When Natural Compounds Meet Nanotechnology: Nature-Inspired Nanomedicines for Cancer Immunotherapy
Abstract
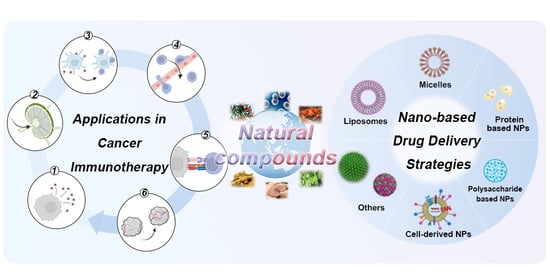
:1. Introduction
2. Immunoregulatory Mechanisms of Natural Compounds in the Cancer-Immunity Cycle
2.1. Natural Compounds Triggered Antigen Release
2.2. Natural Compounds Promoted Antigen Presentation by APCs
2.3. Natural Compounds Promoted T Cell Priming and Activation
2.4. Natural Compounds Promoted T Cell Trafficking and Infiltration into Tumors
2.5. Natural Compounds Promoted Recognition of Cancer Cells
2.6. Natural Compounds Promoted the Killing of Cancer Cells
3. Nature-Inspired Nanomedicine for Cancer Immunotherapy
3.1. Lipid-Based Nanomedicines
3.1.1. Liposomes
3.1.2. High-Density Lipoproteins (HDLs)
3.1.3. Nanoemulsions
3.2. Micelle-Based Nanomedicines
3.3. Polysaccharide-Based Nanomedicines
3.4. Peptide-Based Nanomedicines
3.5. Protein-Based Nanomedicines
3.6. Cell-Derived Nanomedicines
3.6.1. Living Cell-Mediated Nanoparticles
3.6.2. Cell Membrane Camouflaged Nanoparticles (CM-NPs)
3.7. Carrier-Free Nanomedicines
4. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mazumder, A.; Cerella, C.; Diederich, M. Natural scaffolds in anticancer therapy and precision medicine. Biotechnol. Adv. 2018, 36, 1563–1585. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Zhou, H.; Wang, Y.F.; Sang, B.S.; Liu, L. Current Policies and Measures on the Development of Traditional Chinese Medicine in China. Pharmacol. Res. 2021, 163, 105187. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Xu, M.; Zhou, Y.; Li, X.; Wang, Z.; Liu, X.; Meng, X.; Zeng, Y.; Zhang, H. The Status quo and way forwards on the development of Tibetan medicine and the pharmacological research of tibetan materia Medica. Pharmacol. Res. 2020, 155, 104688. [Google Scholar] [CrossRef] [PubMed]
- Rupani, R.; Chavez, A. Medicinal plants with traditional use: Ethnobotany in the Indian subcontinent. Clin. Dermatol. 2018, 36, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Jarić, S.; Kostić, O.; Mataruga, Z.; Pavlović, D.; Pavlović, M.; Mitrović, M.; Pavlović, P. Traditional wound-healing plants used in the Balkan region (Southeast Europe). J. Ethnopharmacol. 2018, 211, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Cock, I.E.; Ndlovu, N.; Van Vuuren, S.F. The use of South African botanical species for the control of blood sugar. J. Ethnopharmacol. 2021, 264, 113234. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, S.; Jin, C.; Cho, S.Y.; Park, S.U.; Jung, W.S.; Moon, S.K.; Park, J.M.; Ko, C.N.; Cho, K.H. Traditional East Asian Herbal Medicine Treatment for Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Pharmaceuticals 2022, 15, 174. [Google Scholar] [CrossRef]
- Yao, C.L.; Zhang, J.Q.; Li, J.Y.; Wei, W.L.; Wu, S.F.; Guo, D.A. Traditional Chinese medicine (TCM) as a source of new anticancer drugs. Nat. Prod. Rep. 2021, 38, 1618–1633. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Sanmamed, M.F.; Chen, L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2018, 175, 313–326. [Google Scholar] [CrossRef] [Green Version]
- Szeto, G.L.; Finley, S.D. Integrative Approaches to Cancer Immunotherapy. Trends Cancer 2019, 5, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.S.; Chen, D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020, 52, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Kubli, S.P.; Berger, T.; Araujo, D.V.; Siu, L.L.; Mak, T.W. Beyond immune checkpoint blockade: Emerging immunological strategies. Nat. Rev. Drug Discov. 2021, 20, 899–919. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.B.; Salama, A.K.S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, K.G.; Vrabel, M.R.; Mantooth, S.M.; Hopkins, J.J.; Wagner, E.S.; Gabaldon, T.A.; Zaharoff, D.A. Localized Interleukin-12 for Cancer Immunotherapy. Front. Immunol. 2020, 11, 575597. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Fang, J.; Fan, X.; Miyata, T.; Hu, X.; Zhang, L.; Zhang, L.; Cui, Y.; Liu, Z.; Wu, X. Advances in Molecular Mechanisms for Traditional Chinese Medicine Actions in Regulating Tumor Immune Responses. Front. Pharmacol. 2020, 11, 1009. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Piazzini, V.; Risaliti, L.; Vanti, G.; Casamonti, M.; Wang, M.; Bergonzi, M.C. Nanocarriers: A Successful Tool to Increase Solubility, Stability and Optimise Bioefficacy of Natural Constituents. Curr. Med. Chem. 2019, 26, 4631–4656. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, H.; Qiao, W.; Cheng, J.; Han, Y.; Yang, X. Nanomedicine-Cum-Carrier by Co-Assembly of Natural Small Products for Synergistic Enhanced Antitumor with Tissues Protective Actions. ACS Appl. Bio Mater. 2020, 12, 42537–42550. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, V.V.; Svistunov, A.A.; Chubarev, V.N.; Dostdar, S.A.; Sokolov, A.V.; Brzecka, A.; Sukocheva, O.; Neganova, M.E.; Klochkov, S.G.; Somasundaram, S.G.; et al. Extracellular vesicles in cancer nanomedicine. Semin. Cancer Biol. 2021, 69, 212–225. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Qiu, N.; Zhou, Q.; Wang, G.; Jiang, H.; Piao, Y.; Zhou, Z.; Tang, J.; Shen, Y. Co-delivery of IOX1 and doxorubicin for antibody-independent cancer chemo-immunotherapy. Nat. Commun. 2021, 12, 2425. [Google Scholar] [CrossRef]
- Hu, Q.; Shang, L.; Wang, M.; Tu, K.; Hu, M.; Yu, Y.; Xu, M.; Kong, L.; Guo, Y.; Zhang, Z. Co-Delivery of Paclitaxel and Interleukin-12 Regulating Tumor Microenvironment for Cancer Immunochemotherapy. Adv. Healthc. Mater. 2020, 9, e1901858. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, N.; Plengsuriyakarn, T.; Na-Bangchang, K. Application of active targeting nanoparticle delivery system for chemotherapeutic drugs and traditional/herbal medicines in cancer therapy: A systematic review. Int. J. Nanomed. 2018, 13, 3921–3935. [Google Scholar] [CrossRef] [Green Version]
- Lagoa, R.; Silva, J.; Rodrigues, J.R.; Bishayee, A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol. Adv. 2020, 38, 107382. [Google Scholar] [CrossRef]
- Kundu, M.; Majumder, R.; Das, C.K.; Mandal, M. Natural products based nanoformulations for cancer treatment: Current evolution in Indian research. Biomed. Mater. 2021, 16, 044101. [Google Scholar] [CrossRef]
- Jiang, M.; Zeng, J.; Zhao, L.; Zhang, M.; Ma, J.; Guan, X.; Zhang, W. Chemotherapeutic drug-induced immunogenic cell death for nanomedicine-based cancer chemo-immunotherapy. Nanoscale 2021, 13, 17218–17235. [Google Scholar] [CrossRef]
