. [Google Scholar] [CrossRef] [Green Version]
- Asahara, T.; Masuda, H.; Takahashi, T.; Kalka, C.; Pastore, C.; Silver, M.; Kearne, M.; Magner, M.; Isner, J.M. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 1999, 85, 221–228. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.; Wang, X.; Nie, Y.; Hu, A.; Liu, H.; Zhang, K.; Zhang, L.; Wu, Q.; Li, K.; Liu, C.; et al. Targeted trapping of endogenous endothelial progenitor cells for myocardial ischemic injury repair through neutrophil-mediated SPIO nanoparticle-conjugated CD34 antibody delivery and imaging. Acta Biomater. 2022. [Google Scholar] [CrossRef]
- Deutsch, M.-A.; Brunner, S.; Grabmaier, U.; David, R.; Ott, I.; Huber, B.C. Cardioprotective potential of human endothelial-colony forming cells from diabetic and nondiabetic donors. Cells 2020, 9, 588. [Google Scholar] [CrossRef] [Green Version]
- Brunt, K.R.; Wu, J.; Chen, Z.; Poeckel, D.; Dercho, R.A.; Melo, L.G.; Funk, C.D.; Ward, C.A.; Li, R.-K. Ex Vivo Akt/HO-1 Gene Therapy to Human Endothelial Progenitor Cells Enhances Myocardial Infarction Recovery. Cell Transplant. 2012, 21, 1443–1461. [Google Scholar] [CrossRef] [Green Version]
- Langer, H.; May, A.E.; Daub, K.; Heinzmann, U.; Lang, P.; Schumm, M.; Vestweber, D.; Massberg, S.; Schönberger, T.; Pfisterer, I.; et al. Adherent Platelets Recruit and Induce Differentiation of Murine Embryonic Endothelial Progenitor Cells to Mature Endothelial Cells In Vitro. Circ. Res. 2006, 98, e2–e10. [Google Scholar] [CrossRef] [Green Version]
- Chavakis, E.; Urbich, C.; Dimmeler, S. Homing and engraftment of progenitor cells: A prerequisite for cell therapy. J. Mol. Cell. Cardiol. 2008, 45, 514–522. [Google Scholar] [CrossRef]
- Steinle, H.; Golombek, S.; Behring, A.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Improving the Angiogenic Potential of EPCs via Engineering with Synthetic Modified mRNAs. Mol. Ther. Nucleic Acids 2018, 13, 387–398. [Google Scholar] [CrossRef] [Green Version]
- Hoenig, M.R.; Bianchi, C.; Sellke, F.W. Hypoxia inducible factor-1 alpha, endothelial progenitor cells, monocytes, cardiovascular risk, wound healing, cobalt and hydralazine: A unifying hypothesis. Curr. Drug Targets 2008, 9, 422–435. [Google Scholar] [CrossRef]
- Kawakami, Y.; Ii, M.; Matsumoto, T.; Kuroda, R.; Kuroda, T.; Kwon, S.M.; Kawamoto, A.; Akimaru, H.; Mifune, Y.; Shoji, T.; et al. SDF-1/CXCR4 axis in Tie2-lineage cells including endothelial progenitor cells contributes to bone fracture healing. J. Bone Miner. Res. 2015, 30, 95–105. [Google Scholar] [CrossRef]
- Liu, Z.J.; Tian, R.; An, W.; Zhuge, Y.; Li, Y.; Shao, H.; Habib, B.; Livingstone, A.S.; Velazquez, O.C. Identification of E-selectin as a novel target for the regulation of postnatal neovascularization: Implications for diabetic wound healing. Ann. Surg. 2010, 252, 625–634. [Google Scholar] [CrossRef] [Green Version]
- Oh, I.-Y.; Yoon, C.-H.; Hur, J.; Kim, J.-H.; Kim, T.-Y.; Lee, C.-S.; Park, K.-W.; Chae, I.-H.; Oh, B.-H.; Park, Y.-B.; et al. Involvement of E-selectin in recruitment of endothelial progenitor cells and angiogenesis in ischemic muscle. Blood 2007, 110, 3891–3899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foubert, P.; Silvestre, J.-S.; Souttou, B.; Barateau, V.; Martin, C.; Ebrahimian, T.G.; Leré-Déan, C.; Contreres, J.O.; Sulpice, E.; Levy, B.I.; et al. PSGL-1–mediated activation of EphB4 increases the proangiogenic potential of endothelial progenitor cells. J. Clin. Investig. 2007, 117, 1527–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avci-Adali, M.; Perle, N.; Ziemer, G.; Wendel, H.P. Current concepts and new developments for autologous in vivo endothelialisation of biomaterials for intravascular applications. Eur. Cells Mater. 2011, 21, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Steinle, H.; Weber, J.; Stoppelkamp, S.; Große-Berkenbusch, K.; Golombek, S.; Weber, M.; Canak-Ipek, T.; Trenz, S.-M.; Schlensak, C.; Avci-Adali, M. Delivery of synthetic mRNAs for tissue regeneration. Adv. Drug Deliv. Rev. 2021, 179, 114007. [Google Scholar] [CrossRef]
