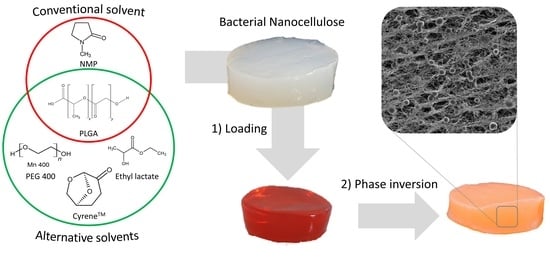
In situ Formation of Polymer Microparticles in Bacterial Nanocellulose Using Alternative and Sustainable Solvents to Incorporate Lipophilic Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of BNC Fleeces
2.2. In Situ Formulation of PLGA Particles in BNC Fleeces
2.3. Scanning Electron Microscopy (SEM)
2.4. Transparency
2.5. Fourier Transform Infrared (FT-IR) Spectroscopy
2.6. Differential Scanning Calorimetry (DSC)
2.7. Compression Stability
2.8. Water Binding Characteristics
2.9. Fluid Release
2.10. High-Performance Liquid Chromatography (HPLC)
2.11. Drug Loading and In Vitro Release
2.12. Data and Statistical Analysis
3. Results and Discussion
3.1. Development of the In Situ Inversion for Selection of Suitable Solvents
3.2. Morphology and Thermal Characteristics of the Polymer Containing BNC
3.3. Interaction with Water and Mechanical Characteristics of Microparticle Containing BNC
3.4. Potential of PLGA Loaded BNC as Drug Delivery System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pötzinger, Y.; Kralisch, D.; Fischer, D. Bacterial nanocellulose: The future of controlled drug delivery? Ther. Deliv. 2017, 8, 753–761. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K. Bacterial nanocellulose: Present status, biomedical applications and future perspectives. Mater. Sci. Eng. C 2019, 104, 109963. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Petzold-Welcke, K.; Kramer, F.; Richter, T.; Raddatz, V.; Fried, W.; Nietzsche, S.; Bellmann, T.; Fischer, D. Biotech nanocellulose: A review on progress in product design and today’s state of technical and medical applications. Carbohydr. Polym. 2020, 254, 117313. [Google Scholar] [CrossRef]
- Numata, Y.; Mazzarino, L.; Borsali, R. A slow-release system of bacterial cellulose gel and nanoparticles for hydrophobic active ingredients. Int. J. Pharm. 2015, 486, 217–225. [Google Scholar] [CrossRef]
- Zmejkoski, D.Z.; Marković, Z.M.; Budimir, M.D.; Zdravković, N.M.; Trišić, D.D.; Bugárová, N.; Danko, M.; Kozyrovska, N.O.; Špitalský, Z.; Kleinová, A.; et al. Photoactive and antioxidant nanochitosan dots/biocellulose hydrogels for wound healing treatment. Mater. Sci. Eng. C 2021, 122, 111925. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.H.; Yang, Y.N.; Ho, Y.C.; Tsai, M.L.; Mi, F.L. Drug release and antioxidant/antibacterial activities of silymarin-zein nanoparticle/bacterial cellulose nanofiber composite films. Carbohydr. Polym. 2018, 180, 286–296. [Google Scholar] [CrossRef]
- Li, N.; Yang, L.; Pan, C.; Saw, P.E.; Ren, M.; Lan, B.; Wu, J.; Wang, X.; Zeng, T.; Zhou, L.; et al. Naturally-occurring bacterial cellulose-hyperbranched cationic polysaccharide derivative/MMP-9 siRNA composite dressing for wound healing enhancement in diabetic rats. Acta Biomater. 2020, 102, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, Y.; Dewaldt, M.; Moritz, S.; Nitzsche, R.; Kralisch, D.; Fischer, D. Controlled extended octenidine release from a bacterial nanocellulose/Poloxamer hybrid system. Eur. J. Pharm. Biopharm. 2017, 112, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Mäder, K.; Lehner, E.; Liebau, A.; Plontke, S.K. Controlled drug release to the inner ear: Concepts, materials, mechanisms, and performance. Hear. Res. 2018, 368, 49–66. [Google Scholar] [CrossRef]