- Casares, N.; Pequignot, M.O.; Tesniere, A.; Ghiringhelli, F.; Roux, S.; Chaput, N.; Schmitt, E.; Hamai, A.; Hervas-Stubbs, S.; Obeid, M.; et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 2005, 202, 1691–1701. [Google Scholar] [CrossRef]
- Giglio, P.; Gagliardi, M.; Tumino, N.; Antunes, F.; Smaili, S.; Cotella, D.; Santoro, C.; Bernardini, R.; Mattei, M.; Piacentini, M.; et al. PKR and GCN2 stress kinases promote an ER stress-independent eIF2α phosphorylation responsible for calreticulin exposure in melanoma cells. Oncoimmunology 2018, 7, e1466765. [Google Scholar] [CrossRef] [Green Version]
- Lau, T.S.; Chan, L.K.Y.; Man, G.C.W.; Wong, C.H.; Lee, J.H.S.; Yim, S.F.; Cheung, T.H.; McNeish, I.A.; Kwong, J. Paclitaxel Induces Immunogenic Cell Death in Ovarian Cancer via TLR4/IKK2/SNARE-Dependent Exocytosis. Cancer Immunol. Res. 2020, 8, 1099–1111. [Google Scholar] [CrossRef]
- Yin, S.Y.; Efferth, T.; Jian, F.Y.; Chen, Y.H.; Liu, C.I.; Wang, A.H.; Chen, Y.R.; Hsiao, P.W.; Yang, N.S. Immunogenicity of mammary tumor cells can be induced by shikonin via direct binding-interference with hnRNPA1. Oncotarget 2016, 7, 43629–43653. [Google Scholar] [CrossRef]
- Lin, S.Y.; Hsieh, S.Y.; Fan, Y.T.; Wei, W.C.; Hsiao, P.W.; Tsai, D.H.; Wu, T.S.; Yang, N.S. Necroptosis promotes autophagy-dependent upregulation of DAMP and results in immunosurveillance. Autophagy 2018, 14, 778–795. [Google Scholar] [CrossRef] [Green Version]
- Kessel, D. Hypericin Accumulation as a Determinant of PDT Efficacy. Photochem. Photobiol. 2020, 96, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yang, L.; Shi, H.; Du, W.; Qi, Y.; Qiu, C.; Liang, X.; Shi, W.; Liu, J. Endoplasmic reticulum-targeting photosensitizer Hypericin confers chemo-sensitization towards oxaliplatin through inducing pro-death autophagy. Int. J. Biochem. Cell Biol. 2017, 87, 54–68. [Google Scholar] [CrossRef]
- Menger, L.; Vacchelli, E.; Adjemian, S.; Martins, I.; Ma, Y.; Shen, S.; Yamazaki, T.; Sukkurwala, A.Q.; Michaud, M.; Mignot, G.; et al. Cardiac glycosides exert anticancer effects by inducing immunogenic cell death. Sci. Transl. Med. 2012, 4, 143ra199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diederich, M.; Muller, F.; Cerella, C. Cardiac glycosides: From molecular targets to immunogenic cell death. Biochem. Pharmacol. 2017, 125, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schneider, N.F.Z.; Cerella, C.; Simões, C.M.O.; Diederich, M. Anticancer and Immunogenic Properties of Cardiac Glycosides. Molecules 2017, 22, 1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zheng, J.; Chen, S.; Meng, F.D.; Ning, J.; Sun, S.L. Oleandrin, a cardiac glycoside, induces immunogenic cell death via the PERK/elF2α/ATF4/CHOP pathway in breast cancer. Cell Death Dis. 2021, 12, 314. [Google Scholar] [CrossRef]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.; Sun, P.; Xu, N.; Liao, M.; Xu, C.; Ding, Y.; Cai, J.; Zhang, Y.; Xie, W. Canagliflozin inhibits p-gp function and early autophagy and improves the sensitivity to the antitumor effect of doxorubicin. Biochem. Pharmacol. 2020, 175, 113856. [Google Scholar] [CrossRef]
- Altomare, C.; Lodrini, A.M.; Milano, G.; Biemmi, V.; Lazzarini, E.; Bolis, S.; Pernigoni, N.; Torre, E.; Arici, M.; Ferrandi, M.; et al. Structural and Electrophysiological Changes in a Model of Cardiotoxicity Induced by Anthracycline Combined With Trastuzumab. Front. Physiol. 2021, 12, 658790. [Google Scholar] [CrossRef]
- Manoury, B.; Maisonneuve, L.; Podsypanina, K. The role of endoplasmic reticulum stress in the MHC class I antigen presentation pathway of dendritic cells. Mol. Immunol. 2022, 144, 44–48. [Google Scholar] [CrossRef]
- Sabado, R.L.; Balan, S.; Bhardwaj, N. Dendritic cell-based immunotherapy. Cell Res. 2017, 27, 74–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.; He, Y.; Huang, J.; Tao, Y.; Liu, S. Metabolism of Dendritic Cells in Tumor Microenvironment: For Immunotherapy. Front. Immunol. 2021, 12, 613492. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Hu, X.; Shan, F.; Hua, H.; Lu, C.; Wang, E.; Liang, Z. Analysis of maturation of murine dendritic cells (DCs) induced by purified Ganoderma lucidum polysaccharides (GLPs). Int. J. Biol. Macromol. 2011, 49, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yang, Y.; Gad, E.; Wenner, C.A.; Chang, A.; Larson, E.R.; Dang, Y.; Martzen, M.; Standish, L.J.; Disis, M.L. Polysaccharide krestin is a novel TLR2 agonist that mediates inhibition of tumor growth via stimulation of CD8 T cells and NK cells. Clin. Cancer Res. 2011, 17, 67–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.L.; Ying, Y.; Xu, Y.L.; Su, J.F.; Luo, H.; Wang, H.F. Effects of Lycium barbarum polysaccharide on tumor microenvironment T-lymphocyte subsets and dendritic cells in H22-bearing mice. Zhong Xi Yi Jie He Xue Bao 2005, 3, 374–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, K.K. Personalized cancer vaccines. Expert Opin. Biol. Ther. 2010, 10, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Cueto, F.J.; Sancho, D. The Flt3L/Flt3 Axis in Dendritic Cell Biology and Cancer Immunotherapy. Cancers 2021, 13, 1525. [Google Scholar] [CrossRef]
- Yang, Y.; Nam, G.-H.; Kim, G.B.; Kim, Y.K.; Kim, I.-S. Intrinsic cancer vaccination. Adv. Drug Deliv. Rev. 2019, 151–152, 2–22. [Google Scholar] [CrossRef]
- Choi, Y.; Shi, Y.; Haymaker, C.L.; Naing, A.; Ciliberto, G.; Hajjar, J. T-cell agonists in cancer immunotherapy. J. Immunother. Cancer 2020, 8, e000966. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Lin, Q.; Zhang, Z.; Zhang, L. Therapeutic strategies for the costimulatory molecule OX40 in T-cell-mediated immunity. Acta Pharm. Sin. B 2020, 10, 414–433. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.-J.; Lu, Z.-Q.; Tang, L.-M.; Wu, Z.-S.; Wang, D.-W.; Zheng, J.-Y.; Qiu, Q.-M. Curcumin inhibits suppressive capacity of naturally occurring CD4+CD25+ regulatory T cells in mice in vitro. Int. Immunopharmacol. 2012, 14, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Yu, L.; Zhao, L.-Z. Curcumin up regulates T helper 1 cells in patients with colon cancer. Am. J. Transl. Res. 2017, 9, 1866–1875. [Google Scholar]