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics—developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Hajj, K.A.; Whitehead, K.A. Tools for translation: Non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017, 2, 1–17. [Google Scholar] [CrossRef]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and challenges in the delivery of mRNA-based vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Z.; Mc Cafferty, S.; Combes, F.; Huysmans, H.; De Temmerman, J.; Gitsels, A.; Vanrompay, D.; Catani, J.P.; Sanders, N.N. mRNA therapeutics deliver a hopeful message. Nano Today 2018, 23, 16–39. [Google Scholar] [CrossRef]
- Avci-Adali, M.; Behring, A.; Steinle, H.; Keller, T.; Krajeweski, S.; Schlensak, C.; Wendel, H.P. In Vitro Synthesis of Modified mRNA for Induction of Protein Expression in Human Cells. J. Vis. Exp. 2014, 93, e51943. [Google Scholar] [CrossRef] [Green Version]
- Hatzopoulos, A.K.; Folkman, J.; Vasile, E.; Eiselen, G.K.; Rosenberg, R.D. Isolation and characterization of endothelial progenitor cells from mouse embryos. Development 1998, 125, 1457–1468. [Google Scholar] [CrossRef]
- Leal, V.; Ribeiro, C.F.; Oliveiros, B.; António, N.; Silva, S. Intrinsic Vascular Repair by Endothelial Progenitor Cells in Acute Coronary Syndromes: An Update Overview. Stem Cell Rev. Rep. 2018, 15, 35–47. [Google Scholar] [CrossRef]
- Takahashi, T.; Kalka, C.; Masuda, H.; Chen, D.; Silver, M.; Kearney, M.; Magner, M.; Isner, J.M.; Asahara, T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat. Med. 1999, 5, 434–438. [Google Scholar] [CrossRef]
- Gill, M.; Dias, S.; Hattori, K.; Rivera, M.L.; Hicklin, D.; Witte, L.; Girardi, L.; Yurt, R.; Himel, H.; Rafii, S. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ. Res. 2001, 88, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Shintani, S.; Murohara, T.; Ikeda, H.; Ueno, T.; Honma, T.; Katoh, A.; Sasaki, K.-I.; Shimada, T.; Oike, Y.; Imaizumi, T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 2001, 103, 2776–2779. [Google Scholar] [CrossRef] [Green Version]
- Hattori, K.; Dias, S.; Heissig, B.; Hackett, N.R.; Lyden, D.; Tateno, M.; Hicklin, D.J.; Zhu, Z.; Witte, L.; Crystal, R.G.; et al. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J. Exp. Med. 2001, 193, 1005–1014. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, J.-I.; Kusano, K.F.; Masuo, O.; Kawamoto, A.; Silver, M.; Murasawa, S.; Bosch-Marce, M.; Masuda, H.; Losordo, D.W.; Isner, J.M.; et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation 2003, 107, 1322–1328. [Google Scholar] [CrossRef] [Green Version]
- Heeschen, C.; Lehmann, R.; Honold, J.; Assmus, B.; Aicher, A.; Walter, D.H.; Martin, H.; Zeiher, A.M.; Dimmeler, S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation 2004, 109, 1615–1622. [Google Scholar] [CrossRef] [Green Version]
- Vasa, M.; Fichtlscherer, S.; Aicher, A.; Adler, K.; Urbich, C.; Martin, H.; Zeiher, A.M.; Dimmeler, S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ. Res. 2001, 89, E1–E7. [Google Scholar] [CrossRef] [Green Version]
