Advances in NIR-Responsive Natural Macromolecular Hydrogel Assembly Drugs for Cancer Treatment
Abstract
:1. Introduction
1.1. The Hazards of Cancer and the Limitations of Traditional Therapy
1.2. Features and Mechanism of Hydrogels for Drug Delivery
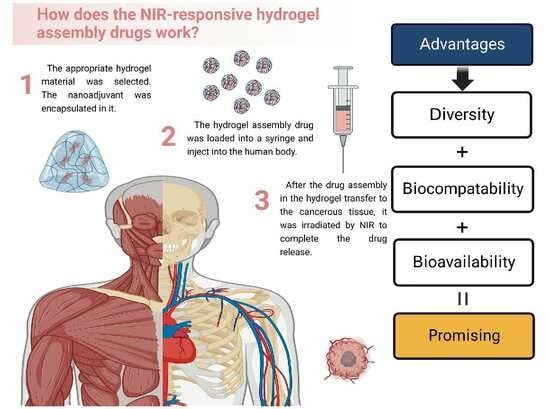
1.3. The Mechanism of NIR-NMHADs
2. The Application of NIR Light to HAD: Properties, Advantages and Controlled Release
2.1. The NIR Light and Properties with Different Wavelength Windows
2.2. Laser Driving Forces in NIR vs. UV and X-ray Irradition
2.3. NIR-Controlled Hydrogel Release Drugs
2.3.1. The Thermosensitive Hydrogel Releases Drugs via NIR Irradiation
2.3.2. Hydrogels Release Drugs through Photochemical Reactions under NIR Irradiation
3. Selection and Application of NIR Functional Hydrogel Materials
3.1. Agarose Hydrogel
3.2. Chitosan Hydrogel
3.3. GelMA Hydrogel
3.4. DNA Hydrogel
4. Hydrogel-Based Phototherapy Combined with Other Therapies
4.1. Hydrogel-Based Phototherapy Combined with Other Therapies
4.2. Photothermal Therapy (PTT) for Cancer Treatment
4.3. Photodynamic Therapy (PDT) for Cancer Treatment
4.4. Hydrogel-Based Phototherapy Combined with Chemotherapy (CT) for Cancer Treatment
4.5. Hydrogel-Based Phototherapy Combined with IT for Cancer Treatment
5. Targeted Drug Delivery Technology for Assisting NIR-HAD Therapy
6. Conclusions and Perspectives
- (1)
- NIR irradiation has space–time controllability and can well control the instantaneous release of drugs in vivo. The study of the NIR-I window is more mature and has a large number of PTA adaptations. NIR-II has stronger tissue penetration and is more promising for clinical application in the future. Compared with other light sources, NIR is almost non-invasive to the human body and can cooperate with many bioengineering technologies. At present, there are two commonly used NIR-mediated hydrogel drug release methods: one is to convert the light energy of NIR into heat energy through a PTA, change the phase state or swelling rate of the hydrogel and achieve the drug release effect. The other is to convert NIR light into UV light using UCNPs. Then, the hydrogel can be cracked by photochemical reaction to complete the release of the drug.
- (2)
- We introduced the commonly used hydrogel matrices, such as agarose, chitosan, DNA and GelMA hydrogels. In addition, we also list many innovative synthetic hydrogels for reference. The temperature-sensitive, photo-sensitive and pH-sensitive properties of these hydrogels well cater to the application of NIR and establish an efficient and safe drug delivery system.
- (3)
- NIR responsive hydrogel assembly drugs for cancer treatment methods are extremely diverse and the many materials that can be wrapped can cooperate with each other to make up for the shortcomings of a single material, synergistic photothermal therapy, photodynamic therapy, chemotherapy and immunotherapy. Driven by NIR irradiation, effective anti-cancer treatments prevent cancer recurrence.
- (4)
- Bioengineering technology can be roughly divided into two categories in NIR-responsive hydrogel assembly drugs: imaging technology and targeted drug delivery technology. Imaging technology makes the process and results of drug treatment in vivo more intuitive to researchers. Targeted drug delivery technology makes the drug delivery process more controllable and efficient. With the help of these technologies, the design of NIR-responsive hydrogel drugs can be optimized for more applications.
