Antioxidant and Cytotoxic Activity of New Polyphenolic Derivatives of Quinazolin-4(3H)-one: Synthesis and In Vitro Activities Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. Synthesis of Intermediate Compounds 1a–d, 2a–d, 3a–d
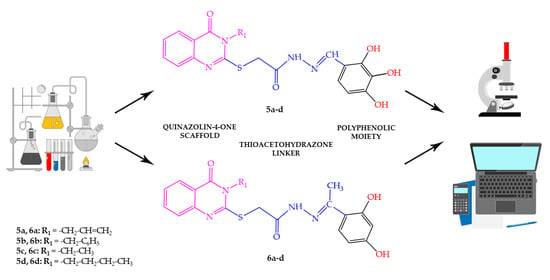
2.1.2. Synthesis of Compounds 5a–d
2.1.3. Synthesis of Compounds 6a–d
2.2. In Vitro Antioxidant, Antiradical and Chelation Assays
2.2.1. Antiradical Assays
2.2.2. Electron Transfer Assays
2.2.3. Transition Metals’ Ions Chelation Assays
2.3. Theoretical Quantum and Thermodynamical Calculations
2.4. In Vitro Cytotoxicity Activity
2.4.1. Cell Cultures
2.4.2. Experimental
2.5. Molecular Properties with Influence on the Pharmacokinetics of Compounds
3. Results
3.1. Chemical Synthesis
3.2. In Vitro Antioxidant, Antiradical and Chelation Assays
3.2.1. Antiradical Assays
ABTS˙+ Radical Scavenging Assay
DPPH˙ Radical Scavenging Assay
NO˙ Radical Scavenging Assay
3.2.2. Electron Transfer Assays
Ferric Reducing Antioxidant Power (FRAP)
Phosphomolybdate Assay for Total Antioxidant Capacity (TAC)
Reducing Power (RP) Assay
Cupric Reducing Antioxidant Capacity (CUPRAC) Assay
3.2.3. Transition Metals’ Ions Chelation Assays
Fe2+ Chelation Assay
Cu2+ Chelation Assay
3.3. Theoretical Quantum and Thermodynamical Calculations
3.4. In Vitro Cytotoxicity Activity
3.5. Molecular Properties with Influence on the Pharmacokinetics of Compounds
4. Discussion
4.1. Chemical Synthesis
4.2. In Vitro Antioxidant, Antiradical and Chelation Assay
4.2.1. Antiradical Assays
4.2.2. Electron Transfer Assays
4.2.3. Transition Metal Ions Chelation Assays
4.3. Theoretical Quantum and Thermodynamical Energy Calculations
4.4. In Vitro Cytotoxicity Activity
4.5. Molecular Properties with Influence on the Pharmacokinetics of Compounds
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajput, R.; Mishra, A.P. A review on biological activity of quinazolinones. Int. J. Pharm. Pharm. Sci. 2012, 4, 66–70. [Google Scholar]
- Wang, Z.; Wang, M.; Yao, X.; Li, Y.; Tan, J.; Wang, L.; Qiao, W.; Geng, Y.; Liu, Y.; Wang, Q. Design, synthesis and antiviral activity of novel quinazolinones. Eur. J. Med. Chem. 2012, 53, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.W.; Yin, X.D.; Li, H.; Ma, K.Y.; Zhang, Z.J.; Zhou, R.; Wang, Y.L.; Hu, G.F.; Liu, Y.Q. Design, Synthesis, and Structure-Activity Relationship of Quinazolinone Derivatives as Potential Fungicides. J. Agric. Food Chem. 2021, 69, 4604–4614. [Google Scholar] [CrossRef]
- Shirish, P.G.; Amol, S. Jagdale Significant Pharmacological / Biological Activities of Novel Quinazoline Derivatives in Medicinal Chemistry. World J. Pharm. Res. 2019, 8, 498–508. [Google Scholar] [CrossRef]
- Asif, M. Chemical Characteristics, Synthetic Methods, and Biological Potential of Quinazoline and Quinazolinone Derivatives. Int. J. Med. Chem. 2014, 2014, 395637. [Google Scholar] [CrossRef] [PubMed]
- Jatav, V.; Mishra, P.; Kashaw, S.; Stables, J.P. CNS depressant and anticonvulsant activities of some novel 3-[5-substituted 1,3,4-thiadiazole-2-yl]-2-styryl quinazoline-4(3H)-ones. Eur. J. Med. Chem. 2008, 43, 1945–1954. [Google Scholar] [CrossRef]