- Zou, J.Y.; Su, C.H.; Luo, H.H.; Lei, Y.Y.; Zeng, B.; Zhu, H.S.; Chen, Z.G. Curcumin converts Foxp3+ regulatory T cells to T helper 1 cells in patients with lung cancer. J. Cell. Biochem. 2018, 119, 1420–1428. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Md Sakib Hossain, D.; Mohanty, S.; Sankar Sen, G.; Chattopadhyay, S.; Banerjee, S.; Chakraborty, J.; Das, K.; Sarkar, D.; Das, T.; et al. Curcumin reverses T cell-mediated adaptive immune dysfunctions in tumor-bearing hosts. Cell Mol. Immunol. 2010, 7, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.X.; Xie, Y.; Ye, Y.P. Advances in saponin-based adjuvants. Vaccine 2009, 27, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Jackson, H.; Parente, P.; Luke, T.; Rizkalla, M.; Tai, T.Y.; Zhu, H.-C.; Mifsud, N.A.; Dimopoulos, N.; Masterman, K.-A.; et al. Immunodominant CD4+ responses identified in a patient vaccinated with full-length NY-ESO-1 formulated with ISCOMATRIX adjuvant. Proc. Natl. Acad. Sci. USA 2004, 101, 9363–9368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waite, D.C.; Jacobson, E.W.; Ennis, F.A.; Edelman, R.; White, B.; Kammer, R.; Anderson, C.; Kensil, C.R. Three double-blind, randomized trials evaluating the safety and tolerance of different formulations of the saponin adjuvant QS-21. Vaccine 2001, 19, 3957–3967. [Google Scholar] [CrossRef]
- Yang, Y.; Paik, J.H.; Cho, D.; Cho, J.-A.; Kim, C.-W. Resveratrol induces the suppression of tumor-derived CD4+CD25+ regulatory T cells. Int. Immunopharmacol. 2008, 8, 542–547. [Google Scholar] [CrossRef]
- Chen, L.; Yang, S.; Liao, W.; Xiong, Y. Modification of Antitumor Immunity and Tumor Microenvironment by Resveratrol in Mouse Renal Tumor Model. Cell Biochem. Biophys. 2015, 72, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.J.; Qi, M.; Li, N.; Lei, Y.H.; Zhang, D.M.; Chen, J.X. Natural products and their derivatives: Promising modulators of tumor immunotherapy. J. Leukoc. Biol. 2020, 108, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Feng, Y.H.; Gao, L.W.; Li, X.Y.; Jin, Q.X.; Wang, Y.Y.; Xu, Y.Y.; Jin, F.; Lu, S.L.; Wei, M.J. Artemisinin enhances the anti-tumor immune response in 4T1 breast cancer cells in vitro and in vivo. Int. Immunopharmacol. 2019, 70, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Schwager, J.; Seifert, N.; Bompard, A.; Raederstorff, D.; Bendik, I. Resveratrol, EGCG and Vitamins Modulate Activated T Lymphocytes. Molecules 2021, 26, 5600. [Google Scholar] [CrossRef]
- Anandappa, A.J.; Wu, C.J.; Ott, P.A. Directing Traffic: How to Effectively Drive T Cells into Tumors. Cancer Discov. 2020, 10, 185–197. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Sun, Z.-J. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics 2021, 11, 5365–5386. [Google Scholar] [CrossRef]
- Manaster, Y.; Shipony, Z.; Hutzler, A.; Kolesnikov, M.; Avivi, C.; Shalmon, B.; Barshack, I.; Besser, M.J.; Feferman, T.; Shakhar, G. Reduced CTL motility and activity in avascular tumor areas. Cancer Immunol. Immun. 2019, 68, 1287–1301. [Google Scholar] [CrossRef]
- Obermajer, N.; Urban, J.; Wieckowski, E.; Muthuswamy, R.; Ravindranathan, R.; Bartlett, D.L.; Kalinski, P. Promoting the accumulation of tumor-specific T cells in tumor tissues by dendritic cell vaccines and chemokine-modulating agents. Nat. Protoc. 2018, 13, 335–357. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.S.; Yang, H.; Chon, H.J.; Kim, C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp. Mol. Med. 2020, 52, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Wallin, J.J.; Bendell, J.C.; Funke, R.; Sznol, M.; Korski, K.; Jones, S.; Hernandez, G.; Mier, J.; He, X.; Hodi, F.S.; et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat. Commun. 2016, 7, 12624. [Google Scholar] [CrossRef]
- Chakraborty, K.; Bose, A.; Pal, S.; Sarkar, K.; Goswami, S.; Ghosh, D.; Laskar, S.; Chattopadhyay, U.; Baral, R. Neem leaf glycoprotein restores the impaired chemotactic activity of peripheral blood mononuclear cells from head and neck squamous cell carcinoma patients by maintaining CXCR3/CXCL10 balance. Int. Immunopharmacol. 2008, 8, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, Z.; Zhu, J.; Chen, X.; Hao, Y.; Yang, R.; Huang, R.; Zhou, J.; Wang, Z.; Xiao, W.; et al. Systems pharmacology dissection of Epimedium targeting tumor microenvironment to enhance cytotoxic T lymphocyte responses in lung cancer. Aging 2021, 13, 2912–2940. [Google Scholar] [CrossRef] [PubMed]
- Williamson, J.D.; Sadofsky, L.R.; Crooks, M.G.; Greenman, J.; Hart, S.P. Bleomycin increases neutrophil adhesion to human vascular endothelial cells independently of upregulation of ICAM-1 and E-selectin. Exp. Lung Res. 2016, 42, 397–407. [Google Scholar] [CrossRef]
- Millrose, M.; Kruse, M.; Flick, B.; Stahlmann, R. Effects of macrolides on proinflammatory epitopes on endothelial cells in vitro. Arch. Toxicol. 2009, 83, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Lapeyre-Prost, A.; Terme, M.; Pernot, S.; Pointet, A.L.; Voron, T.; Tartour, E.; Taieb, J. Immunomodulatory Activity of VEGF in Cancer. Int. Rev. Cell Mol. Biol. 2017, 330, 295–342. [Google Scholar]
- Wang, L.; Wu, H.; Xiong, L.; Liu, X.; Yang, N.; Luo, L.; Qin, T.; Zhu, X.; Shen, Z.; Jing, H.; et al. Quercetin Downregulates Cyclooxygenase-2 Expression and HIF-1alpha/VEGF Signaling-Related Angiogenesis in a Mouse Model of Abdominal Aortic Aneurysm. BioMed Res. Int. 2020, 2020, 9485398. [Google Scholar] [PubMed]
- Wang, F.R.; Jiang, Y.S. Effect of treatment with baicalein on the intracerebral tumor growth and survival of orthotopic glioma models. J. Neurooncol. 2015, 124, 5–11. [Google Scholar] [CrossRef]
- Bae, M.K.; Kim, S.H.; Jeong, J.W.; Lee, Y.M.; Kim, H.S.; Kim, S.R.; Yun, I.; Bae, S.K.; Kim, K.W. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol. Rep. 2006, 15, 1557–1562. [Google Scholar] [CrossRef]
- Chandran, S.S.; Klebanoff, C.A. T cell receptor-based cancer immunotherapy: Emerging efficacy and pathways of resistance. Immunol. Rev. 2019, 290, 127–147. [Google Scholar] [CrossRef]