Author Contributions
Funding
Conflicts of Interest
References
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA A Cancer J Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global burden of primary liver cancer in 2020 and predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Pomerantz, H.; Huang, D.; Weinstock, M.A. Risk of subsequent melanoma after melanoma in situ and invasive melanoma: A population-based study from 1973 to 2011. J. Am. Acad. Dermatol. 2015, 72, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 365, 1687–1717. [CrossRef] [PubMed]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Lønning, P.E.; Nikolaienko, O.; Pan, K.; Kurian, A.W.; Eikesdal, H.P.; Pettinger, M.; Anderson, G.L.; Prentice, R.L.; Chlebowski, R.T.; Knappskog, S. Constitutional BRCA1 Methylation and Risk of Incident Triple-Negative Breast Cancer and High-grade Serous Ovarian Cancer. JAMA Oncol. 2022, 8, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E. Update on Survival in Osteosarcoma. Orthop. Clin. N. Am. 2016, 47, 283–292. [Google Scholar] [CrossRef]
- Ailia, M.J.; Thakur, N.; Abdul-Ghafar, J.; Jung, C.K.; Yim, K.; Chong, Y. Current Trend of Artificial Intelligence Patents in Digital Pathology: A Systematic Evaluation of the Patent Landscape. Cancers 2022, 14, 2400. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Zhang, H.; Yuan, X.; Chen, T.; Pei, Z.; Ji, X. Two-Dimensional Nanomaterial-based catalytic Medicine: Theories, advanced catalyst and system design. Adv. Drug Deliv. Rev. 2022, 184, 114241. [Google Scholar] [CrossRef]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Romero, A.; Pérez-Puyana, V. Novel Trends in Hydrogel Development for Biomedical Applications: A Review. Polymers 2022, 14, 3023. [Google Scholar] [CrossRef] [PubMed]
- Kalita, H.; Patowary, M. Biocompatible Polymer Nano-Constructs: A Potent Platform for Cancer Theranostics. Technol. Cancer Res. Treat. 2023, 22, 153303382311603. [Google Scholar] [CrossRef] [PubMed]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qu, S.; Suo, Z.; Yang, W. Functional hydrogel coatings. Natl. Sci. Rev. 2021, 8, nwaa254. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.A.; Kim, E.-S.; Kong, H.J.; Mooney, D.J. Material-based deployment enhances efficacy of endothelial progenitor cells. Proc. Natl. Acad. Sci. USA 2008, 105, 14347–14352. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, F.; Yu, Y.; Chen, G.; Shang, L.; Sun, L.; Zhao, Y. Bio-inspired clamping microneedle arrays from flexible ferrofluid-configured moldings. Sci. Bull. 2019, 64, 1110–1117. [Google Scholar] [CrossRef]
- Lin, C.-C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Boontheekul, T.; Kong, H.-J.; Mooney, D.J. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 2005, 26, 2455–2465. [Google Scholar] [CrossRef] [PubMed]
- Brannon-Peppas, L.; Peppas, N.A. Equilibrium swelling behavior of pH-sensitive hydrogels. Chem. Eng. Sci. 1991, 46, 715–722. [Google Scholar] [CrossRef]
- Amsden, B. Solute Diffusion within Hydrogels. Mechanisms and Models. Macromolecules 1998, 31, 8382–8395. [Google Scholar] [CrossRef]
- MacArthur, J.W.; Purcell, B.P.; Shudo, Y.; Cohen, J.E.; Fairman, A.; Trubelja, A.; Patel, J.; Hsiao, P.; Yang, E.; Lloyd, K.; et al. Sustained Release of Engineered Stromal Cell–Derived Factor 1-α From Injectable Hydrogels Effectively Recruits Endothelial Progenitor Cells and Preserves Ventricular Function After Myocardial Infarction. Circulation 2013, 128, S79–S86. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Obara, K.; Ishizuka, T.; Fujita, M.; Sato, M.; Masuoka, K.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; et al. Controlled release of fibroblast growth factors and heparin from photocrosslinked chitosan hydrogels and subsequent effect on in vivo vascularization. J. Biomed. Mater. Res. 2003, 64A, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Zhao, X.; Zhou, J.; Suo, Z. A theory of coupled diffusion and large deformation in polymeric gels. J. Mech. Phys. Solids 2008, 56, 1779–1793. [Google Scholar] [CrossRef]
- Yan, B.; Boyer, J.-C.; Habault, D.; Branda, N.R.; Zhao, Y. Near Infrared Light Triggered Release of Biomacromolecules from Hydrogels Loaded with Upconversion Nanoparticles. J. Am. Chem. Soc. 2012, 134, 16558–16561. [Google Scholar] [CrossRef]
- Obaidat, A.A.; Park, K. Characterization of protein release through glucose-sensitive hydrogel membranes. Biomaterials 1997, 18, 801–806. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Li, Q.; Yu, C.; Chu, W. Natural Polymer-based Stimuli-responsive Hydrogels. Curr. Med. Chem. 2020, 27, 2631–2657. [Google Scholar] [CrossRef]
- Xian, S.; Webber, M.J. Temperature-responsive supramolecular hydrogels. J. Mater. Chem. B 2020, 8, 9197–9211. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; He, J.; Bochani, S.; Nosrati, V.; Shahbazi, M.-A.; Guo, B. Multifunctional Photoactive Hydrogels for Wound Healing Acceleration. ACS Nano 2021, 15, 18895–18930. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, X.; Yoon, J. Organelle-Targeted Photosensitizers for Precision Photodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 19543–19571. [Google Scholar] [CrossRef]