- Zayed, F.M.; Hassan, H.M. Synthesis and biological evaluation studies of novel quinazolinone derivatives as antibacterial and anti-inflammatory agents. Saudi Pharm. J. 2014, 22, 157–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakesh, K.P.; Manukumar, H.M.; Gowda, D.C. Schiff’s bases of quinazolinone derivatives: Synthesis and SAR studies of a novel series of potential anti-inflammatory and antioxidants. Bioorg. Med. Chem. Lett. 2015, 25, 1072–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, C.; You, J.; Li, X.F.; You, R.; Weng, Y.; Li, J.; Wang, Y. Design, synthesis and anticoccidial activity of a series of 3-(2-(2-methoxyphenyl)-2-oxoethyl) quinazolinone derivatives. Pestic. Biochem. Physiol. 2010, 97, 194–198. [Google Scholar] [CrossRef]
- Birhan, Y.S.; Bekhit, A.A.; Hymete, A. Synthesis and antileishmanial evaluation of some 2,3-disubstituted-4(3H)-quinazolinone derivatives. Org. Med. Chem. Lett. 2014, 4, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, Y.; Luo, H.; Zhang, Y.; Gao, F.; Wang, J.; Zheng, J. Novel Approaches for the Solid-Phase Synthesis of Dihydroquinazoline-2(1H)-One Derivatives and Biological Evaluation as Potential Anticancer Agents. Molecules 2022, 27, 8577. [Google Scholar] [CrossRef] [PubMed]
- Muzza, M.; Pogliaghi, G.; Colombo, C.; Carbone, E.; Cirello, V.; Palazzo, S.; Frattini, F.; Gentilini, D.; Gazzano, G.; Persani, L.; et al. Oxidative Stress Correlates with More Aggressive Features in Thyroid Cancer. Cancers 2022, 14, 5857. [Google Scholar] [CrossRef] [PubMed]
- Mossakowska, B.J.; Fabisiewicz, A.; Tudek, B.; Siedlecki, J.A. Possible Mechanisms of Resistance Development to Photodynamic Therapy (PDT) In Vulvar Cancer Cells. Int. J. Mol. Sci. 2022, 23, 14689. [Google Scholar] [CrossRef]
- Tavleeva, M.M.; Belykh, E.S.; Rybak, A.V.; Rasova, E.E.; Chernykh, A.A.; Ismailov, Z.B.; Velegzhaninov, I.O. Effects of Antioxidant Gene Overexpression on Stress Resistance and Malignization In Vitro and In Vivo: A Review. Antioxidants 2022, 11, 2316. [Google Scholar] [CrossRef] [PubMed]
- Pele, R.; Marc, G.; Stana, A.; Ionuț, I.; Nastasă, C.; Tiperciuc, B.; Oniga, I.; Pîrnău, A.; Vlase, L.; Oniga, O. Synthesis of New Phenolic Derivatives of Quinazolin-4(3H)-One as Potential Antioxidant Agents—In Vitro Evaluation and Quantum Studies. Molecules 2022, 27, 2599. [Google Scholar] [CrossRef] [PubMed]
- Palierse, E.; Masse, S.; Laurent, G.; Le Griel, P.; Mosser, G.; Coradin, T.; Jolivalt, C. Synthesis of Hybrid Polyphenol/Hydroxyapatite Nanomaterials with Anti-Radical Properties. Nanomaterials 2022, 12, 3588. [Google Scholar] [CrossRef] [PubMed]
- Karwasra, R.; Ahmad, S.; Bano, N.; Qazi, S.; Raza, K.; Singh, S.; Varma, S. Macrophage-Targeted Punicalagin Nanoengineering to Alleviate Methotrexate-Induced Neutropenia: A Molecular Docking, DFT, and MD Simulation Analysis. Molecules 2022, 27, 6034. [Google Scholar] [CrossRef]