- Ariza, M.E.; Ramakrishnan, R.; Singh, N.P.; Chauhan, A.; Nagarkatti, P.S.; Nagarkatti, M. Bryostatin-1, a Naturally Occurring Antineoplastic Agent, Acts as a Toll-like Receptor 4 (TLR-4) Ligand and Induces Unique Cytokines and Chemokines in Dendritic Cells. J. Biol. Chem. 2011, 286, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Hardman, C.; Ho, S.; Shimizu, A.; Luu-Nguyen, Q.; Sloane, J.L.; Soliman, M.S.A.; Marsden, M.D.; Zack, J.A.; Wender, P.A. Synthesis and evaluation of designed PKC modulators for enhanced cancer immunotherapy. Nat. Commun. 2020, 11, 1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Van der Jeught, K.; Zhou, Z.; Zhang, L.; Yu, T.; Sun, Y.; Li, Y.; Wan, C.; So, K.M.; Liu, D.; et al. Atractylenolide I enhances responsiveness to immune checkpoint blockade therapy by activating tumor antigen presentation. J. Clin. Investig. 2021, 131, e146832. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lostao, L.; Anel, A.; Pardo, J. How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clin. Cancer Res. 2015, 21, 5047–5056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaddepally, R.K.; Kharel, P.; Pandey, R.; Garje, R.; Chandra, A.B. Review of Indications of FDA-Approved Immune Checkpoint Inhibitors per NCCN Guidelines with the Level of Evidence. Cancers 2020, 12, 738. [Google Scholar] [CrossRef] [Green Version]
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2021, 184, 5309–5337. [Google Scholar] [CrossRef]
- Coombs, M.R.P.; Harrison, M.E.; Hoskin, D.W. Apigenin inhibits the inducible expression of programmed death ligand 1 by human and mouse mammary carcinoma cells. Cancer Lett. 2016, 380, 424–433. [Google Scholar] [CrossRef]
- Jiang, Z.B.; Wang, W.J.; Xu, C.; Xie, Y.J.; Wang, X.R.; Zhang, Y.Z.; Huang, J.M.; Huang, M.; Xie, C.; Liu, P.; et al. Luteolin and its derivative apigenin suppress the inducible PD-L1 expression to improve anti-tumor immunity in KRAS-mutant lung cancer. Cancer Lett. 2021, 515, 36–48. [Google Scholar] [CrossRef]
- Ma, Y.; Yan, G.; Guo, J.; Li, F.; Zheng, H.; Wang, C.; Chen, Y.; Ye, Y.; Dai, H.; Qi, Z.; et al. Berberine Prolongs Mouse Heart Allograft Survival by Activating T Cell Apoptosis via the Mitochondrial Pathway. Front. Immunol. 2021, 12, 616074. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Zhang, N.; Yin, M.; Dong, J.; Zeng, Q.; Mao, G.; Song, D.; Liu, L.; Deng, H. Berberine diminishes cancer cell PD-L1 expression and facilitates antitumor immunity via inhibiting the deubiquitination activity of CSN5. Acta Pharm. Sin. B 2020, 10, 2299–2312. [Google Scholar] [CrossRef]
- Rawangkan, A.; Wongsirisin, P.; Namiki, K.; Iida, K.; Kobayashi, Y.; Shimizu, Y.; Fujiki, H.; Suganuma, M. Green Tea Catechin Is an Alternative Immune Checkpoint Inhibitor that Inhibits PD-L1 Expression and Lung Tumor Growth. Molecules 2018, 23, 2071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravindran Menon, D.; Li, Y.; Yamauchi, T.; Osborne, D.G.; Vaddi, P.K.; Wempe, M.F.; Zhai, Z.; Fujita, M. EGCG Inhibits Tumor Growth in Melanoma by Targeting JAK-STAT Signaling and Its Downstream PD-L1/PD-L2-PD1 Axis in Tumors and Enhancing Cytotoxic T-Cell Responses. Pharmaceuticals 2021, 14, 1081. [Google Scholar] [CrossRef] [PubMed]
- Verdura, S.; Cuyàs, E.; Cortada, E.; Brunet, J.; Lopez-Bonet, E.; Martin-Castillo, B.; Bosch-Barrera, J.; Encinar, J.A.; Menendez, J.A. Resveratrol targets PD-L1 glycosylation and dimerization to enhance antitumor T-cell immunity. Aging 2020, 12, 8–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Z.; Vong, C.T.; Chen, F.; Tan, H.; Zhang, C.; Wang, N.; Cui, L.; Wang, Y.; Feng, Y. Immunomodulatory potential of natural products from herbal medicines as immune checkpoints inhibitors: Helping to fight against cancer via multiple targets. Med. Res. Rev. 2022, 42, 1246–1279. [Google Scholar] [CrossRef] [PubMed]
- Yentz, S.; Smith, D. Indoleamine 2,3-Dioxygenase (IDO) Inhibition as a Strategy to Augment Cancer Immunotherapy. BioDrugs 2018, 32, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, G.C.; Malachowski, W.J.; Mondal, A.; Scherle, P.; Muller, A.J. Indoleamine 2,3-Dioxygenase and Its Therapeutic Inhibition in Cancer. Nat. Rev. Mol. Cell Biol. 2018, 336, 175–203. [Google Scholar]
- Jeong, Y.I.; Jung, I.D.; Lee, J.S.; Lee, C.M.; Lee, J.D.; Park, Y.M. (−)-Epigallocatechin gallate suppresses indoleamine 2,3-dioxygenase expression in murine dendritic cells: Evidences for the COX-2 and STAT1 as potential targets. Biochem. Biophys. Res. Commun. 2007, 354, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.; DuHadaway, J.B.; Gaspari, P.; Sutanto-Ward, E.; Munn, D.H.; Mellor, A.L.; Malachowski, W.P.; Prendergast, G.C.; Muller, A.J. A key in vivo antitumor mechanism of action of natural product-based brassinins is inhibition of indoleamine 2,3-dioxygenase. Oncogene 2008, 27, 2851–2857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjeldsen, J.W.; Lorentzen, C.L.; Martinenaite, E.; Ellebaek, E.; Donia, M.; Holmstroem, R.B.; Klausen, T.W.; Madsen, C.O.; Ahmed, S.M.; Weis-Banke, S.E.; et al. A phase 1/2 trial of an immune-modulatory vaccine against IDO/PD-L1 in combination with nivolumab in metastatic melanoma. Nat. Med. 2021, 27, 2212–2223. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Koudelka, Š.; Turánek, J. Liposomal paclitaxel formulations. J. Control. Release 2012, 163, 322–334. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Gu, Z.; Da Silva, C.G.; Van der Maaden, K.; Ossendorp, F.; Cruz, L.J. Liposome-Based Drug Delivery Systems in Cancer Immunotherapy. Pharmaceutics 2020, 12, 1054. [Google Scholar] [CrossRef] [PubMed]
- Zahednezhad, F.; Saadat, M.; Valizadeh, H.; Zakeri-Milani, P.; Baradaran, B. Liposome and immune system interplay: Challenges and potentials. J. Control. Release 2019, 305, 194–209. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, S.; Ding, Y.; Chen, D.; Dahiru, N.S.; Tang, H.; Xu, H.; Ji, M.; Wang, X.; Li, Z.; et al. A bio-responsive, cargo-catchable gel for postsurgical tumor treatment via ICD-based immunotherapy. J. Control. Release 2022, 346, 212–225. [Google Scholar] [CrossRef]
- Deng, B.; Ma, B.; Ma, Y.; Cao, P.; Leng, X.; Huang, P.; Zhao, Y.; Ji, T.; Lu, X.; Liu, L. Doxorubicin and CpG loaded liposomal spherical nucleic acid for enhanced Cancer treatment. J. Nanobiotechnol. 2022, 20, 140. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Yu, H.; Chen, M. Glioma-targeted multifunctional nanoparticles to co-deliver camptothecin and curcumin for enhanced chemo-immunotherapy. Biomater. Sci. 2022, 10, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dai, Q.; Yang, Q.; Bao, X.; Zhou, Y.; Zhong, H.; Wu, L.; Wang, T.; Zhang, Z.; Lu, Y.; et al. Therapeutic nucleus-access BNCT drug combined CD47-targeting gene editing in glioblastoma. J. Nanobiotechnol. 2022, 20, 102. [Google Scholar] [CrossRef]