- Babayekhorasani, F.; Hosseini, M.; Spicer, P.T. Molecular and Colloidal Transport in Bacterial Cellulose Hydrogels. Biomacromolecules 2022, 23, 2404–2414. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Prakash, N.; Datta, D. Biopolymers Based on Carboxylic Acids Derived from Renewable Resources. In Biopolymers; Wiley: Hoboken, NJ, USA, 2011; pp. 167–182. [Google Scholar]
- Si, W.; Yang, Q.; Zong, Y.; Ren, G.; Zhao, L.; Hong, M.; Xin, Z. Toward Understanding the Effect of Solvent Evaporation on the Morphology of PLGA Microspheres by Double Emulsion Method. Ind. Eng. Chem. Res. 2021, 60, 9196–9205. [Google Scholar] [CrossRef]
- Klinger-Strobel, M.; Ernst, J.; Lautenschläger, C.; Pletz, M.W.; Fischer, D.; Makarewicz, O. A blue fluorescent labeling technique utilizing micro-and nanoparticles for tracking in LIVE/DEAD® stained pathogenic biofilms of Staphylococcus aureus and Burkholderia cepacia. Int. J. Nanomed. 2016, 11, 575. [Google Scholar] [CrossRef]
- Wang, R.; Bao, Q.; Clark, A.G.; Wang, Y.; Zhang, S.; Burgess, D.J. Characterization and in vitro release of minocycline hydrochloride microspheres prepared via coacervation. Int. J. Pharm. 2022, 628, 122292. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Maltesen, M.J.; Andersen, S.K.; Bjerregaard, S.; Foged, C.; Rantanen, J.; Yang, M. One-Step Production of Protein-Loaded PLGA Microparticles via Spray Drying Using 3-Fluid Nozzle. Pharm. Res. 2014, 31, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Damiati, S.A.; Rossi, D.; Joensson, H.N.; Damiati, S. Artificial intelligence application for rapid fabrication of size-tunable PLGA microparticles in microfluidics. Sci. Rep. 2020, 10, 19517. [Google Scholar] [CrossRef] [PubMed]
- Lagreca, E.; Onesto, V.; Di Natale, C.; La Manna, S.; Netti, P.A.; Vecchione, R. Recent advances in the formulation of PLGA microparticles for controlled drug delivery. Prog. Biomater. 2020, 9, 153–174. [Google Scholar] [CrossRef] [PubMed]
- Sherstneva, A.A.; Demina, T.S.; Monteiro, A.P.F.; Akopova, T.A.; Grandfils, C.; Ilangala, A.B. Biodegradable Microparticles for Regenerative Medicine: A State of the Art and Trends to Clinical Application. Polymers 2022, 14, 1314. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.T.; Faulhammer, E.; Dieplinger, J.; Dekner, M.; Makert, C.; Nieder, M.; Paudel, A. Progress in spray-drying of protein pharmaceuticals: Literature analysis of trends in formulation and process attributes. Dry. Technol. 2021, 39, 1415–1446. [Google Scholar] [CrossRef]
- Jo, Y.K.; Lee, D. Biopolymer Microparticles Prepared by Microfluidics for Biomedical Applications. Small 2020, 16, 1903736. [Google Scholar] [CrossRef]
- Reverchon, E.; Adami, R.; Cardea, S.; Porta, G.D. Supercritical fluids processing of polymers for pharmaceutical and medical applications. J. Supercrit. Fluids 2009, 47, 484–492. [Google Scholar] [CrossRef]
- Barbero-Colmenar, E.; Guastaferro, M.; Baldino, L.; Cardea, S.; Reverchon, E. Supercritical CO2 Assisted Electrospray to Produce Poly(lactic-co-glycolic Acid) Nanoparticles. ChemEngineering 2022, 6, 66. [Google Scholar] [CrossRef]
- Perrut, M.; Clavier, J.-Y. Supercritical Fluid Formulation: Process Choice and Scale-up. Ind. Eng. Chem. Res. 2003, 42, 6375–6383. [Google Scholar] [CrossRef]
- ICH Guideline Q3C(R8), Impurities: Guideline for Residual Solvents. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use; ICH: Geneva, Switzerland, 2021; Volume 4, pp. 1–51.
