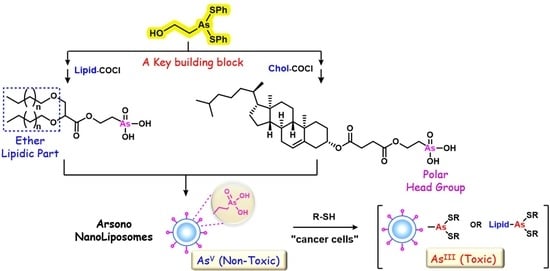
Synthesis of Novel Arsonolipids and Development of Novel Arsonoliposome Types
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthetic Procedures
2.3. NMR Sample Preparation—Operation
2.4. Liposome Preparation Procedures
2.5. Liposome Size Distribution, ζ-Potential, and Stability Studies
2.6. Liposome Integrity Studies
2.7. Cytotoxicity Study
3. Results and Discussion
3.1. Synthetic Part
3.2. Comparison of Novel Arsonoliposome with Conventional Arsonoliposomes
3.2.1. Arsono Liposome Physicochemical Properties and Integrity (In Vitro)
3.2.2. Anticancer Potential of Novel Arsonoliposomes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ali, N.; Hoque, M.A.; Haque, A.; Salam, K.A.; Karim, M.R.; Rahman, A.; Islam, K.; Saud, Z.A.; Khalek, M.A.; Akhand, A.A.; et al. Association between Arsenic Exposure and Plasma Cholinesterase Activity: A Population Based Study in Bangladesh. Environ. Health 2010, 9, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Argos, M.; Kalra, T.; Rathouz, P.J.; Chen, Y.; Pierce, B.; Parvez, F.; Islam, T.; Ahmed, A.; Rakibuz-Zaman, M.; Hasan, R.; et al. Arsenic Exposure from Drinking Water, and All-Cause and Chronic-Disease Mortalities in Bangladesh (HEALS): A Prospective Cohort Study. Lancet 2010, 376, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Mazumder, D.N.G.; Haque, R.; Ghosh, N.; De Binay, K.; Santra, A.; Chakraborty, D.; Smith, A.H. Arsenic Levels in Drinking Water and the Prevalence of Skin Lesions in West Bengal, India. Int. J. Epidemiol. 1998, 27, 871–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazumder, D.N.G. Effect of Chronic Intake of Arsenic-Contaminated Water on Liver. Toxicol. Appl. Pharmacol. 2005, 206, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Meliker, J.R.; Wahl, R.L.; Cameron, L.L.; Nriagu, J.O. Arsenic in Drinking Water and Cerebrovascular Disease, Diabetes Mellitus, and Kidney Disease in Michigan: A Standardized Mortality Ratio Analysis. Environ. Health 2007, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Tapio, S.; Grosche, B. Arsenic in the Aetiology of Cancer. Mutat. Res. 2006, 612, 215–246. [Google Scholar] [CrossRef]
- Vahidnia, A.; Romijn, F.; van der Voet, G.B.; de Wolff, F.A. Arsenic-Induced Neurotoxicity in Relation to Toxicokinetics: Effects on Sciatic Nerve Proteins. Chem. Biol. Interact. 2008, 176, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Jeng, J.S.; Yip, P.K.; Chen, C.L.; Hsu, L.I.; Hsueh, Y.M.; Chiou, H.Y.; Wu, M.M.; Chen, C.J. Biological Gradient between Long-Term Arsenic Exposure and Carotid Atherosclerosis. Circulation 2002, 105, 1804–1809. [Google Scholar] [CrossRef] [Green Version]
- Mathews, V.; Chendamarai, E.; George, B.; Viswabandya, A.; Srivastava, A. Treatment of Acute Promyelocytic Leukemia with Single-Agent Arsenic Trioxide. Mediterr. J. Hematol. Infect. Dis. 2011, 3, e2011056. [Google Scholar] [CrossRef] [Green Version]
- Lengfelder, E.; Hofmann, W.K.; Nowak, D. Impact of Arsenic Trioxide in the Treatment of Acute Promyelocytic Leukemia. Leukemia 2012, 26, 433–442. [Google Scholar] [CrossRef] [Green Version]