- Li, M.; Yang, Y.; Wei, J.; Cun, X.; Lu, Z.; Qiu, Y.; Zhang, Z.; He, Q. Enhanced chemo-immunotherapy against melanoma by inhibition of cholesterol esterification in CD8+ T cells. Nanomedicine 2018, 14, 2541–2550. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tai, X.; Shi, K.; Ruan, S.; Qiu, Y.; Zhang, Z.; Xiang, B.; He, Q. A New Concept of Enhancing Immuno-Chemotherapeutic Effects against B16F10 Tumor via Systemic Administration by Taking Advantages of the Limitation of EPR Effect. Theranostics 2016, 6, 2141–2160. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, X.-G.; Yang, P.-P.; Qiao, Z.-Y.; Wang, H. Tumor Microenvironmental pH and Enzyme Dual Responsive Polymer-Liposomes for Synergistic Treatment of Cancer Immuno-Chemotherapy. Biomacromolecules 2019, 20, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, D.; Li, W.; Song, B.; Chen, C.; Chen, D.; Hu, H. Enhancing TNBC Chemo-immunotherapy via combination reprogramming tumor immune microenvironment with Immunogenic Cell Death. Int. J. Pharm. 2021, 598, 120333. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Liu, Y.; Zheng, Z.; Ran, W.; Zhai, Y.; Yin, Q.; Zhang, P.; Li, Y. Cocktail Strategy Based on Spatio-Temporally Controlled Nano Device Improves Therapy of Breast Cancer. Adv. Mater. 2019, 31, e1806202. [Google Scholar] [CrossRef] [PubMed]
- Khayrani, A.C.; Mahmud, H.; Oo, A.K.K.; Zahra, M.H.; Oze, M.; Du, J.; Alam, M.J.; Afify, S.M.; Quora, H.A.A.; Shigehiro, T.; et al. Targeting Ovarian Cancer Cells Overexpressing CD44 with Immunoliposomes Encapsulating Glycosylated Paclitaxel. Int. J. Mol. Sci. 2019, 20, 1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, D.; Que, H.; Chen, L.; Lan, T.; Hong, W.; He, C.; Yang, J.; Wei, Y.; Wei, X. Lymph-Node-Targeted Cholesterolized TLR7 Agonist Liposomes Provoke a Safe and Durable Antitumor Response. Nano. Lett. 2021, 21, 7960–7969. [Google Scholar] [CrossRef]
- Miranda, A.; Hamilton, P.T.; Zhang, A.W.; Pattnaik, S.; Becht, E.; Mezheyeuski, A.; Bruun, J.; Micke, P.; de Reynies, A.; Nelson, B.H. Cancer stemness, intratumoral heterogeneity, and immune response across cancers. Proc. Natl. Acad. Sci. USA 2019, 116, 9020–9029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Qiu, Q.; Gao, X.; Yan, X.; Fan, C.; Luo, X.; Liu, X.; Wang, S.; Lai, X.; Song, Y.; et al. Sialic acid conjugate-modified liposomal platform modulates immunosuppressive tumor microenvironment in multiple ways for improved immune checkpoint blockade therapy. J. Control. Release 2021, 337, 393–406. [Google Scholar] [CrossRef]
- Hirsch, H.A.; Iliopoulos, D.; Tsichlis, P.N.; Struhl, K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009, 69, 7507–7511. [Google Scholar] [CrossRef] [Green Version]
- Ben-Aicha, S.; Badimon, L.; Vilahur, G. Advances in HDL: Much More than Lipid Transporters. Int. J. Mol. Sci. 2020, 21, 732. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Chifodya, K.; Han, G.; Jiang, W.; Chen, Y.; Shi, Y.; Xu, Q.; Xi, Y.; Wang, J.; Zhou, J.; et al. High-density lipoprotein in Alzheimer’s disease: From potential biomarkers to therapeutics. J. Control. Release 2021, 338, 56–70. [Google Scholar] [CrossRef]
- Han, Y.; Ding, B.; Zhao, Z.; Zhang, H.; Sun, B.; Zhao, Y.; Jiang, L.; Zhou, J.; Ding, Y. Immune lipoprotein nanostructures inspired relay drug delivery for amplifying antitumor efficiency. Biomaterials 2018, 185, 205–218. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, H.; Han, Y.; Jin, Y.; Wang, G.; Sun, C.; Jiang, W.; Han, G.; Sun, B.; Jiang, Z.; et al. Natural discoidal lipoproteins with tiny modification for tumor extracellular dissociation in antitumor chemoimmunotherapy. Biomaterials 2021, 275, 120859. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Shechter, I.; Anantharamaiah, G.M.; Reddy, S.T.; Van Lenten, B.J.; Fogelman, A.M. Structure and function of HDL mimetics. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 164–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuai, R.; Yuan, W.; Son, S.; Nam, J.; Xu, Y.; Fan, Y.; Schwendeman, A.; Moon, J.J. Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci. Adv. 2018, 4, eaao1736. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Zhang, W.; Tang, J.; Zhou, Z.; Liu, X.; Shen, Y. Improving safety of cancer immunotherapy via delivery technology. Biomaterials 2021, 265, 120407. [Google Scholar] [CrossRef]
- Mohammadi, A.; Jafari, S.M.; Esfanjani, A.F.; Akhavan, S. Application of nano-encapsulated olive leaf extract in controlling the oxidative stability of soybean oil. Food Chem. 2016, 190, 513–519. [Google Scholar] [CrossRef]
- Gharehbeglou, P.; Jafari, S.M.; Homayouni, A.; Hamishekar, H.; Mirzaei, H. Fabrication of double W1/O/W2 nano-emulsions loaded with oleuropein in the internal phase (W1) and evaluation of their release rate. Food Hydrocoll. 2019, 89, 44–55. [Google Scholar] [CrossRef]
- Xue, F.; Li, X.; Qin, L.; Liu, X.; Li, C.; Adhikari, B. Anti-aging properties of phytoconstituents and phyto-nanoemulsions and their application in managing aging-related diseases. Adv. Drug Deliv. Rev. 2021, 176, 113886. [Google Scholar] [CrossRef]
- Jia, L.; Pang, M.; Fan, M.; Tan, X.; Wang, Y.; Huang, M.; Liu, Y.; Wang, Q.; Zhu, Y.; Yang, X. A pH-responsive Pickering Nanoemulsion for specified spatial delivery of Immune Checkpoint Inhibitor and Chemotherapy agent to Tumors. Theranostics 2020, 10, 9956–9969. [Google Scholar] [CrossRef]
- Qiu, N.; Liu, Y.; Liu, Q.; Chen, Y.; Shen, L.; Hu, M.; Zhou, X.; Shen, Y.; Gao, J.; Huang, L. Celastrol nanoemulsion induces immunogenicity and downregulates PD-L1 to boost abscopal effect in melanoma therapy. Biomaterials 2021, 269, 120604. [Google Scholar] [CrossRef]
- Feng, S.-T.; Li, J.; Luo, Y.; Yin, T.; Cai, H.; Wang, Y.; Dong, Z.; Shuai, X.; Li, Z.-P. pH-sensitive nanomicelles for controlled and efficient drug delivery to human colorectal carcinoma LoVo cells. PLoS ONE 2014, 9, e100732. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, C.; Qin, Y.; Wang, Z.; Wang, L.; Wei, X.; Zhou, Y.; Li, Q.; Zhou, H.; Wang, W.; et al. Coordinating antigen cytosolic delivery and danger signaling to program potent cross-priming by micelle-based nanovaccine. Cell Discov. 2017, 3, 17007. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, F.; Hou, L.; Shen, L.; Zhang, X.; Wang, D.; Huang, L. Nanocarrier-Mediated Chemo-Immunotherapy Arrested Cancer Progression and Induced Tumor Dormancy in Desmoplastic Melanoma. ACS Nano 2018, 12, 7812–7825. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, G.; Wang, S.; Yu, G.; Yang, Z.; Lin, L.; Zhou, Z.; Liu, Y.; Dai, Y.; Zhang, F.; et al. In Situ Dendritic Cell Vaccine for Effective Cancer Immunotherapy. ACS Nano 2019, 13, 3083–3094. [Google Scholar] [CrossRef]