- Darlami, O.; Shin, D. Total Synthesis of Resvebassianol A, a Metabolite of Resveratrol by Beauveria bassiana. Antioxidants 2021, 10, 1509. [Google Scholar] [CrossRef]
- Momchilova, A.; Pankov, R.; Staneva, G.; Pankov, S.; Krastev, P.; Vassileva, E.; Hazarosova, R.; Krastev, N.; Robev, B.; Nikolova, B.; et al. Resveratrol Affects Sphingolipid Metabolism in A549 Lung Adenocarcinoma Cells. Int. J. Mol. Sci. 2022, 23, 10870. [Google Scholar] [CrossRef]
- Ramadan, S.K.; Elrazaz, E.Z.; Abouzid, K.A.M.; El-Naggar, A.M. Design, synthesis and in silico studies of new quinazolinone derivatives as antitumor PARP-1 inhibitors. RSC Adv. 2020, 10, 29475–29492. [Google Scholar] [CrossRef] [PubMed]
- Hagar, M.; Soliman, S.M.; Ibid, F.; El Ashry, E.S.H. Synthesis, molecular structure and spectroscopic studies of some new quinazolin-4(3H)-one derivatives; an account on the N- versus S-Alkylation. J. Mol. Struct. 2016, 1108, 667–679. [Google Scholar] [CrossRef]
- El-Azab, A.S.; Abdel-Hamide, S.G.; Sayed-Ahmed, M.M.; Hassan, G.S.; El-Hadiyah, T.M.; Al-Shabanah, O.A.; Al-Deeb, O.A.; El-Subbagh, H.I. Novel 4(3H)-quinazolinone analogs: Synthesis and anticonvulsant activity. Med. Chem. Res. 2013, 22, 2815–2827. [Google Scholar] [CrossRef]
- Haghighijoo, Z.; Firuzi, O.; Hemmateenejad, B.; Emami, S.; Edraki, N.; Miri, R. Synthesis and biological evaluation of quinazolinone-based hydrazones with potential use in Alzheimer’s disease. Bioorg. Chem. 2017, 74, 126–133. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Marc, G.; Stana, A.; Franchini, A.H.; Vodnar, D.C.; Barta, G.; Tertiş, M.; Şanta, I.; Cristea, C.; Pîrnău, A.; Ciorîţă, A.; et al. Phenolic Thiazoles with Antioxidant and Antiradical Activity. Synthesis, In Vitro Evaluation, Toxicity, Electrochemical Behavior, Quantum Studies and Antimicrobial Screening. Antioxidants 2021, 10, 1707. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Hamada, N.; Abdo, N. Synthesis, Characterization, Antimicrobial Screening and Free-Radical Scavenging Activity of Some Novel Substituted Pyrazoles. Molecules 2015, 20, 10468–10486. [Google Scholar] [CrossRef] [Green Version]
- Hellal, K.; Maulidiani, M.; Ismail, I.S.; Tan, C.P.; Abas, F. Antioxidant, α-Glucosidase, and Nitric Oxide Inhibitory Activities of Six Algerian Traditional Medicinal Plant Extracts and 1H-NMR-Based Metabolomics Study of the Active Extract. Molecules 2020, 25, 1247. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 15–27. [Google Scholar] [CrossRef]
- Stana, A.; Vodnar, D.C.; Marc, G.; Benedec, D.; Tiperciuc, B.; Tamaian, R.; Oniga, O. Antioxidant activity and antibacterial evaluation of new thiazolin-4-one derivatives as potential tryptophanyl-tRNA synthetase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 898–908. [Google Scholar] [CrossRef] [Green Version]