- Cohen, M.H.; Hirschfeld, S.; Honig, S.F.; Ibrahim, A.; Johnson, J.R.; O’Leary, J.J.; White, R.M.; Williams, G.A.; Pazdur, R. Drug Approval Summaries: Arsenic Trioxide, Tamoxifen Citrate, Anastrazole, Paclitaxel, Bexarotene. Oncologist 2001, 6, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Liu, L.; Zheng, T.; Yin, D. An Evidence-Based Perspective of Arsenic Trioxide (As2O3) for Cancer Patients. In Evidence-Based Anticancer Materia Medica; Cho, W.C.S., Ed.; Springer Science+Business Media B.V.: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2011; pp. 37–64. [Google Scholar] [CrossRef]
- Khairul, I.; Wang, Q.Q.; Jiang, Y.H.; Wang, C.; Naranmandura, H. Metabolism, Toxicity and Anticancer Activities of Arsenic Compounds. Oncotarget 2017, 8, 23905–23926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Orazio, G.; Parisi, G.; Policano, C.; Mechelli, R.; Codacci Pisanelli, G.; Pitaro, M.; Ristori, G.; Salvetti, M.; Nicotra, F.; La Ferla, B. Arsenical C-Glucoside Derivatives with Promising Antitumor Activity. Eur. J. Org. Chem. 2015, 2015, 4620–4623. [Google Scholar] [CrossRef]
- Lloyd, N.C.; Morgan, H.W.; Nicholson, B.K.; Ronimus, R.S. The Composition of Ehrlich’s Salvarsan: Resolution of a Century-Old Debate. Angew. Chem.—Int. Ed. 2005, 44, 941–944. [Google Scholar] [CrossRef] [Green Version]
- Mandal, B.K.; Ogra, Y.; Suzuki, K.T. Identification of Dimethylarsinous and Monomethylarsonous Acids in Human Urine of the Arsenic-Affected Areas in West Bengal, India. Chem. Res. Toxicol. 2001, 14, 371–378. [Google Scholar] [CrossRef]
- Ioannou, P.V.; Tsivgoulis, G.M. A High Yield Procedure for the Preparation of Arsonolipids (2,3-Diacyloxypropylarsonic Acids). Chem. Phys. Lipids 2010, 163, 51–55. [Google Scholar] [CrossRef]
- Ioannou, P.V. Arsonolipids, Pseudo Arsonolipids, Arsinolipids and Arsonoliposomes: Preparations, Biophysical, Biochemical and Biological Aspects. Main Group Chem. 2018, 17, 111–132. [Google Scholar] [CrossRef]
- Long, J.W.; Ray, W.J. Kinetics and Thermodynamics of the Formation of Glucose Arsenate. Reaction of Glucose Arsenate with Phosphoglucomutase. Biochemistry 1973, 12, 3932–3937. [Google Scholar] [CrossRef]
- Fatouros, D.; Gortzi, O.; Klepetsanis, P.; Antimisiaris, S.G.; Stuart, M.C.A.; Brisson, A.; Ioannou, P.V. Preparation and Properties of Arsonolipid Containing Liposomes. Chem. Phys. Lipids 2001, 109, 75–89. [Google Scholar] [CrossRef]
- Gortzi, O.; Klepetsanis, P.; Antimisiaris, S.G.; Ioannou, P.V. Capability of Arsonolipids to Transport Divalent Cations: A Pressman Cell Study. Chem. Phys. Lipids 2001, 112, 21–29. [Google Scholar] [CrossRef]
- Fatouros, D.G.; Klepetsanis, P.; Ioannou, P.V.; Antimisiaris, S.G. The Effect of PH on the Electrophoretic Behaviour of a New Class of Liposomes: Arsonoliposomes. Int. J. Pharm. 2005, 288, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Fatouros, D.G.; Piperoudi, S.; Gortzi, O.; Ioannou, P.V.; Frederik, P.; Antimisiaris, S.G. Physical Stability of Sonicated Arsonoliposomes: Effect of Calcium Ions. J. Pharm. Sci. 2005, 94, 46–55. [Google Scholar] [CrossRef]