- Lv, Z.; He, S.; Wang, Y.; Zhu, X. Noble Metal Nanomaterials for NIR-Triggered Photothermal Therapy in Cancer. Adv. Healthc. Mater. 2021, 10, 2001806. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Song, J.; Liu, D.; Wang, K.; Qi, J. NIR-II AIEgens with Photodynamic Effect for Advanced Theranostics. Molecules 2022, 27, 6649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, S.; Zhang, Z.; Ji, L.; Zhang, J.; Wang, Q.; Guo, T.; Ni, S.; Cai, R.; Mu, X.; et al. Recent Progress on NIR-II Photothermal Therapy. Front. Chem. 2021, 9, 728066. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wang, X.; Shao, J.; Wang, W.; Mou, X.; Dong, X. NIR-II Organic Nanotheranostics for Precision Oncotherapy. Small 2021, 17, 2102646. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Zhang, X.; Wang, L.; Feng, W.; Li, Q. Beyond the Visible: Bioinspired Infrared Adaptive Materials. Adv. Mater. 2021, 33, 2004754. [Google Scholar] [CrossRef]
- Zhao, J.; Zhong, D.; Zhou, S. NIR-I-to-NIR-II fluorescent nanomaterials for biomedical imaging and cancer therapy. J. Mater. Chem. B 2018, 6, 349–365. [Google Scholar] [CrossRef]
- Zhu, S.; Yung, B.C.; Chandra, S.; Niu, G.; Antaris, A.L.; Chen, X. Near-Infrared-II (NIR-II) Bioimaging via Off-Peak NIR-I Fluorescence Emission. Theranostics 2018, 8, 4141–4151. [Google Scholar] [CrossRef]
- Zheng, F.; Huang, X.; Ding, J.; Bi, A.; Wang, S.; Chen, F.; Zeng, W. NIR-I Dye-Based Probe: A New Window for Bimodal Tumor Theranostics. Front. Chem. 2022, 10, 859948. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Qin, Q.; Li, Z.; Wang, Y.; Liu, X.; Liu, Y.; Huan, S.; Zhang, X.; Song, G. Tongue cancer tailored photosensitizers for NIR-II fluorescence imaging guided precise treatment. Nano Today 2022, 45, 101550. [Google Scholar] [CrossRef]
- Zhou, R.; Ohulchanskyy, T.Y.; Xu, Y.; Ziniuk, R.; Xu, H.; Liu, L.; Qu, J. Tumor-Microenvironment-Activated NIR-II Nanotheranostic Platform for Precise Diagnosis and Treatment of Colon Cancer. ACS Appl. Mater. Interfaces 2022, 14, 23206–23218. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Mitsunaga, M.; Ito, K.; Kobayashi, H.; Saruta, M. Cancer neovasculature-targeted near-infrared photoimmunotherapy (NIR-PIT) for gastric cancer: Different mechanisms of phototoxicity compared to cell membrane-targeted NIR-PIT. Gastric Cancer 2020, 23, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, R.; Barros, A.S.; Guedes, S.; Caixeta, D.C.; Sabino-Silva, R. Diagnostic and monitoring applications using near infrared (NIR) spectroscopy in cancer and other diseases. Photodiagnosis Photodyn. Ther. 2023, 42, 103633. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, C. Near infrared spectroscopy: A mature analytical technique with new perspectives—A review. Anal. Chim. Acta 2018, 1026, 8–36. [Google Scholar] [CrossRef]
- Luo, S.; Wu, J.; Jia, Z.; Tang, P.; Sheng, J.; Xie, C.; Liu, C.; Gan, D.; Hu, D.; Zheng, W.; et al. An Injectable, Bifunctional Hydrogel with Photothermal Effects for Tumor Therapy and Bone Regeneration. Macromol. Biosci. 2019, 19, 1900047. [Google Scholar] [CrossRef]
- Chen, D.; Chen, C.; Huang, C.; Chen, T.; Liu, Z. Injectable Hydrogel for NIR-II Photo-Thermal Tumor Therapy and Dihydroartemisinin-Mediated Chemodynamic Therapy. Front. Chem. 2020, 8, 251. [Google Scholar] [CrossRef]
- Ruan, C.; Liu, C.; Hu, H.; Guo, X.-L.; Jiang, B.-P.; Liang, H.; Shen, X.-C. NIR-II light-modulated thermosensitive hydrogel for light-triggered cisplatin release and repeatable chemo-photothermal therapy. Chem. Sci. 2019, 10, 4699–4706. [Google Scholar] [CrossRef]
- Li, B.; Wang, W.; Zhao, L.; Yan, D.; Li, X.; Gao, Q.; Zheng, J.; Zhou, S.; Lai, S.; Feng, Y.; et al. Multifunctional AIE Nanosphere-Based “Nanobomb” for Trimodal Imaging-Guided Photother-mal/Photodynamic/Pharmacological Therapy of Drug-Resistant Bacterial Infections. ACS Nano 2023, 17, 4601–4618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, H.; Dong, C.; Wang, J.; Zhang, T.; Huang, L.; Ni, D.; Luo, Y. 3D printed hydrogel/bioceramics core/shell scaffold with NIR-II triggered drug release for chemo-photothermal therapy of bone tumors and enhanced bone repair. Chem. Eng. J. 2023, 461, 141855. [Google Scholar] [CrossRef]
- Jia, T.; Li, D.; Du, J.; Fang, X.; Gerasimov, V.; Ågren, H.; Chen, G. A bimodal type of AgPd Plasmonic Blackbody Nanozyme with boosted catalytic efficacy and synergized photothermal therapy for efficacious tumor treatment in the second biological window. J. Nanobiotechnol. 2022, 20, 424. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Qu, X. Biosystem-Inspired Engineering of Nanozymes for Biomedical Applications. Adv. Mater. 2023, 2211147. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, Y.; Zhang, X.; Ma, J.; Wang, M. Double-crosslinked bifunctional hydrogels with encapsulated anti-cancer drug for bone tumor cell ablation and bone tissue regeneration. Colloids Surf. B Biointerfaces 2022, 213, 112364. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Alami-Milani, M.; Geranmayeh, M.H.; Barar, J.; Erfan-Niya, H.; Omidi, Y. Dual thermo-and pH-sensitive injectable hydrogels of chitosan/(poly(N-isopropylacrylamide-co-itaconic acid)) for doxorubicin delivery in breast cancer. Int. J. Biol. Macromol. 2019, 128, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Han, Y.; Zhang, H.; Tu, W.; Zhang, S. Radiotherapy-Induced Digestive Injury: Diagnosis, Treatment and Mechanisms. Front. Oncol. 2021, 11, 757973. [Google Scholar] [CrossRef]