- Marc, G.; Stana, A.; Oniga, S.D.; Pîrnău, A.; Vlase, L.; Oniga, O. New Phenolic Derivatives of Thiazolidine-2,4-dione with Antioxidant and Antiradical Properties: Synthesis, Characterization, In Vitro Evaluation, and Quantum Studies. Molecules 2019, 24, 2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Apak, R. The main and modified CUPRAC methods of antioxidant measurement. TrAC Trends Anal. Chem. 2011, 30, 652–664. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.; Bektaşoğlu, B.; Berker, K.; Özyurt, D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mic, M.; Pîrnău, A.; Floare, C.G.; Marc, G.; Franchini, A.H.; Oniga, O.; Vlase, L.; Bogdan, M. Synthesis and molecular interaction study of a diphenolic hidrazinyl-thiazole compound with strong antioxidant and antiradical activity with HSA. J. Mol. Struct. 2021, 1244, 131278. [Google Scholar] [CrossRef]
- Cesari, L.; Mutelet, F.; Canabady-Rochelle, L. Antioxidant properties of phenolic surrogates of lignin depolymerisation. Ind. Crops Prod. 2019, 129, 480–487. [Google Scholar] [CrossRef]
- Wu, H.-C.; Shiau, C.-Y.; Chen, H.-M.; Chiou, T.-K. Antioxidant activities of carnosine, anserine, some free amino acids and their combination. J. Food Drug Anal. 2020, 11, 13. [Google Scholar] [CrossRef]
- Grozav, A.; Porumb, I.-D.; Găină, L.; Filip, L.; Hanganu, D. Cytotoxicity and Antioxidant Potential of Novel 2-(2-((1H-indol-5yl)methylene)-hydrazinyl)-thiazole Derivatives. Molecules 2017, 22, 260. [Google Scholar] [CrossRef] [Green Version]
- Antonijević, M.R.; Simijonović, D.M.; Avdović, E.H.; Ćirić, A.; Petrović, Z.D.; Marković, J.D.; Stepanić, V.; Marković, Z.S. Green One-Pot Synthesis of Coumarin-Hydroxybenzohydrazide Hybrids and Their Antioxidant Potency. Antioxidants 2021, 10, 1106. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pop, A.; Fizeșan, I.; Vlase, L.; Rusu, M.E.; Cherfan, J.; Babota, M.; Gheldiu, A.M.; Tomuta, I.; Popa, D.S. Enhanced recovery of phenolic and tocopherolic compounds from walnut (Juglans regia L.) male flowers based on process optimization of ultrasonic assisted-extraction: Phytochemical profile and biological activities. Antioxidants 2021, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Delaney, J.S. ESOL: Estimating Aqueous Solubility Directly from Molecular Structure. J. Chem. Inf. Comput. Sci. 2004, 44, 1000–1005. [Google Scholar] [CrossRef]





| Compound | % of ABTS˙+ Scavenging | IC50 (µg/mL) | IC50 (µM) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.83 µg/mL | 1.67 µg/mL | 3.33 µg/mL | 4.99 µg/mL | 6.66 µg/mL | 9.99 µg/mL | 13.32 µg/mL | |||
| 5a | 38.14 | 51.47 | 72.00 | + | + | + | + | 1.65 | 3.87 |
| 5b | 34.65 | 49.32 | 68.62 | 85.98 | + | + | + | 1.91 | 4.01 |
| 5c | 39.05 | 52.25 | 72.57 | + | + | + | + | 1.60 | 3.86 |
| 5d | 41.98 | 52.25 | 67.46 | 81.06 | + | + | + | 1.55 | 3.50 |
| 6a | 27.03 | 30.08 | 37.19 | 42.27 | 50.03 | 60.55 | 70.24 | 7.17 | 16.89 |
| 6b | 26.26 | 33.32 | 44.60 | 55.88 | 68.23 | 90.98 | + | 4.13 | 8.70 |
| 6c | 29.08 | 34.73 | 43.19 | 54.47 | 68.58 | 91.60 | + | 4.06 | 9.84 |
| 6d | 29.08 | 33.32 | 40.37 | 50.24 | 57.59 | 75.41 | 85.79 | 5.17 | 11.74 |
| Ascorbic acid | 60.97 | 73.24 | 87.73 | + | + | + | + | 2.01 | 11.41 |
| Trolox | 38.66 | 53.16 | 66.54 | 94.57 | + | + | + | 4.66 | 18.62 |