- Piperoudi, S.; Fatouros, D.; Ioannou, P.V.; Frederik, P.; Antimisiaris, S.G. Incorporation of PEG-lipids in arsonoliposomes results in formation of highly stable arsenic-containing vesicles. Chem. Phys. Lipids 2006, 139, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Serves, S.V.; Ioannou, P.V. Carbonic Anhydrase Inhibitors—Part 33. Isozyme II Inhibition with 2,3-Dihydroxypropylarsonic Acids and Arsonolipids. J. Inorg. Biochem. 1996, 62, 207–212. [Google Scholar] [CrossRef]
- Rogers, J.; Yu, B.Z.; Serves, S.V.; Tsivgoulis, G.M.; Sotiropoulos, D.N.; Ioannou, P.V.; Jain, M.K. Kinetic Basis for the Substrate Specificity during Hydrolysis of Phospholipids by Secreted Phospholipase A2. Biochemistry 1996, 35, 9375–9384. [Google Scholar] [CrossRef]
- Gortzi, O.; Papadimitriou, E.; Kontoyannis, C.G.; Antimisiaris, S.G.; Ioannou, P.V. Arsonoliposomes, a Novel Class of Arsenic-Containing Liposomes: Effect of Palmitoyl-Arsonolipid-Containing Liposomes on the Viability of Cancer and Normal Cells in Culture. Pharm. Res. 2002, 19, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Gortzi, O.; Antimisiaris, S.G.; Klepetsanis, P.; Papadimitriou, E.; Ioannou, P.V. Arsonoliposomes: Effect of Arsonolipid Acyl Chain Length and Vesicle Composition on Their Toxicity towards Cancer and Normal Cells in Culture. Eur. J. Pharm. Sci. 2003, 18, 175–183. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Ioannou, P.V.; Loiseau, P.M. In-Vitro Antileishmanial and Trypanocidal Activities of Arsonoliposomes and Preliminary in-Vivo Distribution in BALB/c Mice. J. Pharm. Pharmacol. 2010, 55, 647–652. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Klepetsanis, P.; Zachariou, V.; Giannopoulou, E.; Ioannou, P.V. In Vivo Distribution of Arsenic after i.p. Injection of Arsonoliposomes in Balb-c Mice. Int. J. Pharm. 2005, 289, 151–158. [Google Scholar] [CrossRef]
- Favretto, M.E.; Marouf, S.; Ioannou, P.; Antimisiaris, S.G.; Parker, T.L.; Kallinteri, P. Arsonoliposomes for the Potential Treatment of Medulloblastoma. Pharm. Res. 2009, 26, 2237–2246. [Google Scholar] [CrossRef]
- Haikou, M.N.; Zagana, P.; Ioannou, P.V.; Antimisiaris, S.G. Arsonoliposome interaction with thiols: Effect of pegylation and arsonolipid content of arsonoliposomes on their integrity during incubation in glutathione. J. Nanosci. Nanotechnol. 2006, 6, 2974–2978. [Google Scholar] [CrossRef] [PubMed]
- Antimisiaris, S.G.; Ioannou, P.V.; Wei, M.X. Treatment of Tumors Prostate with Arsonoliposomes. U.S. Patent 2012/0015023 A1, 19 January 2012. [Google Scholar]
- Zagana, P.; Mourtas, S.; Basta, A.; Antimisiaris, S.G. Preparation, Physicochemical Properties, and in Vitro Toxicity towards Cancer Cells of Novel Types of Arsonoliposomes. Pharmaceutics 2020, 12, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantzari, M.; Gartziou, F.; Lambrou, E.; Mourtas, S.; Zagana, P.; Antimisiaris, S.G. Novel TNBC-Targeted DOX-Arsonoliposomes. Proceedings 2021, 78, 17. [Google Scholar] [CrossRef]
- Le Gall, T.; Berchel, M.; Le Hir, S.; Fraix, A.; Salaün, J.Y.; Férec, C.; Lehn, P.; Jaffrès, P.A.; Montier, T. Arsonium-Containing Lipophosphoramides, Poly-Functional Nano-Carriers for Simultaneous Antibacterial Action and Eukaryotic Cell Transfection. Adv. Healthc. Mater. 2013, 2, 1513–1524. [Google Scholar] [CrossRef] [Green Version]