- Pfeifer, G.P. Mechanisms of UV-induced mutations and skin cancer. Genome Instab. Dis. 2020, 1, 99–113. [Google Scholar] [CrossRef]
- Kawai, J.; Ishii, H.; Ida, H. Pyroelectric X-ray application to X-ray absorption and emission spectroscopies. X-ray Spectrom. 2012, 41, 216–218. [Google Scholar] [CrossRef]
- Yang, Y.; Tan, H.; Cheng, B.; Fan, J.; Yu, J.; Ho, W. Near-Infrared-Responsive Photocatalysts. Small Methods 2021, 5, 2001042. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Deng, Y.; Zhang, Y.; Sui, M.; Deng, J.; Zhou, S. Degradation of antipyrine by UV, UV/H2O2 and UV/PS. J. Hazard. Mater. 2013, 260, 1008–1016. [Google Scholar] [CrossRef]
- Deng, J.; Xun, X.; Zheng, W.; Su, Y.; Zheng, L.; Wang, C.; Su, M. Sequential delivery of bismuth nanoparticles and doxorubicin by injectable macroporous hydrogels for combined anticancer kilovoltage X-ray radio- and chemo-therapy. J. Mater. Chem. B 2018, 6, 7966–7973. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, G. NIR light-responsive nanocarriers for controlled release. J. Photochem. Photobiol. C Photochem. Rev. 2021, 47, 100420. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F. Upconversion Luminescent Materials: Advances and Applications. Chem. Rev. 2015, 115, 395–465. [Google Scholar] [CrossRef] [PubMed]
- Le, M.; Huang, W.; Chen, K.-F.; Lin, C.; Cai, L.; Zhang, H.; Jia, Y.-G. Upper critical solution temperature polymeric drug carriers. Chem. Eng. J. 2022, 432, 134354. [Google Scholar] [CrossRef]
- Lima-Sousa, R.; De Melo-Diogo, D.; Alves, C.G.; Cabral, C.S.D.; Miguel, S.P.; Mendonça, A.G.; Correia, I.J. Injectable in situ forming thermo-responsive graphene based hydrogels for cancer chemo-photothermal therapy and NIR light-enhanced antibacterial applications. Mater. Sci. Eng. C 2020, 117, 111294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, T.; Tian, Y.; Zhang, C.; Ge, K.; Zhang, J.; Chang, J.; Wang, H. Gold nanorods-mediated efficient synergistic immunotherapy for detection and inhibition of postoperative tumor recurrence. Acta Pharm. Sin. B 2021, 11, 1978–1992. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Q.; Shim, G.; Oh, Y.-K. Melanin-loaded CpG DNA hydrogel for modulation of tumor immune microenvironment. J. Control. Release 2021, 330, 540–553. [Google Scholar] [CrossRef]
- Gulfam, M.; Jo, S.-H.; Vu, T.T.; Ali, I.; Rizwan, A.; Joo, S.-B.; Park, S.-H.; Lim, K.T. NIR-degradable and biocompatible hydrogels derived from hyaluronic acid and coumarin for drug delivery and bio-imaging. Carbohydr. Polym. 2023, 303, 120457. [Google Scholar] [CrossRef]
- Liu, B.; Sun, J.; Zhu, J.; Li, B.; Ma, C.; Gu, X.; Liu, K.; Zhang, H.; Wang, F.; Su, J.; et al. Injectable and NIR-Responsive DNA–Inorganic Hybrid Hydrogels with Outstanding Photothermal Therapy. Adv. Mater. 2020, 32, 2004460. [Google Scholar] [CrossRef]
- Han, R.-L.; Shi, J.-H.; Liu, Z.-J.; Hou, Y.-F.; Wang, Y. Near-Infrared Light-Triggered Hydrophobic-to-Hydrophilic Switch Nanovalve for On-Demand Cancer Therapy. ACS Biomater. Sci. Eng. 2018, 4, 3478–3486. [Google Scholar] [CrossRef]
- Mahmood, A.; Patel, D.; Hickson, B.; DesRochers, J.; Hu, X. Recent Progress in Biopolymer-Based Hydrogel Materials for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 1415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xia, L.-Y.; Chen, X.; Chen, Z.; Wu, F.-G. Hydrogel-based phototherapy for fighting cancer and bacterial infection. Sci. China Mater. 2017, 60, 487–503. [Google Scholar] [CrossRef]
- Yazdi, M.K.; Taghizadeh, A.; Taghizadeh, M.; Stadler, F.J.; Farokhi, M.; Mottaghitalab, F.; Zarrintaj, P.; Ramsey, J.D.; Seidi, F.; Saeb, M.R.; et al. Agarose-based biomaterials for advanced drug delivery. J. Control. Release 2020, 326, 523–543. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Z.; Wei, S.; He, F.; Li, Z.; Wang, H.-H.; Huang, Y.; Nie, Z. Near-infrared light-controllable MXene hydrogel for tunable on-demand release of therapeutic proteins. Acta Biomater. 2021, 130, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.; Yang, R.; Zhang, L.; Zhang, L.; Liu, G.; Xu, Z.; Kang, Y.; Xue, P. Injectable and Natural Humic Acid/Agarose Hybrid Hydrogel for Localized Light-Driven Photothermal Ablation and Chemotherapy of Cancer. ACS Biomater. Sci. Eng. 2018, 4, 4266–4277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zeng, W.; Chen, Z.; Suo, W.; Quan, H.; Tan, Z.-J. An intratumoral injectable nanozyme hydrogel for hypoxia-resistant thermoradiotherapy. Colloids Surf. B Biointerfaces 2021, 207, 112026. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejska, M.; Jankowska, K.; Klak, M.; Wszoła, M. Chitosan as an Underrated Polymer in Modern Tissue Engineering. Nanomaterials 2021, 11, 3019. [Google Scholar] [CrossRef]