- Iwaguro, H.; Yamaguchi, J.-I.; Kalka, C.; Murasawa, S.; Masuda, H.; Hayashi, S.-I.; Silver, M.; Li, T.; Isner, J.M.; Asahara, T. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation 2002, 105, 732–738. [Google Scholar] [CrossRef]
- Shimpo, M.; Ikeda, U.; Maeda, Y.; Takahashi, M.; Miyashita, H.; Mizukami, H.; Urabe, M.; Kume, A.; Takizawa, T.; Shibuya, M.; et al. AAV-mediated VEGF gene transfer into skeletal muscle stimulates angiogenesis and improves blood flow in a rat hindlimb ischemia model. Cardiovasc. Res. 2002, 53, 993–1001. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Wang, B.; Wang, C.; He, B.; Fan, H.; Shao, Q.; Gao, L.; Liu, Y.; Yan, G.; Pu, J. In vivo enhancement of angiogenesis by adenoviral transfer of HIF-1alpha-modified endothelial progenitor cells (Ad-HIF-1alpha-modified EPC for angiogenesis). Int. J. Biochem. Cell Biol. 2008, 40, 2284–2295. [Google Scholar] [CrossRef]
- Kuliszewski, M.A.; Kobulnik, J.; Lindner, J.R.; Stewart, D.J.; Leong-Poi, H. Vascular gene transfer of SDF-1 promotes endothelial progenitor cell engraftment and enhances angiogenesis in ischemic muscle. Mol. Ther. 2011, 19, 895–902. [Google Scholar] [CrossRef]
- Hiasa, K.I.; Ishibashi, M.; Ohtani, K.; Inoue, S.; Zhao, Q.; Kitamoto, S.; Sata, M.; Ichiki, T.; Takeshita, A.; Egashira, K. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: Next-generation chemokine therapy for therapeutic neovascularization. Circulation 2004, 109, 2454–2461. [Google Scholar]
- Sundararaman, S.; Miller, T.J.; Pastore, J.M.; Kiedrowski, M.; Aras, R.; Penn, M.S. Plasmid-based transient human stromal cell-derived factor-1 gene transfer improves cardiac function in chronic heart failure. Gene Ther. 2011, 18, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Chavakis, E.; Aicher, A.; Heeschen, C.; Sasaki, K.I.; Kaiser, R.; El Makhfi, N.; Urbich, C.; Peters, T.; Scharffetter-Kochanek, K.; Zeiher, A.M.; et al. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J. Exp. Med. 2005, 201, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ip, J.E.; Huang, J.; Zhang, L.; Matsushita, K.; Liew, C.C.; Pratt, R.E.; Dzau, V.J. Essential role of ICAM-1/CD18 in mediating EPC recruitment, angiogenesis, and repair to the infarcted myocardium. Circ. Res. 2006, 99, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Yoon, C.-H.; Hur, J.; Oh, I.-Y.; Park, K.-W.; Kim, T.-Y.; Shin, J.-H.; Kim, J.-H.; Lee, C.-S.; Chung, J.-K.; Park, Y.-B.; et al. Intercellular adhesion molecule-1 is upregulated in ischemic muscle, which mediates trafficking of endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1066–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiss, C.; Keymel, S.; Niesler, U.; Ziemann, J.; Kelm, M.; Kalka, C. Impaired progenitor cell activity in age-related endothelial dysfunction. J. Am. Coll. Cardiol. 2005, 45, 1441–1448. [Google Scholar] [CrossRef] [Green Version]
- Jie, K.E.; Goossens, M.H.; van Oostrom, O.; Lilien, M.R.; Verhaar, M.C. Circulating endothelial progenitor cell levels are higher during childhood than in adult life. Atherosclerosis 2009, 202, 345–347. [Google Scholar] [CrossRef]
- Madonna, R.; Novo, G.; Balistreri, C.R. Cellular and molecular basis of the imbalance between vascular damage and repair in ageing and age-related diseases: As biomarkers and targets for new treatments. Mech. Ageing Dev. 2016, 159, 22–30. [Google Scholar] [CrossRef]