- Kigawa, J.; Minagawa, Y.; Kanamori, Y.; Itamochi, H.; Cheng, X.; Okada, M.; Oishi, T.; Terakawa, N. Glutathione Concentration May Be a Useful Predictor of Response to Second-Line Chemotherapy in Patients with Ovarian Cancer. Cancer 1998, 82, 697–702. [Google Scholar] [CrossRef]
- Tsivgoulis, G.M.; Sotiropoulos, D.N.; Ioannou, P.V. Rac-1,2-Diacyloxypropyl-3-Arsonic Acids: Arsonolipid Analogues of Phosphonolipids. Phosphorus Sulfur Silicon Relat. Elem. 1991, 63, 329–334. [Google Scholar] [CrossRef]
- Serves, S.V.; Tsivgoulis, G.M.; Sotiropoulos, D.N.; Ioannou, P.V. Synthesis of (R) and (S)-1,2-Diacyloxypropyl-3-Arsonic Acids: Optically Active Arsonolipids. Phosphorus Sulfur Silicon Relat. Elem. 1992, 71, 99–105. [Google Scholar] [CrossRef]
- Serves, S.V.; Sotiropoulos, D.N.; Ioannou, P.V.; Jain, M.K. One Pot Synthesis of Arsonolipids via Thioarsenite Precursors. Phosphorus Sulfur Silicon Relat. Elem. 1993, 81, 181–190. [Google Scholar] [CrossRef]
- Ralph, S.J. Arsenic-Based Antineoplastic Drugs and Their Mechanisms of Action. Met. Based. Drugs 2008, 2008, 260146. [Google Scholar] [CrossRef]
- Anderson, M.; Omri, A. The Effect of Different Lipid Components on the In Vitro Stability and Release Kinetics of Liposome Formulations. Drug Deliv. 2004, 11, 33–39. [Google Scholar] [CrossRef]
- Ulmer, C.Z.; Koelmel, J.P.; Jones, C.M.; Garrett, T.J.; Aristizabal-Henao, J.J.; Vesper, H.W.; Bowden, J.A. A Review of Efforts to Improve Lipid Stability during Sample Preparation and Standardization Efforts to Ensure Accuracy in the Reporting of Lipid Measurements. Lipids 2021, 56, 3–16. [Google Scholar] [CrossRef]
- Grit, M.; Crommelin, D.J.A. Chemical Stability of Liposomes: Implications for Their Physical Stability. Chem. Phys. Lipids 1993, 64, 3–18. [Google Scholar] [CrossRef]
- Tsivgoulis, G.M.; Ioannou, P.V. A Reinvestigation of the Synthesis of Arsonolipids (2,3-Diacyloxypropylarsonic Acids). Chem. Phys. Lipids 2008, 152, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kumar, B.S.; Negi, A.S. Current Status on Development of Steroids as Anticancer Agents. J. Steroid Biochem. Mol. Biol. 2013, 137, 242–270. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, H.; Santos, C.; Silva, A. Cholesterol-Based Compounds: Recent Advances in Synthesis and Applications. Molecules 2018, 24, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radwan, A.A.; Alanazi, F.K. Targeting Cancer Using Cholesterol Conjugates. Saudi Pharm. J. 2014, 22, 3–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talsma, H.; Van Steenbergen, M.J.; Borchert, J.C.H.; Crommelin, D.J.A. A Novel Technique for the One-Step Preparation of Liposomes and Nonionic Surfactant Vesicles without the Use of Organic Solvents. Liposome Formation in a Continuous Gas Stream: The ‘Bubble’ Method. J. Pharm. Sci. 1994, 83, 276–280. [Google Scholar] [CrossRef]
- Stewart, J.C.M. Colorimetric Determination of Phospholipids with Ammonium Ferrothiocyanate. Anal. Biochem. 1980, 104, 10–14. [Google Scholar] [CrossRef]
- Mourtas, S.; Christodoulou, P.; Klepetsanis, P.; Gatos, D.; Barlos, K.; Antimisiaris, S.G. Preparation of Benzothiazolyl-Decorated Nanoliposomes. Molecules 2019, 24, 1540. [Google Scholar] [CrossRef] [Green Version]