- Sabino, I.J.; Lima-Sousa, R.; Alves, C.G.; Melo, B.L.; Moreira, A.F.; Correia, I.J.; De Melo-Diogo, D. Injectable in situ forming hydrogels incorporating dual-nanoparticles for chemo-photothermal therapy of breast cancer cells. Int. J. Pharm. 2021, 600, 120510. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, W.; Zhao, J.; Wu, C.; Ye, C.; Huang, M.; Wang, S. Preparation of injectable temperature-sensitive chitosan-based hydrogel for combined hyperthermia and chemotherapy of colon cancer. Carbohydr. Polym. 2019, 222, 115039. [Google Scholar] [CrossRef]
- Feng, C.; Ouyang, J.; Tang, Z.; Kong, N.; Liu, Y.; Fu, L.; Ji, X.; Xie, T.; Farokhzad, O.C.; Tao, W. Germanene-Based Theranostic Materials for Surgical Adjuvant Treatment: Inhibiting Tumor Recurrence and Wound Infection. Matter 2020, 3, 127–144. [Google Scholar] [CrossRef]
- Yin, X.; Fan, T.; Zheng, N.; Yang, J.; Yan, L.; He, S.; Ai, F.; Hu, J. Palladium nanoparticle based smart hydrogels for NIR light-triggered photothermal/photodynamic therapy and drug release with wound healing capability. Nanoscale Adv. 2023, 5, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Zhao, N.; Wang, C.; Yuan, W. Injectable self-healing polysaccharide hydrogel loading CuS and pH-responsive DOX@ZIF-8 nanoparticles for synergistic photothermal-photodynamic-chemo therapy of cancer. J. Mater. Sci. Technol. 2022, 127, 245–255. [Google Scholar] [CrossRef]
- Yue, K.; Santiago, G.T.-D.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Yuan, X.; Zhao, Y.; Cai, Q.; Wang, Y.; Luo, R.; Yu, S.; Wang, Y.; Han, J.; Ge, L.; et al. Injectable GelMA Cryogel Microspheres for Modularized Cell Delivery and Potential Vascularized Bone Regeneration. Small 2021, 17, 2006596. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Shoichet, M.S. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat. Mater. 2004, 3, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chan-Park, M.B. A biomimetic hydrogel based on methacrylated dextran-graft-lysine and gelatin for 3D smooth muscle cell culture. Biomaterials 2010, 31, 1158–1170. [Google Scholar] [CrossRef]
- Van Den Steen, P.E.; Dubois, B.; Nelissen, I.; Rudd, P.M.; Dwek, R.A.; Opdenakker, G. Biochemistry and Molecular Biology of Gelatinase B or Matrix Metalloproteinase-9 (MMP-9). Crit. Rev. Biochem. Mol. Biol. 2002, 37, 375–536. [Google Scholar] [CrossRef]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef]
- Aubin, H.; Nichol, J.W.; Hutson, C.B.; Bae, H.; Sieminski, A.L.; Cropek, D.M.; Akhyari, P.; Khademhosseini, A. Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials 2010, 31, 6941–6951. [Google Scholar] [CrossRef]
- Benton, J.A.; DeForest, C.A.; Vivekanandan, V.; Anseth, K.S. Photocrosslinking of Gelatin Macromers to Synthesize Porous Hydrogels That Promote Valvular Interstitial Cell Function. Tissue Eng. Part A 2009, 15, 3221–3230. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, L.; Liao, J.; Tan, Y.; Ouyang, K.; Ning, C.; Ni, G.; Tan, G. Cell-laden photocrosslinked GelMA–DexMA copolymer hydrogels with tunable mechanical properties for tissue engineering. J. Mater. Sci. Mater. Med. 2014, 25, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, Y.; Sun, M.; Jin, M.; Xia, W.; Yang, H.; Wang, T. Effect of Freezing Process on the Microstructure of Gelatin Methacryloyl Hydrogels. Front. Bioeng. Biotechnol. 2021, 9, 810155. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, W.; Zhang, R.; Zhang, C.; Yan, J.; Feng, J.; Rosenholm, J.M.; Shi, T.; Shen, X.; Zhang, H. Minimally invasive injection of biomimetic Nano@Microgel for in situ ovarian cancer treatment through enhanced photodynamic reactions and photothermal combined therapy. Mater. Today Bio 2023, 20, 100663. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; He, Z.; Liu, F.; Feng, J.; Huang, C.; Sun, X.; Deng, H. Hybrid Hydrogels for Synergistic Periodontal Antibacterial Treatment with Sustained Drug Release and NIR-Responsive Photothermal Effect. Int. J. Nanomed. 2020, 15, 5377–5387. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, Y.; Liu, P.; Luo, D. Bioresponsive DNA Hydrogels: Beyond the Conventional Stimuli Responsiveness. Acc. Chem. Res. 2017, 50, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Jian, X.; Feng, X.; Luo, Y.; Li, F.; Tan, J.; Yin, Y.; Liu, Y. Development, Preparation, and Biomedical Applications of DNA-Based Hydrogels. Front. Bioeng. Biotechnol. 2021, 9, 661409. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Ma, N.; Yang, X.; Ling, G.; Yu, J.; Zhang, P. Preparation of intelligent DNA hydrogel and its applications in biosensing. Eur. Polym. J. 2020, 137, 109951. [Google Scholar] [CrossRef]
- Wang, X.; Wu, B.; Zhang, Y.; Dou, X.; Zhao, C.; Feng, C. Polydopamine-doped supramolecular chiral hydrogels for postoperative tumor recurrence inhibition and simultaneously enhanced wound repair. Acta Biomater. 2022, 153, 204–215. [Google Scholar] [CrossRef]
- Fan, M.; Li, M.; Wang, X.; Liao, Y.; Wang, H.; Rao, J.; Yang, Y.; Wang, Q. Injectable Thermosensitive Iodine-Loaded Starch- g -poly(N. -isopropylacrylamide) Hydrogel for Cancer Photothermal Therapy and Anti-Infection. Macromol. Rapid Commun. 2022, 43, 2200203. [Google Scholar] [CrossRef]