| Compound | % of DPPH˙ Scavenging | IC50 (µg/mL) | IC50 (µM) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1.25 µg/mL | 2.5 µg/mL | 3.75 µg/mL | 5 µg/mL | 7.5 µg/mL | 10 µg/mL | 12.5 µg/mL | 15 µg/mL | |||
| 5a | 41.09 | 49.94 | 54.93 | 62.06 | 73.89 | + | + | + | 2.76 | 6.47 |
| 5b | 37.55 | 45.21 | 51.00 | 56.36 | 69.66 | 81.12 | + | + | 3.62 | 7.60 |
| 5c | 44.32 | 55.40 | 64.87 | 73.44 | 90.15 | + | + | + | 1.85 | 4.46 |
| 5d | 41.12 | 53.03 | 63.53 | 71.70 | 87.99 | + | + | + | 2.18 | 4.93 |
| 6a | - | - | - | - | - | - | 12.28 | 21.34 | >15 | >30 |
| 6b | - | - | - | - | - | - | - | 11.59 | >15 | >30 |
| 6c | - | - | - | - | - | - | 10.11 | 14.89 | >15 | >30 |
| 6d | - | - | - | - | - | - | 10.99 | 13.87 | >15 | >30 |
| Ascorbic acid | 35.11 | 47.45 | 55.71 | 64.21 | 79.16 | 94.39 | + | + | 2.83 | 16.07 |
| Trolox | 17.01 | 28.53 | 40.42 | 53.87 | 75.85 | 94.85 | + | + | 4.68 | 18.70 |
| Compound | % of NO˙ Scavenged |
|---|---|
| 5a | 38.39 |
| 5b | 36.75 |
| 5c | 43.21 |
| 5d | 35.14 |
| 6a | 31.82 |
| 6b | 33.31 |
| 6c | 30.62 |
| 6d | 30.71 |
| Gentisic acid | 48.14 |
| Compound | % of Activity of Ascorbic Acid | % of Activity of Trolox | ||||||
|---|---|---|---|---|---|---|---|---|
| FRAP | TAC | RP | CUPRAC | FRAP | TAC | RP | CUPRAC | |
| 5a | 86.18 | 91.43 | 63.20 | 132.79 | 98.88 | 176.42 | 92.09 | 126.56 |
| 5b | 67.05 | 81.34 | 58.80 | 97.31 | 76.93 | 156.96 | 85.68 | 92.75 |
| 5c | 83.40 | 84.73 | 49.93 | 116.41 | 95.70 | 163.49 | 72.75 | 110.95 |
| 5d | 80.80 | 61.09 | 49.54 | 111.66 | 92.71 | 117.87 | 72.18 | 106.43 |
| 6a | 22.81 | 48.91 | 21.09 | 13.46 | 26.17 | 94.38 | 30.73 | 12.83 |
| 6b | 18.51 | 52.36 | 19.91 | 23.74 | 21.24 | 101.03 | 29.01 | 22.63 |
| 6c | 25.85 | 57.03 | 19.89 | 30.52 | 29.65 | 110.05 | 28.98 | 29.09 |
| 6d | 24.36 | 39.72 | 16.84 | 11.80 | 27.95 | 76.63 | 24.53 | 11.25 |
| Compound | Chelation Capacity (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 17.70 µg/mL | 20.59 µg/mL | 29.41 µg/mL | 44.11 µg/mL | 58.82 µg/mL | 88.23 µg/mL | 117.64 µg/mL | 257.46 µg/mL | 343.28 µg/mL | |
| 5a | - | - | - | - | 18.44 | 34.12 | 45.93 | 71.76 | 86.30 |
| 5b | - | - | - | - | - | - | - | 19.02 | 35.16 |
| 5c | - | - | - | - | - | - | 20.27 | 65.75 | 89.24 |
| 5d | - | - | - | - | - | - | 58.18 | 71.56 | 80.50 |
| 6a | - | - | - | - | - | - | - | - | - |
| 6b | - | - | - | - | - | - | - | - | - |
| 6c | - | - | - | - | - | - | - | - | - |
| 6d | - | - | - | - | - | - | - | - | - |
| EDTA-Na2 | 1.32 | 20.59 | 42.89 | 95.10 | + | + | + | + | + |
| Compound | Chelation Capacity (%) | ||
|---|---|---|---|
| 3.36 µg/mL | 8.40 µg/mL | 16.80 µg/mL | |
| 5a | 20.33 | 30.76 | 40.29 |
| 5b | 11.17 | 25.27 | 40.20 |
| 5c | 13.06 | 23.23 | 32.60 |
| 5d | 14.19 | 28.04 | 41.42 |
| 6a | 10.96 | 20.64 | 31.79 |
| 6b | 6.77 | 17.17 | 30.49 |
| 6c | 7.47 | 16.75 | 26.72 |
| 6d | 5.98 | 11.84 | 20.42 |
| EDTA-Na2 | 10.39 | 22.68 | 44.51 |
| Compound | Frontier Orbitals (eV) | X-H BDE (kcal/mol) | ||||||
|---|---|---|---|---|---|---|---|---|
| HOMO | LUMO | gap | H1 | H2 | H3 | H4 | H5 | |
| 5a | −5.52 | −1.62 | 3.90 | 78.08 | 72.00 | 78.27 | 88.90 | 101.09 |