- Sanada, F.; Taniyama, Y.; Azuma, J.; Iekushi, K.; Dosaka, N.; Yokoi, T.; Koibuchi, N.; Kusunoki, H.; Aizawa, Y.; Morishita, R. Hepatocyte Growth Factor, but not Vascular Endothelial Growth Factor, Attenuates Angiotensin II–Induced Endothelial Progenitor Cell Senescence. Hypertension 2009, 53, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Thum, T.; Hoeber, S.; Froese, S.; Klink, I.; Stichtenoth, D.O.; Galuppo, P.; Jakob, M.; Tsikas, D.; Anker, S.D.; Poole-Wilson, P.A.; et al. Age-dependent impairment of endothelial progenitor cells is corrected by growth hormone mediated increase of insulin-like growth factor-1. Circ. Res. 2007, 100, 434–443. [Google Scholar] [CrossRef] [Green Version]
- Xia, W.H.; Chen, L.; Liang, J.W.; Zhang, X.Y.; Su, C.; Tong, X.; He, J.; Li, Y.; Cao, Z.; Lin, X.F.; et al. BMP4/Id2 signaling pathway is a novel therapeutic target for late outgrowth endothelial progenitor cell-mediated endothelial injury repair. Int. J. Cardiol. 2017, 228, 796–804. [Google Scholar] [CrossRef]
- Li, X.; Xue, X.; Sun, Y.; Chen, L.; Zhao, T.; Yang, W.; Chen, Y.; Zhang, Z. MicroRNA-326-5p enhances therapeutic potential of endothelial progenitor cells for myocardial infarction. Stem Cell Res. Ther. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Z.; Qi, Y.; Zhang, W.; Zhang, C.; Jiang, M.; Deng, S.; Wang, H. Exosomes from SIRT1-overexpressing ADSCs restore cardiac function by improving angiogenic function of EPCs. Mol. Ther. Nucleic Acids 2020, 21, 737–750. [Google Scholar] [CrossRef]
- Masuda, H.; Tanaka, R.; Fujimura, S.; Ishikawa, M.; Akimaru, H.; Shizuno, T.; Sato, A.; Okada, Y.; Iida, Y.; Itoh, J.; et al. Vasculogenic Conditioning of Peripheral Blood Mononuclear Cells Promotes Endothelial Progenitor Cell Expansion and Phenotype Transition of Anti-Inflammatory Macrophage and T Lymphocyte to Cells With Regenerative Potential. J. Am. Heart Assoc. 2014, 3, e000743. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, R.; Masuda, H.; Fujimura, S.; Ito-Hirano, R.; Arita, K.; Kakinuma, Y.; Hagiwara, H.; Kado, M.; Hayashi, A.; Mita, T.; et al. Quality-quantity control culture enhances vasculogenesis and wound healing efficacy of human diabetic peripheral blood CD34+ cells. Stem Cells Transl. Med. 2018, 7, 428–438. [Google Scholar] [CrossRef]
- Chruewkamlow, N.; Pruekprasert, K.; Phutthakunphithak, P.; Acharayothin, O.; Prapassaro, T.; Hongku, K.; Hahtapornsawan, S.; Puangpunngam, N.; Chinsakchai, K.; Wongwanit, C.; et al. Novel culture media enhances mononuclear cells from patients with chronic limb-threatening ischemia to increase vasculogenesis and anti-inflammatory effect. Stem Cell Res. Ther. 2021, 12, 1–8. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canjuga, D.; Steinle, H.; Mayer, J.; Uhde, A.-K.; Klein, G.; Wendel, H.P.; Schlensak, C.; Avci-Adali, M. Homing of mRNA-Modified Endothelial Progenitor Cells to Inflamed Endothelium. Pharmaceutics 2022, 14, 1194. https://doi.org/10.3390/pharmaceutics14061194
Canjuga D, Steinle H, Mayer J, Uhde A-K, Klein G, Wendel HP, Schlensak C, Avci-Adali M. Homing of mRNA-Modified Endothelial Progenitor Cells to Inflamed Endothelium. Pharmaceutics. 2022; 14(6):1194. https://doi.org/10.3390/pharmaceutics14061194
Chicago/Turabian StyleCanjuga, Denis, Heidrun Steinle, Jana Mayer, Ann-Kristin Uhde, Gerd Klein, Hans Peter Wendel, Christian Schlensak, and Meltem Avci-Adali. 2022. "Homing of mRNA-Modified Endothelial Progenitor Cells to Inflamed Endothelium" Pharmaceutics 14, no. 6: 1194. https://doi.org/10.3390/pharmaceutics14061194
APA StyleCanjuga, D., Steinle, H., Mayer, J., Uhde, A. -K., Klein, G., Wendel, H. P., Schlensak, C., & Avci-Adali, M. (2022). Homing of mRNA-Modified Endothelial Progenitor Cells to Inflamed Endothelium. Pharmaceutics, 14(6), 1194. https://doi.org/10.3390/pharmaceutics14061194