- Hao, Y.; Chung, C.K.; Gu, Z.; Schomann, T.; Dong, X.; Veld, T.; Camps, M.G.M.; Dijke, P.T.; Ossendorp, F.A.; Cruz, L.J. Combinatorial therapeutic approaches of photodynamic therapy and immune checkpoint blockade for colon cancer treatment. Mol. Biomed. 2022, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhang, Q.; Luo, Y.; He, Z.; Tian, X.; Battaglia, G. Thermosensitive nanocomposite gel for intra-tumoral two-photon photodynamic therapy. J. Control. Release 2019, 298, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Céspedes-Valenzuela, D.N.; Sánchez-Rentería, S.; Cifuentes, J.; Gómez, S.C.; Serna, J.A.; Rueda-Gensini, L.; Ostos, C.; Muñoz-Camargo, C.; Cruz, J.C. Novel Photo- and Thermo-Responsive Nanocomposite Hydrogels Based on Functionalized rGO and Modified SIS/Chitosan Polymers for Localized Treatment of Malignant Cutaneous Melanoma. Front. Bioeng. Biotechnol. 2022, 10, 947616. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yang, X.; Liu, R.; Zhao, D.; Guo, C.; Zhu, A.; Wen, M.; Liu, Z.; Qu, G.; Meng, H. Pathological Mechanism of Photodynamic Therapy and Photothermal Therapy Based on Nanoparticles. Int. J. Nanomed. 2020, 15, 6827–6838. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Yang, T.; O'Hagan, J.; Zhang, S.; Donnelly, R.F. Photothermal therapy. J. Control. Release 2020, 325, 52–71. [Google Scholar] [CrossRef]
- Chu, Y.; Xu, X.-Q.; Wang, Y. Ultradeep Photothermal Therapy Strategies. J. Phys. Chem. Lett. 2022, 13, 9564–9572. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, X.; Wang, X.; Guan, X.; Zhang, W.; Ma, J. Recent advances in selective photothermal therapy of tumor. J. Nanobiotechnol. 2021, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Zavahir, S.; Sobolčiak, P.; Krupa, I.; Han, D.S.; Tkac, J.; Kasak, P. Ti3C2Tx MXene-Based Light-Responsive Hydrogel Composite for Bendable Bilayer Photoactuator. Nanomaterials 2020, 10, 1419. [Google Scholar] [CrossRef]
- He, T.; Lv, S.; Wei, D.; Feng, R.; Yang, J.; Yan, Y.; Liu, L.; Wu, L. Photothermal Conversion of Hydrogel-Based Biomaterial. Chem. Rec. 2023, 23, e202300184. [Google Scholar] [CrossRef]
- Huang, K.; Liu, W.; Wei, W.; Zhao, Y.; Zhuang, P.; Wang, X.; Wang, Y.; Hu, Y.; Dai, H. Photothermal Hydrogel Encapsulating Intelligently Bacteria-Capturing Bio-MOF for Infectious Wound Healing. ACS Nano. 2022, 16, 19491–19508. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fu, R.; Duan, Z.; Zhu, C.; Fan, D. Artificial Nonenzymatic Antioxidant MXene Nanosheet-Anchored Injectable Hydrogel as a Mild Photothermal-Controlled Oxygen Release Platform for Diabetic Wound Healing. ACS Nano. 2022, 16, 7486–7502. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.-D.; Xiao, M.-T. Polysaccharide-based hydrogel with photothermal effect for accelerating wound healing. Carbohydr. Polym. 2023, 299, 120228. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, X.; Wang, W.; Qu, X.; Song, X.; Zhang, Y.; Zhong, L.; Yang, D.-P.; Dong, X.; Zhao, Y. Injectable hydrogel for postoperative synergistic photothermal-chemodynamic tumor and anti-infection therapy. Biomaterials 2022, 280, 121289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; He, G.; Yu, Y.; Zhang, Y.; Li, X.; Wang, S. Design of Biocompatible Chitosan/Polyaniline/Laponite Hydrogel with Photothermal Conversion Capability. Biomolecules 2022, 12, 1089. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, S.; Li, X.; Wang, X. Smart MXene/agarose hydrogel with photothermal property for controlled drug release. Int. J. Biol. Macromol. 2021, 190, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Lei, S.; Song, S.; Xia, X.; Qi, J.; Liu, J.; Zhao, H. Guanosine-based Hydrogel Integrating Photothermal Effect of PDA-AuNPs through Dynamic Borate Bond for Photothermal Therapy of Cancer. Chem. Asian J. 2022, 17, e202200302. [Google Scholar] [CrossRef]
- Tan, W.; Chen, S.; Xu, Y.; Chen, M.; Liao, H.; Niu, C. Temperature-Sensitive Nanocarbon Hydrogel for Photothermal Therapy of Tumors. Int. J. Nanomed. 2023, 18, 6137–6151. [Google Scholar] [CrossRef]
- Lu, S.; Wu, Y.; Liu, Y.; Sun, X.; Li, J.; Li, J. Multifunctional Photothermal Hydrogel in the Second Near-Infrared Window for Localized Tumor Therapy. ACS Appl. Bio Mater. 2023, 6, 4694–4702. [Google Scholar] [CrossRef]
- Chau, A.L.; Getty, P.T.; Rhode, A.R.; Bates, C.M.; Hawker, C.J.; Pitenis, A.A. Superlubricity of pH-responsive hydrogels in ex. treme environments. Front. Chem. 2022, 10, 891519. [Google Scholar] [CrossRef]