| 5b | −5.53 | −1.64 | 3.89 | 77.95 | 71.99 | 78.28 | 86.01 | 100.98 |
| 5c | −5.53 | −1.60 | 3.93 | 77.98 | 71.97 | 78.26 | 89.91 | 106.64 |
| 5d | −5.51 | −1.58 | 3.93 | 78.03 | 71.98 | 78.28 | 89.63 | 101.03 |
| 6a | −5.42 | −1.58 | 3.84 | 84.54 | N/A | 79.45 | 83.21 | N/A |
| 6b | −5.39 | −1.64 | 3.75 | 84.61 | N/A | 79.49 | 83.39 | N/A |
| 6c | −5.40 | −1.57 | 3.83 | 84.64 | N/A | 79.45 | 83.19 | N/A |
| 6d | −5.39 | −1.55 | 3.84 | 84.44 | N/A | 79.21 | 83.12 | N/A |
| Compound | Conformation | Compound | Conformation |
|---|---|---|---|
| 5a |  | 6a |  |
| 5b |  | 6b |  |
| 5c |  | 6c |  |
| 5d |  | 6d |  |
| Cell Line | IC50 (µg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Compounds | Reference | ||||||||
| 5a | 5b | 5c | 5d | 6a | 6b | 6c | 6d | Doxorubicin | |
| A549 | 30.91 | 69.49 | 49.09 | 42.14 | 35.68 | 42.19 | >100 | 32.42 | 0.54 |
| LNCaP | 45.64 | 69.97 | 35.92 | 56.80 | 43.37 | 30.40 | >100 | 24.27 | 0.74 |
| BJ | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >7.5 |
| Compound | MW | Rotatable Bonds | HBA | HBD | TPSA (Å2) | MLogP | Solubility (µg/mL) | Lipinski Violations |
|---|---|---|---|---|---|---|---|---|
| 5a | 426.45 | 8 | 7 | 4 | 162.34 | 1.29 | 61.10 | 0 |
| 5b | 476.50 | 8 | 7 | 4 | 162.34 | 2.02 | 7.91 | 0 |
| 5c | 414.44 | 7 | 7 | 4 | 162.34 | 0.72 | 86.80 | 0 |
| 5d | 442.49 | 9 | 7 | 4 | 162.34 | 1.17 | 25.00 | 0 |
| 6a | 424.47 | 8 | 6 | 3 | 142.11 | 2.02 | 27.00 | 0 |
| 6b | 474.53 | 8 | 6 | 3 | 142.11 | 2.73 | 3.54 | 0 |
| 6c | 412.46 | 7 | 6 | 3 | 142.11 | 1.46 | 38.90 | 0 |
| 6d | 440.52 | 9 | 6 | 3 | 142.11 | 1.90 | 11.00 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pele, R.; Marc, G.; Ionuț, I.; Nastasă, C.; Fizeșan, I.; Pîrnău, A.; Vlase, L.; Palage, M.; Oniga, S.; Oniga, O. Antioxidant and Cytotoxic Activity of New Polyphenolic Derivatives of Quinazolin-4(3H)-one: Synthesis and In Vitro Activities Evaluation. Pharmaceutics 2023, 15, 136. https://doi.org/10.3390/pharmaceutics15010136
Pele R, Marc G, Ionuț I, Nastasă C, Fizeșan I, Pîrnău A, Vlase L, Palage M, Oniga S, Oniga O. Antioxidant and Cytotoxic Activity of New Polyphenolic Derivatives of Quinazolin-4(3H)-one: Synthesis and In Vitro Activities Evaluation. Pharmaceutics. 2023; 15(1):136. https://doi.org/10.3390/pharmaceutics15010136
Chicago/Turabian StylePele, Raluca, Gabriel Marc, Ioana Ionuț, Cristina Nastasă, Ionel Fizeșan, Adrian Pîrnău, Laurian Vlase, Mariana Palage, Smaranda Oniga, and Ovidiu Oniga. 2023. "Antioxidant and Cytotoxic Activity of New Polyphenolic Derivatives of Quinazolin-4(3H)-one: Synthesis and In Vitro Activities Evaluation" Pharmaceutics 15, no. 1: 136. https://doi.org/10.3390/pharmaceutics15010136
APA StylePele, R., Marc, G., Ionuț, I., Nastasă, C., Fizeșan, I., Pîrnău, A., Vlase, L., Palage, M., Oniga, S., & Oniga, O. (2023). Antioxidant and Cytotoxic Activity of New Polyphenolic Derivatives of Quinazolin-4(3H)-one: Synthesis and In Vitro Activities Evaluation. Pharmaceutics, 15(1), 136. https://doi.org/10.3390/pharmaceutics15010136