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guides. Sustain. Chem. Process. 2016, 4, 7. [Google Scholar] [CrossRef]
- Soni, J.; Sahiba, N.; Sethiya, A.; Agarwal, S. Polyethylene glycol: A promising approach for sustainable organic synthesis. J. Mol. Liq. 2020, 315, 113766. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef]
- Polyethylene glycol [MAK Value Documentation, 1998]. In The MAK-Collection for Occupational Health and Safety; Wiley: Hoboken, NJ, USA,, 1998; pp. 248–270.
- Fuertges, F.; Abuchowski, A. The clinical efficacy of poly(ethylene glycol)-modified proteins. J. Control. Release 1990, 11, 139–148. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Quimidroga Alternative Solvents to Traditional Solvents. Available online: https://www.quimidroga.com/en/2022/03/08/alternative-solvents-to-traditional-solvents/ (accessed on 19 December 2022).
- Sherwood, J.; Constantinou, A.; Moity, L.; McElroy, C.R.; Farmer, T.J.; Duncan, T.; Raverty, W.; Hunt, A.J.; Clark, J.H. Dihydrolevoglucosenone (Cyrene) as a bio-based alternative for dipolar aprotic solvents. Chem. Commun. 2014, 50, 9650–9652. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes—A review. Green Chem. 2011, 13, 2658–2671. [Google Scholar] [CrossRef]
- Zhang, J.; White, G.B.; Ryan, M.D.; Hunt, A.J.; Katz, M.J. Dihydrolevoglucosenone (Cyrene) As a Green Alternative to N,N-Dimethylformamide (DMF) in MOF Synthesis. ACS Sustain. Chem. Eng. 2016, 4, 7186–7192. [Google Scholar] [CrossRef]
- Clary, J.J.; Feron, V.J.; van Velthuijsen, J.A. Safety Assessment of Lactate Esters. Regul. Toxicol. Pharmacol. 1998, 27, 88–97. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA)—Ethyl Lactate; WHO: Geneva, Switzerland, 2004.
- Blaskovich, M.A.T.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J.; et al. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 7. [Google Scholar] [CrossRef]
- Gilbert, N.C.; Gerstmeier, J.; Schexnaydre, E.E.; Börner, F.; Garscha, U.; Neau, D.B.; Werz, O.; Newcomer, M.E. Structural and mechanistic insights into 5-lipoxygenase inhibition by natural products. Nat. Chem. Biol. 2020, 16, 783–790. [Google Scholar] [CrossRef]
- Beekmann, U.; Schmölz, L.; Lorkowski, S.; Werz, O.; Thamm, J.; Fischer, D.; Kralisch, D. Process control and scale-up of modified bacterial cellulose production for tailor-made anti-inflammatory drug delivery systems. Carbohydr. Polym. 2020, 236, 116062. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Ni, Z.; Hessler, N.; Wesarg, F.; Muller, F.A.; Kralisch, D.; Fischer, D. The biopolymer bacterial nanocellulose as drug delivery system: Investigation of drug loading and release using the model protein albumin. J. Pharm. Sci. 2013, 102, 579–592. [Google Scholar] [CrossRef]
- Karl, B.; Alkhatib, Y.; Beekmann, U.; Bellmann, T.; Blume, G.; Steiniger, F.; Thamm, J.; Werz, O.; Kralisch, D.; Fischer, D. Development and characterization of bacterial nanocellulose loaded with Boswellia serrata extract containing nanoemulsions as natural dressing for skin diseases. Int. J. Pharm. 2020, 587, 119635. [Google Scholar] [CrossRef] [PubMed]
- Zahel, P.; Beekmann, U.; Eberlein, T.; Schmitz, M.; Werz, O.; Kralisch, D. Bacterial Cellulose—Adaptation of a Nature-Identical Material to the Needs of Advanced Chronic Wound Care. Pharmaceuticals 2022, 15, 683. [Google Scholar] [CrossRef] [PubMed]
- ICH Guideline Q2 (R1), Validation of Analytical Procedures: Text and Methodology International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use; ICH: Geneva, Switzerland, 2005; Volume 4, pp. 1–17.