- Nasir, A.; Khan, A.; Li, J.; Naeem, M.; Khalil, A.A.K.; Khan, K.; Qasim, M. Nanotechnology, A Tool for Diagnostics and Treatment of Cancer. Curr. Top. Med. Chem. 2021, 21, 1360–1376. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Zhang, X.; Zhang, S.; Wei, G.; Su, Z. Biomedical and bioactive engineered nanomaterials for targeted tumor photothermal therapy: A review. Mater. Sci. Eng. C 2019, 104, 109891. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, P.; Zhou, C.; Zhang, T.; Zhou, T.; Men, D.; Jiang, G.; Hang, L. Gold nanobipyramid@copper sulfide nanotheranostics for image-guided NIR-II photo/chemodynamic cancer therapy with enhanced immune response. Acta Biomater. 2023, 158, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Korupalli, C.; Kalluru, P.; Nuthalapati, K.; Kuthala, N.; Thangudu, S.; Vankayala, R. Recent Advances of Polyaniline-Based Biomaterials for Phototherapeutic Treatments of Tumors and Bacterial Infections. Bioengineering 2020, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Sun, Y.; Li, C.; Lin, H.; Huang, Q.; Li, C. Facile synthesis of phycocyanin/polydopamine hierarchical nanocomposites for synergizing PTT/PDT against cancer. RSC Adv. 2022, 12, 34815–34821. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Zhu, H.; Kong, Y.; Shen, Q. Injectable Nanomedicine–Hydrogel for NIR Light Photothermal–Chemo Combination Therapy of Tumor. Polymers 2022, 14, 5547. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Fang, K.; Hu, X.; Yang, J.; Jiang, Z.; Feng, L.; Li, R.; Rao, Y.; Shi, S.; Dong, C. NIR-light-controlled G-quadruplex hydrogel for synergistically enhancing photodynamic therapy via sustained delivery of metformin and catalase-like activity in breast cancer. Mater. Today Bio 2022, 16, 100375. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Jin, J.-O. Attachable Hydrogel Containing Indocyanine Green for Selective Photothermal Therapy against Melanoma. Biomolecules 2020, 10, 1124. [Google Scholar] [CrossRef]
- Yin, J.; Han, Q.; Zhang, J.; Liu, Y.; Gan, X.; Xie, K.; Xie, L.; Deng, Y. MXene-Based Hydrogels Endow Polyetheretherketone with Effective Osteogenicity and Combined Treatment of Osteosarcoma and Bacterial Infection. ACS Appl. Mater. Interfaces 2020, 12, 45891–45903. [Google Scholar] [CrossRef]
- Luo, G.; Xu, Z.; Zhong, H.; Shao, H.; Liao, H.; Liu, N.; Jiang, X.; Zhang, Y.; Ji, X. Biodegradable photothermal thermosensitive hydrogels treat osteosarcoma by reprogramming macrophages. Biomater. Sci. 2023, 11, 2818–2827. [Google Scholar] [CrossRef]
- Shen, J.; Lin, M.; Ding, M.; Yu, N.; Yang, C.; Kong, D.; Sun, H.; Xie, Z. Tumor immunosuppressive microenvironment modulating hydrogels for second near-infrared photothermal-immunotherapy of cancer. Mater. Today Bio 2022, 16, 100416. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wang, W.; Xu, Z.; Zhang, P.; Gu, J.; Xu, Z.; Xi, J.; Fan, L. CaCO3-assistant synthesis of pH/near-infrared light-responsive and injectable sodium alginate hydrogels for melanoma synergistic treatment. J. Colloid Interface Sci. 2023, 633, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, B.; Chen, T.; Huang, Y.; Xiao, Z.; Jin, Y. Chemo-photothermal immunotherapy for eradication of orthotopic tumors and inhibition of metastasis by intratumoral injection of polydopamine versatile hydrogels. Acta Pharm. Sin. B 2022, 12, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Ran, S.; Cheng, H.; Zhang, M.; Sun, W.; Wan, Y.; Shao, C.; Zhu, Z. Polydopamine-modified ZIF-8 nanoparticles as a drug carrier for combined chemo-photothermal osteosarcoma therapy. Colloids Surf. B Biointerfaces 2022, 216, 112507. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, X.; Zhang, X.; Qu, D.; Xue, L.; Mo, R.; Zhang, C. Nanocomposite hydrogel incorporating gold nanorods and paclitaxel-loaded chitosan micelles for combination photothermal–chemotherapy. Int. J. Pharm. 2016, 497, 210–221. [Google Scholar] [CrossRef] [PubMed]
- GhavamiNejad, A.; SamariKhalaj, M.; Aguilar, L.E.; Park, C.H.; Kim, C.S. pH/NIR Light-Controlled Multidrug Release via a Mussel-Inspired Nanocomposite Hydrogel for Chemo-Photothermal Cancer Therapy. Sci. Rep. 2016, 6, 33594. [Google Scholar] [CrossRef] [PubMed]
- Theune, L.E.; Buchmann, J.; Wedepohl, S.; Molina, M.; Laufer, J.; Calderón, M. NIR- and thermo-responsive semi-interpenetrated polypyrrole nanogels for imaging guided combinational photothermal and chemotherapy. J. Control. Release 2019, 311–312, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.; Fan, Q.; Dai, H.; Zhou, X.; Xu, J.; Ma, Q.; Maruyama, A.; Wang, C. Physiologically triggered injectable red blood cell-based gel for tumor photoablation and enhanced cancer immunotherapy. Biomaterials 2021, 271, 120724. [Google Scholar] [CrossRef]
- Dong, X.; Liang, J.; Yang, A.; Qian, Z.; Kong, D.; Lv, F. Fluorescence imaging guided CpG nanoparticles-loaded IR820-hydrogel for synergistic photothermal immunotherapy. Biomaterials 2019, 209, 111–125. [Google Scholar] [CrossRef]
- Costa, F.J.P.; Nave, M.; Lima-Sousa, R.; Alves, C.G.; Melo, B.L.; Correia, I.J.; De Melo-Diogo, D. Development of Thiol-Maleimide hydrogels incorporating graphene-based nanomaterials for cancer chemo-photothermal therapy. Int. J. Pharm. 2023, 635, 122713. [Google Scholar] [CrossRef]
- Wang, S.; Chen, B.; Ouyang, L.; Wang, D.; Tan, J.; Qiao, Y.; Ge, S.; Ruan, J.; Zhuang, A.; Liu, X.; et al. A Novel Stimuli-Responsive Injectable Antibacterial Hydrogel to Achieve Synergetic Photothermal/Gene-Targeted Therapy towards Uveal Melanoma. Adv. Sci. 2021, 8, 2004721. [Google Scholar] [CrossRef] [PubMed]
- Appidi, T.; Rajalakshmi, P.S.; Chinchulkar, S.A.; Pradhan, A.; Begum, H.; Shetty, V.; Srivastava, R.; Ganesan, P.; Rengan, A.K. A plasmon-enhanced fluorescent gold coated novel lipo-polymeric hybrid nanosystem: Synthesis, characterization and application for imaging and photothermal therapy of breast cancer. Nanoscale 2022, 14, 9112–9123. [Google Scholar] [CrossRef] [PubMed]