- Zhao, M.; Li, J.; Liu, J.; Xu, M.; Ji, H.; Wu, S.; Chen, D.; Hu, H. Charge-switchable nanoparticles enhance Cancer immunotherapy based on mitochondrial dynamic regulation and immunogenic cell death induction. J. Control. Release 2021, 335, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Feng, Z.; Wang, C.; Su, Q.; Song, H.; Zhang, C.; Huang, P.; Liang, X.-J.; Dong, A.; Kong, D.; et al. Co-localized delivery of nanomedicine and nanovaccine augments the postoperative cancer immunotherapy by amplifying T-cell responses. Biomaterials 2020, 230, 119649. [Google Scholar] [CrossRef]
- Qi, J.; Jin, F.; You, Y.; Du, Y.; Liu, D.; Xu, X.; Wang, J.; Zhu, L.; Chen, M.; Shu, G.; et al. Synergistic effect of tumor chemo-immunotherapy induced by leukocyte-hitchhiking thermal-sensitive micelles. Nat. Commun. 2021, 12, 4755. [Google Scholar] [CrossRef]
- Qiu, X.; Qu, Y.; Guo, B.; Zheng, H.; Meng, F.; Zhong, Z. Micellar paclitaxel boosts ICD and chemo-immunotherapy of metastatic triple negative breast cancer. J. Control. Release 2022, 341, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, T.; Ma, X.; Yang, Q.C.; Yang, L.L.; Yang, S.C.; Liang, M.; Xu, Z.; Sun, Z.J. Microenvironment-Responsive Prodrug-Induced Pyroptosis Boosts Cancer Immunotherapy. Adv. Sci. 2021, 8, e2101840. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, M.; Zhou, J.; Liu, Y.; Zong, Q.; Yuan, Y. Tumor-Acidity and Bioorthogonal Chemistry-Mediated On-Site Size Transformation Clustered Nanosystem to Overcome Hypoxic Resistance and Enhance Chemoimmunotherapy. ACS Nano 2022, 16, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Xiao, Z.; Wang, Y.; Huang, J.; An, Y.; Wang, X.; Shuai, X. Codelivery of Anti-PD-1 Antibody and Paclitaxel with Matrix Metalloproteinase and pH Dual-Sensitive Micelles for Enhanced Tumor Chemoimmunotherapy. Small 2020, 16, e1906832. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Li, X.; Yao, M.; Niu, P.; Yuan, X.; Li, K.; Chen, M.; Fu, Z.; Duan, X.; Liu, H.; et al. Programmable prodrug micelle with size-shrinkage and charge-reversal for chemotherapy-improved IDO immunotherapy. Biomaterials 2020, 241, 119901. [Google Scholar] [CrossRef]
- Xiao, Z.; Su, Z.; Han, S.; Huang, J.; Lin, L.; Shuai, X. Dual pH-sensitive nanodrug blocks PD-1 immune checkpoint and uses T cells to deliver NF-κB inhibitor for antitumor immunotherapy. Sci. Adv. 2020, 6, eaay7785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, Z.; Chen, B.; Yin, Q.; Pan, M.; Xia, H.; Zhang, B.; Yan, Y.; Jiang, Z.; Zhang, Q.; et al. Cooperative Self-Assembled Nanoparticle Induces Sequential Immunogenic Cell Death and Toll-Like Receptor Activation for Synergistic Chemo-immunotherapy. Nano Lett. 2021, 21, 4371–4380. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Jiang, X.; Hunziker, P. Carbohydrate-based amphiphilic nano delivery systems for cancer therapy. Nanoscale 2016, 8, 16091–16156. [Google Scholar] [CrossRef]
- Lin, N.; Huang, J.; Dufresne, A. Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: A review. Nanoscale 2012, 4, 3274–3294. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Qi, S.; Yu, X.; Bai, B.; Zhang, X.; Mao, Z.; Huang, F.; Yu, G. A Hybrid Supramolecular Polymeric Nanomedicine for Cascade-Amplified Synergetic Cancer Therapy. Angew. Chem. 2022, 134, e202203786. [Google Scholar]
- Hu, H.; Yuan, W.; Liu, F.-S.; Cheng, G.; Xu, F.-J.; Ma, J. Redox-Responsive Polycation-Functionalized Cotton Cellulose Nanocrystals for Effective Cancer Treatment. ACS Appl. Mater. Interfaces 2015, 7, 8942–8951. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Thambi, T.; Choi, K.Y.; Son, S.; Ko, H.; Lee, M.C.; Jo, D.-G.; Chae, Y.S.; Kang, Y.M.; Lee, J.Y.; et al. Bioreducible Shell-Cross-Linked Hyaluronic Acid Nanoparticles for Tumor-Targeted Drug Delivery. Biomacromolecules 2015, 16, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhang, C.; Qiu, W.X.; Dong, X.; Zheng, D.W.; Wu, W.; Zhang, X.Z. PD-1 Blockade for Improving the Antitumor Efficiency of Polymer-Doxorubicin Nanoprodrug. Small 2018, 14, e1802403. [Google Scholar] [CrossRef]
- Qi, G.-B.; Gao, Y.-J.; Wang, L.; Wang, H. Self-Assembled Peptide-Based Nanomaterials for Biomedical Imaging and Therapy. Adv. Mater. 2018, 30, 1703444. [Google Scholar] [CrossRef]
- Jin, H.; Wan, C.; Zou, Z.; Zhao, G.; Zhang, L.; Geng, Y.; Chen, T.; Huang, A.; Jiang, F.; Feng, J.-P.; et al. Tumor Ablation and Therapeutic Immunity Induction by an Injectable Peptide Hydrogel. ACS Nano 2018, 12, 3295–3310. [Google Scholar] [CrossRef]
- De Santis, E.; Ryadnov, M.G. Peptide self-assembly for nanomaterials: The old new kid on the block. Chem. Soc. Rev. 2015, 44, 8288–8300. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Su, H.; Xu, D.; Dai, W.; Zhang, W.; Wang, Z.; Anderson, C.F.; Zheng, M.; Oh, R.; Wan, F.; et al. Tumour sensitization via the extended intratumoural release of a STING agonist and camptothecin from a self-assembled hydrogel. Nat. Biomed. Eng. 2020, 4, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yi, Y.; Luo, Z.; Gong, X.; Jiang, Y.; Hou, D.; Zhang, L.; Liu, Z.; Wang, M.; Wang, J.; et al. Selenopeptide Nanomedicine Activates Natural Killer Cells for Enhanced Tumor Chemoimmunotherapy. Adv. Mater. 2022, 34, 2108167. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, Q.; Guo, J.; Zhao, X.; Zhong, Z. Robust and smart polypeptide-based nanomedicines for targeted tumor therapy. Adv. Drug Deliv. Rev. 2020, 160, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.L.; Dai, X.; Yang, J.; Zhang, B.; Zhao, D.H.; Li, C.Q.; Yin, Z.Y.; Zhao, Y.D.; Liu, B. Injectable polypeptide-engineered hydrogel depot for amplifying the anti-tumor immune effect induced by chemo-photothermal therapy. J. Mater. Chem. B 2020, 8, 8623–8633. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Yao, S.; Fan, L.; Fang, Z.; Miao, W.; Hu, Z.; Wang, Z. Fibroblast Activation Protein-α Responsive Peptide Assembling Prodrug Nanoparticles for Remodeling the Immunosuppressive Microenvironment and Boosting Cancer Immunotherapy. Small 2022, 18, e2106296. [Google Scholar] [CrossRef]