- Moritz, S.; Wiegand, C.; Wesarg, F.; Hessler, N.; Müller, F.A.; Kralisch, D.; Hipler, U.C.; Fischer, D. Active wound dressings based on bacterial nanocellulose as drug delivery system for octenidine. Int. J. Pharm. 2014, 471, 45–55. [Google Scholar] [CrossRef]
- Bellmann, T.; Luber, R.; Kischio, L.; Karl, B.; Pötzinger, Y.; Beekmann, U.; Kralisch, D.; Wiegand, C.; Fischer, D. Bacterial nanocellulose patches as a carrier for hydrating formulations to improve the topical treatment of nail diseases. Int. J. Pharm. 2022, 628, 122267. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Fernández-Carballido, A.; Martin-Sabroso, C.; Torres-Suárez, A.I. Stability characteristics of cannabidiol for the design of pharmacological, biochemical and pharmaceutical studies. J. Chromatogr. B 2020, 1150, 122188. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Higuchi, T. Rate of Release of Medicaments from Ointment Bases Containing Drugs in Suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Xiao, Y.; Zhang, S.; Wang, J. Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 2019, 7, 843–855. [Google Scholar] [CrossRef]
- Pircher, N.; Veigel, S.; Aigner, N.; Nedelec, J.M.; Rosenau, T.; Liebner, F. Reinforcement of bacterial cellulose aerogels with biocompatible polymers. Carbohydr. Polym. 2014, 111, 505–513. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Huang, J.; Lin, N.; Ahmad, I.; Dufresne, A.; Thomas, S. Recent developments on nanocellulose reinforced polymer nanocomposites: A review. Polymer 2017, 132, 368–393. [Google Scholar] [CrossRef]
- Camargo, J.A.; Sapin, A.; Daloz, D.; Maincent, P. Ivermectin-loaded microparticles for parenteral sustained release: In vitro characterization and effect of some formulation variables. J. Microencapsul. 2010, 27, 609–617. [Google Scholar] [CrossRef]
- Bilati, U.; Allémann, E.; Doelker, E. Nanoprecipitation versus emulsion-based techniques for the encapsulation of proteins into biodegradable nanoparticles and process-related stability issues. AAPS PharmSciTech 2005, 6, E594–E604. [Google Scholar] [CrossRef]
- Sartor, O. Eligard: Leuprolide acetate in a novel sustained-release delivery system. Urology 2003, 61 (Suppl. S2), 25–31. [Google Scholar] [CrossRef]
- Moreau, J.P.; Vachon, P.J.; Huneau, M.C. Elevated glycemia and local inflammation after injecting N-methyl-2-pyrrolidone (NMP) into the marginal ear vein of rabbits. Contemp. Top. Lab. Anim. Sci. 2001, 40, 38–40. [Google Scholar] [PubMed]
- Sitarek, K.; Stetkiewicz, J. Assessment of Reproductive Toxicity and Gonadotoxic Potential of N-Methyl-2-Pyrrolidone in Male Rats. Int. J. Occup. Med. Environ. Health 2008, 21, 73–80. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Ali, M.E.; Lamprecht, A. Polyethylene glycol as an alternative polymer solvent for nanoparticle preparation. Int. J. Pharm. 2013, 456, 135–142. [Google Scholar] [CrossRef]