- Mourtas, S.; Mavroidi, B.; Marazioti, A.; Kannavou, M.; Sagnou, M.; Pelecanou, M.; Antimisiaris, S.G. Liposomes Decorated with 2-(4′-Aminophenyl)Benzothiazole Effectively Inhibit Aβ 1–42 Fibril Formation and Exhibit in Vitro Brain-Targeting Potential. Biomacromolecules 2020, 21, 4685–4698. [Google Scholar] [CrossRef]
- Morgan, D.M.L. Tetrazolium (MTT) Assay for Cellular Viability and Activity. In Polyamine Protocols; Morgan, D.M.L., Ed.; Humana Press: Totowa, NJ, USA, 1998; pp. 179–184. [Google Scholar]
- Tsivgoulis, G.M.; Sotiropoulos, D.N.; Ioannou, P.V. 1,2-Dihydroxypropyl-3-Arsonic Acid: A Key Intermediate for Arsonolipids. Phosphorus Sulfur Silicon Relat. Elem. 1991, 57, 189–193. [Google Scholar] [CrossRef]
- Meyer, G. Ueber Einige Anomale Reaktionen. Ber. Dtsch. Chem. Ges. 1883, 16, 1439–1443. [Google Scholar] [CrossRef]
- Ioannou, P.V. On C--As Bond Formation: Preparation of Aliphatic Arsonic Acids. Phosphorus Sulfur Silicon Relat. Elem. 2002, 177, 1–14. [Google Scholar] [CrossRef]
- Tsivgoulis, G.; Sotiropoulos, D.; Ioannou, P. Decomposition of Alkylarsonic Acids by Acylating Agents. Phosphorus Sulfur Silicon Relat. Elem. 1991, 55, 165–168. [Google Scholar] [CrossRef]
- Tsivgoulis, G.M.; Ioannou, P.V. Esterification Equilibrium Constants of Arsonic and Arsinic Acids. Mon. Chem. 2012, 143, 1603–1608. [Google Scholar] [CrossRef]
- Argenziano, M.; Lombardi, C.; Ferrara, B.; Trotta, F.; Caldera, F.; Blangetti, M.; Koltai, H.; Kapulnik, Y.; Yarden, R.; Gigliotti, L.; et al. Glutathione/pH-responsive nanosponges enhance strigolactone delivery to prostate cancer cells. Oncotarget 2018, 9, 35813–35829. [Google Scholar] [CrossRef] [Green Version]
- Gamcsik, M.P.; Kasibhatla, M.S.; Teeter, S.D.; Colvin, O.M. Glutathione levels in human tumors. Biomarkers 2012, 17, 671–691. [Google Scholar] [CrossRef]
- Jing, T.; Lim, T.; Ruanm, Z.; Yan, L. pH-and glutathione-stepwise responsive polypeptide nanogel for smart and efficient drug delivery. J. Mater. Sci. 2018, 53, 14933–14943. [Google Scholar] [CrossRef]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mourtas, S.; Papadia, K.; Kordopati, G.G.; Ioannou, P.V.; Antimisiaris, S.G.; Tsivgoulis, G.M. Synthesis of Novel Arsonolipids and Development of Novel Arsonoliposome Types. Pharmaceutics 2022, 14, 1649. https://doi.org/10.3390/pharmaceutics14081649
Mourtas S, Papadia K, Kordopati GG, Ioannou PV, Antimisiaris SG, Tsivgoulis GM. Synthesis of Novel Arsonolipids and Development of Novel Arsonoliposome Types. Pharmaceutics. 2022; 14(8):1649. https://doi.org/10.3390/pharmaceutics14081649
Chicago/Turabian StyleMourtas, Spyridon, Konstantina Papadia, Golfo G. Kordopati, Panayiotis V. Ioannou, Sophia G. Antimisiaris, and Gerasimos M. Tsivgoulis. 2022. "Synthesis of Novel Arsonolipids and Development of Novel Arsonoliposome Types" Pharmaceutics 14, no. 8: 1649. https://doi.org/10.3390/pharmaceutics14081649
APA StyleMourtas, S., Papadia, K., Kordopati, G. G., Ioannou, P. V., Antimisiaris, S. G., & Tsivgoulis, G. M. (2022). Synthesis of Novel Arsonolipids and Development of Novel Arsonoliposome Types. Pharmaceutics, 14(8), 1649. https://doi.org/10.3390/pharmaceutics14081649