- Sai, D.L.; Lee, J.; Nguyen, D.L.; Kim, Y.-P. Tailoring photosensitive ROS for advanced photodynamic therapy. Exp. Mol. Med. 2021, 53, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Barbora, A.; Yazbak, F.; Lyssenko, S.; Nave, V.; Nakonechny, F.; Ishai, P.B.; Minnes, R. Second harmonic generation nanoparticles enables Near-Infrared Photodynamic Therapy from visible light reactive photo-sensitizer conjugates. PLoS ONE 2022, 17, e0274954. [Google Scholar] [CrossRef]
- Guo, D.; Xu, S.; Huang, Y.; Jiang, H.; Yasen, W.; Wang, N.; Su, Y.; Qian, J.; Li, J.; Zhang, C.; et al. Platinum(IV) complex-based two-in-one polyprodrug for a combinatorial chemo-photodynamic therapy. Biomaterials 2018, 177, 67–77. [Google Scholar] [CrossRef]







| NIR | UV | X-ray | Citation | |
|---|---|---|---|---|
| Wavelength/nm | 780–2526 | 10–400 | 0.01–10 | [59] |
| Depth of penetration | 50–80 mm | The derims can be reached | Whole body | [60] |
| Harm to human body | Hardly any | Pruritus, dermatitis and skin cancer | Cells will be ionized, affecting cell activity and resulting in cell death | [61] |
| Hydrogel | Property | Cancer | Citation |
|---|---|---|---|
| LEFPG | more cell adhesion and proliferation were supported | melanoma | [99] |
| PNSI | easy to prepare and has good biocompatibility | melanoma | [100] |
| P407 | safe and suitable for ICI;approved by the FDA | colon cancer | [101] |
| F127 | high drug loading capacity and low toxicity | hepatocellular carcinama | [102] |
| SISMA/ChiMA | suitable for in situ deposition and room-temperature polymerization | melanoma | [103] |
| OSA/HPCS | injectable and high biocompatibility | breast cancer | [83] |
| Photothermal Agent | Cancer | Mechanism of Material Action | Citation |
|---|---|---|---|
| ICG | Breast cancer | The high photothermal efficiency maintains the high concentration of metformin in the tumor, which provides an excellent synergy between PDT and IT | [117] |
| Melanoma | Combination of targeted drug therapy and PTT | [118] | |
| MXenes | Osteosarcoma | Thermal ablation of residual tumor cells can effectively promote bone tissue regeneration and has antibacterial properties | [119] |
| HTA | Osteosarcoma | Induction of apoptosis in osteosarcoma-related cells and thermal ablation of tumors | [120] |
| SPIIN | Breast cancer | Tumor growth and metastasis to the lung were inhibited by 1064 nm light irradiation | [121] |
| PDA NPS | Melanoma | Dual regulation of pH and NIR can improve the tumor’s hypoxic environment and maintain drug concentration | [122] |
| Breast cancer | Cooperates with PPT and IT | [123] | |
| Osteosarcoma | High photothermal effect and good biocompatibility provide a good carrier for drug delivery | [124] | |
| DOX/DOPA-rGO | Breast cancer | Combined photothermal and CT effectively reducing the viability to 21% | [125] |
| Au | Melanoma | Gene-targeted therapy for ocular melanoma under photothermal synergy | [126] |
| Breast cancer | The gold coating was attached to enhance the fluorescence signal for easy diagnosis, and the photothermal conversion efficiency reached 65% | [127] |
| Photosensitizer Name | Mechanism of Action | Citation |
|---|---|---|
| PpIX | ROS is produced to kill tumors, and ROS prodrug release is stimulated to enhance anti-tumor immunity | [131] |
| ROS was generated to achieve local PDT treatment, and hydrogel cross-linking was induced to achieve drug release under NIR regulation | [132] | |
| T1-PPa | Two-photon infrared light irradiation improves the depth of light penetration and prevents Ps aggregation | [121] |
| ICG | PDT in combination with IT for colon cancer; the production of large amounts of ROS and the production of NO inhibit the proliferation of cancer cells | [122] |
| UZC@HA | Targeted therapy, without damage to normal tissues, induces the apoptosis of breast-cancer-related cells | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Pan, B.; Wang, T.; Yang, H.; Vance, D.; Li, X.; Zhao, H.; Hu, X.; Yang, T.; Chen, Z.; et al. Advances in NIR-Responsive Natural Macromolecular Hydrogel Assembly Drugs for Cancer Treatment. Pharmaceutics 2023, 15, 2729. https://doi.org/10.3390/pharmaceutics15122729
Zhao C, Pan B, Wang T, Yang H, Vance D, Li X, Zhao H, Hu X, Yang T, Chen Z, et al. Advances in NIR-Responsive Natural Macromolecular Hydrogel Assembly Drugs for Cancer Treatment. Pharmaceutics. 2023; 15(12):2729. https://doi.org/10.3390/pharmaceutics15122729
Chicago/Turabian StyleZhao, Chenyu, Boyue Pan, Tianlin Wang, Huazhe Yang, David Vance, Xiaojia Li, Haiyang Zhao, Xinru Hu, Tianchang Yang, Zihao Chen, and et al. 2023. "Advances in NIR-Responsive Natural Macromolecular Hydrogel Assembly Drugs for Cancer Treatment" Pharmaceutics 15, no. 12: 2729. https://doi.org/10.3390/pharmaceutics15122729
APA StyleZhao, C., Pan, B., Wang, T., Yang, H., Vance, D., Li, X., Zhao, H., Hu, X., Yang, T., Chen, Z., Hao, L., Liu, T., & Wang, Y. (2023). Advances in NIR-Responsive Natural Macromolecular Hydrogel Assembly Drugs for Cancer Treatment. Pharmaceutics, 15(12), 2729. https://doi.org/10.3390/pharmaceutics15122729