- Jain, A.; Singh, S.K.; Arya, S.K.; Kundu, S.C.; Kapoor, S. Protein Nanoparticles: Promising Platforms for Drug Delivery Applications. ACS Biomater. Sci. Eng. 2018, 4, 3939–3961. [Google Scholar] [CrossRef]
- Kuan, S.L.; Bergamini, F.R.G.; Weil, T. Functional protein nanostructures: A chemical toolbox. Chem. Soc. Rev. 2018, 47, 9069–9105. [Google Scholar] [CrossRef] [Green Version]
- Kratz, F. A clinical update of using albumin as a drug vehicle—A commentary. J. Control. Release 2014, 190, 331–336. [Google Scholar] [CrossRef]
- Lee-Chang, C.; Bodogai, M.; Martin-Montalvo, A.; Wejksza, K.; Sanghvi, M.; Moaddel, R.; de Cabo, R.; Biragyn, A. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. J. Immunol. 2013, 191, 4141–4151. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, X.; Xu, Y.; Han, X.; Wang, M.; Gong, T.; Zhang, Z.R.; John Kao, W.; Fu, Y. Hierarchical assembly of hyaluronan coated albumin nanoparticles for pancreatic cancer chemoimmunotherapy. Nanoscale 2019, 11, 16476–16487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, J.; Xin, X.; Du, X.; Zhao, D.; Qin, C.; Han, X.; Huo, M.; Yang, L.; Yin, L. Dual Targeting of Cancer Cells and MMPs with Self-Assembly Hybrid Nanoparticles for Combination Therapy in Combating Cancer. Pharmaceutics 2021, 13, 1990. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chisholm, J.; Zhuang, J.; Xiao, Y.; Duncan, G.; Chen, X.; Suk, J.S.; Hanes, J. Protein nanocages that penetrate airway mucus and tumor tissue. Proc. Natl. Acad. Sci. USA 2017, 114, E6595–E6602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, H.; Xu, X.; Wang, L.; Mo, R. Advances in living cell-based anticancer therapeutics. Biomater. Sci. 2020, 8, 2344–2365. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, B.; He, Z.; Tu, B.; Zhao, P.; Wang, H.; Asrorov, A.; Muhitdinov, B.; Jiang, J.; Huang, Y. Nanotherapeutic macrophage-based immunotherapy for the peritoneal carcinomatosis of lung cancer. Nanoscale 2022, 14, 2304–2315. [Google Scholar] [CrossRef]
- Li, T.F.; Li, K.; Zhang, Q.; Wang, C.; Yue, Y.; Chen, Z.; Yuan, S.J.; Liu, X.; Wen, Y.; Han, M.; et al. Dendritic cell-mediated delivery of doxorubicin-polyglycerol-nanodiamond composites elicits enhanced anti-cancer immune response in glioblastoma. Biomaterials 2018, 181, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Teng, X.; Wang, Y.; Yang, C.; Yan, X.; Li, J. Neutrophil Delivered Hollow Titania Covered Persistent Luminescent Nanosensitizer for Ultrosound Augmented Chemo/Immuno Glioblastoma Therapy. Adv. Sci. 2021, 8, e2004381. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, H.; Song, X.; Jiang, A.; Deng, Y.; Yang, C.; Sun, D.; Jiang, K.; Yang, F.; Zheng, Y. Adoptive CD8+ T-cell grafted with liposomal immunotherapy drugs to counteract the immune suppressive tumor microenvironment and enhance therapy for melanoma. Nanoscale 2021, 13, 15789–15803. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Ye, H.; Wang, K.; Zhao, J.; Wang, H.; Song, J.; Fan, X.; Lu, Y.; Cao, L.; Wan, B.; et al. Bioengineered Platelets Combining Chemotherapy and Immunotherapy for Postsurgical Melanoma Treatment: Internal Core-Loaded Doxorubicin and External Surface-Anchored Anti-PD-L1 Antibody Backpacks. Nano Lett. 2022, 22, 3141–3150. [Google Scholar] [CrossRef]
- Wu, H.H.; Zhou, Y.; Tabata, Y.; Gao, J.Q. Mesenchymal stem cell-based drug delivery strategy: From cells to biomimetic. J. Control. Release 2019, 294, 102–113. [Google Scholar] [CrossRef]
- Wei, B.; Pan, J.; Yuan, R.; Shao, B.; Wang, Y.; Guo, X.; Zhou, S. Polarization of Tumor-Associated Macrophages by Nanoparticle-Loaded Escherichia coli Combined with Immunogenic Cell Death for Cancer Immunotherapy. Nano Lett. 2021, 21, 4231–4240. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Rao, L.; Yao, H.; Wang, Z.; Ning, P.; Chen, X. Engineering Macrophages for Cancer Immunotherapy and Drug Delivery. Adv. Mater. 2020, 32, e2002054. [Google Scholar] [CrossRef]
- Takayama, Y.; Kusamori, K.; Tsukimori, C.; Shimizu, Y.; Hayashi, M.; Kiyama, I.; Katsumi, H.; Sakane, T.; Yamamoto, A.; Nishikawa, M. Anticancer drug-loaded mesenchymal stem cells for targeted cancer therapy. J. Control. Release 2021, 329, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Fliervoet, L.A.L.; Mastrobattista, E. Drug delivery with living cells. Adv. Drug Deliv. Rev. 2016, 106, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Zhang, M.; Wei, A.; Yin, F.; Wang, Y.; Hu, K.; Jiang, J. Doxorubicin and PD-L1 siRNA co-delivery with stem cell membrane-coated polydopamine nanoparticles for the targeted chemoimmunotherapy of PCa bone metastases. Nanoscale 2021, 13, 8998–9008. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, J.; Chen, X.; Liu, W.; Chen, T. Cell Membrane Coating Technology: A Promising Strategy for Biomedical Applications. Nanomicro Lett. 2019, 11, 100. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.; Wang, J.; Zhou, M.; Rao, Z.; Ling, X. A cell membrane vehicle co-delivering sorafenib and doxorubicin remodel the tumor microenvironment and enhance immunotherapy by inducing immunogenic cell death in lung cancer cells. J. Mater. Chem. B 2020, 8, 7755–7765. [Google Scholar] [CrossRef]
- Kang, M.; Hong, J.; Jung, M.; Kwon, S.P.; Song, S.Y.; Kim, H.Y.; Lee, J.R.; Kang, S.; Han, J.; Koo, J.H.; et al. T-Cell-Mimicking Nanoparticles for Cancer Immunotherapy. Adv. Mater. 2020, 32, e2003368. [Google Scholar] [CrossRef]
- Liu, R.; An, Y.; Jia, W.; Wang, Y.; Wu, Y.; Zhen, Y.; Cao, J.; Gao, H. Macrophage-mimic shape changeable nanomedicine retained in tumor for multimodal therapy of breast cancer. J. Control. Release 2020, 321, 589–601. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Lin, J.; Wu, H.; Yao, Y.; Zhang, J.; Yang, C. T cell membrane cloaking tumor microenvironment-responsive nanoparticles with a smart “membrane escape mechanism” for enhanced immune-chemotherapy of melanoma. Biomater. Sci. 2021, 9, 3453–3464. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Li, G.; Zang, W.; Zhou, X.; Shi, K.; Zhai, Y. Pure drug nano-assemblies: A facile carrier-free nanoplatform for efficient cancer therapy. Acta Pharm. Sin. B 2022, 12, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; An, F.F.; Zhang, X.; Chen, X.; Lee, C.S. Preparation and size control of sub-100 nm pure nanodrugs. Nano Lett. 2015, 15, 313–318. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, W.; Chen, R.; Chelora, J.; Wan, Y.; Cui, X.; Zhang, X.; Zhang, W.; Chen, X.; Xie, H.Y.; et al. Green Mass Production of Pure Nanodrugs via an Ice-Template-Assisted Strategy. Nano Lett. 2019, 19, 658–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, L.; Zhang, B.; Xu, A.; Shen, Z.; Guo, Y.; Zhao, R.; Yao, H.; Shao, J.W. Carrier-Free, Pure Nanodrug Formed by the Self-Assembly of an Anticancer Drug for Cancer Immune Therapy. Mol. Pharm. 2018, 15, 2466–2478. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, W.; Li, X.; Zhao, H.; Zhang, H.; Dong, A.; Yang, X. A directed co-assembly of herbal small molecules into carrier-free nanodrugs for enhanced synergistic antitumor efficacy. J. Mater. Chem. B 2021, 9, 1040–1048. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Zhi, K.; Yang, X. Exploration of the Natural Active Small-Molecule Drug-Loading Process and Highly Efficient Synergistic Antitumor Efficacy. ACS Appl. Mater. Interfaces 2020, 12, 6827–6839. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, R.; Huang, X.; Luo, R.; Xue, J.; Gao, J.; Liu, W.; Liu, F.; Feng, F.; Qu, W. Self-Delivered and Self-Monitored Chemo-Photodynamic Nanoparticles with Light-Triggered Synergistic Antitumor Therapies by Downregulation of HIF-1α and Depletion of GSH. ACS Appl. Mater. Interfaces 2020, 12, 5680–5694. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, Y.; Pan, Y.; Xue, X.; Zhang, X.; Kumar, A.; Liang, X.-J. Synergistically Enhanced Therapeutic Effect of a Carrier-Free HCPT/DOX Nanodrug on Breast Cancer Cells through Improved Cellular Drug Accumulation. Mol. Pharm. 2015, 12, 2237–2244. [Google Scholar] [CrossRef]
- Feng, B.; Niu, Z.; Hou, B.; Zhou, L.; Li, Y.; Yu, H. Enhancing Triple Negative Breast Cancer Immunotherapy by ICG-Templated Self-Assembly of Paclitaxel Nanoparticles. Adv. Funct. Mater. 2020, 30, 1906605. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Li, K.; Shen, S.; Liu, Z.; Wu, D. Ultralong Circulating Lollipop-Like Nanoparticles Assembled with Gossypol, Doxorubicin, and Polydopamine via π–π Stacking for Synergistic Tumor Therapy. Adv. Funct. Mater. 2019, 29, 1805582. [Google Scholar] [CrossRef] [Green Version]






| Drug Combination | Loading Strategies | Improved Effect | Disease Models | Ref. |
|---|---|---|---|---|
| DOX and carborane (CB) | DOX-CB conjugates were entrapped in the lipid bilayer | Coupling boron neutron capture therapy with immunotherapy | Glioblastoma models | [107] |
| DOX and CpG | DOPE-DOX-conjugate and DOPE-MMP-9 responsive peptide-CpG conjugate self-assemble into NPs | Co-delivery of chemotherapeutics with adjuvants | E.G7-OVA tumor models | [105] |
| PTX and αGC | PTX and glycolipid αGC were co-encapsulated in the lipid bilayer | Co-delivery of chemotherapeutics with adjuvants | B16F10 melanoma xenograft and lung metastasis models | [108,109] |
| CPT and Cur | CPT and Cur were entrapped in the lipid bilayer | Cur could downregulate the CPT-induced elevated PD-L1 expression and reduce Treg-mediated immunosuppression | Glioblastoma models | [106] |
| DOX and PD-L1 inhibitor | DOX was encapsulated inside and DSPE-PEG2000-MMP-responsive peptide-PD-L1 inhibitor was inserted into the lipid bilayer | Combination of cancer immunotherapy and chemotherapy to enhance the anti-tumor effect | B16F10 melanoma models | [110] |
| DOX and silybin (SLN) | DOX-loaded liposomes and SLN-loaded liposomes | SLN-loaded liposomes could change stromal structures and abrogate immunosuppression when in combination with DOX-loaded liposomes | Triple-negative breast cancer | [111] |
| IOX1 and DOX | IOX1-loaded liposomes and DOX-loaded liposomes | IOX1 could inhibit P-gp of cancer cells to enhance DOX-triggered ICD | Triple-negative 4T1 breast cancer models | [21] |
| PTX, thioridazine (THZ), and HY19991 (HY) | PTX-loaded micelles, THZ, and HY were entrapped in the aqueous core | Co-delivery of therapeutics against bulk tumor cells, cancer stem cells, and immune checkpoints | Breast cancer models | [112] |
| Cells | Key Characteristics | Therapeutics | Disease Models | Ref. |
|---|---|---|---|---|
| Macrophage | Tumor targeting; phagocytosis; immunoregulatory effect | CEL NPs | Abdominal metastasis of lung cancer models | [166] |
| DCs | Tumor targeting; immune response; blood-brain-barrier (BBB) penetration | Dox-polyglycerol-nanodiamond composites | Glioblastoma models | [167] |
| Neutrophils | Tumor targeting; BBB penetration | PTX and aPD-1 co-loaded NPs | Glioblastoma models | [168] |
| T cells | Tumor targeting; immune response | Liposomal immune regulators | Subcutaneous B16-OVA tumor models | [169] |
| Platelets | High abundance in the blood; endothelium adhesion; tumor targeting and penetration; production of platelet secretory granules | Dox and cross-linked aPD-L1 nanogels | Postsurgical melanoma models | [170] |
| Mesenchymal stem cells | Tumor targeting; relatively abundant sources; immune regulation | Dox-loaded liposomes | Subcutaneous tumor and lung metastasis models | [171] |
| Bacteria | Tumor targeting; inducing macrophage polarization; colonization in tumor tissues | PLGA-R848 and PLGA-DOX | Orthotopic breast cancer models | [172] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Jin, Y.; Song, M.; Zhao, Y.; Zhang, H. When Natural Compounds Meet Nanotechnology: Nature-Inspired Nanomedicines for Cancer Immunotherapy. Pharmaceutics 2022, 14, 1589. https://doi.org/10.3390/pharmaceutics14081589
Yu L, Jin Y, Song M, Zhao Y, Zhang H. When Natural Compounds Meet Nanotechnology: Nature-Inspired Nanomedicines for Cancer Immunotherapy. Pharmaceutics. 2022; 14(8):1589. https://doi.org/10.3390/pharmaceutics14081589
Chicago/Turabian StyleYu, Linna, Yi Jin, Mingjie Song, Yu Zhao, and Huaqing Zhang. 2022. "When Natural Compounds Meet Nanotechnology: Nature-Inspired Nanomedicines for Cancer Immunotherapy" Pharmaceutics 14, no. 8: 1589. https://doi.org/10.3390/pharmaceutics14081589
APA StyleYu, L., Jin, Y., Song, M., Zhao, Y., & Zhang, H. (2022). When Natural Compounds Meet Nanotechnology: Nature-Inspired Nanomedicines for Cancer Immunotherapy. Pharmaceutics, 14(8), 1589. https://doi.org/10.3390/pharmaceutics14081589