- Grune, C.; Zens, C.; Czapka, A.; Scheuer, K.; Thamm, J.; Hoeppener, S.; Jandt, K.D.; Werz, O.; Neugebauer, U.; Fischer, D. Sustainable preparation of anti-inflammatory atorvastatin PLGA nanoparticles. Int. J. Pharm. 2021, 599, 120404. [Google Scholar] [CrossRef]
- Kempe, S.; Metz, H.; Pereira, P.G.C.; Mäder, K. Non-invasive in vivo evaluation of in situ forming PLGA implants by benchtop magnetic resonance imaging (BT-MRI) and EPR spectroscopy. Eur. J. Pharm. Biopharm. 2010, 74, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Camp, J.E. Bio-available Solvent Cyrene: Synthesis, Derivatization, and Applications. ChemSusChem 2018, 11, 3048–3055. [Google Scholar] [CrossRef]
- Grune, C.; Thamm, J.; Werz, O.; Fischer, D. Cyrene™ as an Alternative Sustainable Solvent for the Preparation of Poly(lactic-co-glycolic acid) Nanoparticles. J. Pharm. Sci. 2021, 110, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, I.; Gutiérrez, C.; Rodríguez, J.F.; de Lucas, A.; García, M.T. Production of biodegradable PLGA foams processed with high pressure CO2. J. Supercrit. Fluids 2020, 164, 104886. [Google Scholar] [CrossRef]
- Dávila, M.J.; Aparicio, S.; Alcalde, R. Thermophysical Properties of Binary and Ternary Mixtures Containing Lactams and Methanol. Ind. Eng. Chem. Res. 2009, 48, 10065–10076. [Google Scholar] [CrossRef]
- Han, F.; Zhang, J.; Chen, G.; Wei, X. Density, Viscosity, and Excess Properties for Aqueous Poly(ethylene glycol) Solutions from (298.15 to 323.15) K. J. Chem. Eng. Data 2008, 53, 2598–2601. [Google Scholar] [CrossRef]
- Milescu, R.A.; McElroy, C.R.; Farmer, T.J.; Williams, P.M.; Walters, M.J.; Clark, J.H. Fabrication of PES/PVP Water Filtration Membranes Using Cyrene®, a Safer Bio-Based Polar Aprotic Solvent. Adv. Polym. Technol. 2019, 2019, 9692859. [Google Scholar] [CrossRef]
- Bajić, D.M.; Živković, E.M.; Jovanović, J.; Šerbanović, S.P.; Kijevčanin, M.L. Experimental measurements and modelling of volumetric properties, refractive index and viscosity of binary systems of ethyl lactate with methyl ethyl ketone, toluene and n-methyl-2-pirrolidone at 288.15–323.15K and atmospheric pressure. New UNIFAC–VISCO and ASOG–VISCO interaction parameters. Fluid Phase Equilibria 2015, 399, 50–65. [Google Scholar] [CrossRef]
- Lee, E.S.; Oh, Y.T.; Youn, Y.S.; Nam, M.; Park, B.; Yun, J.; Kim, J.H.; Song, H.-T.; Oh, K.T. Binary mixing of micelles using Pluronics for a nano-sized drug delivery system. Colloids Surf. B Biointerfaces 2011, 82, 190–195. [Google Scholar] [CrossRef]
- Bernardelli de Mattos, I.; Nischwitz, S.P.; Tuca, A.-C.; Groeber-Becker, F.; Funk, M.; Birngruber, T.; Mautner, S.I.; Kamolz, L.-P.; Holzer, J.C.J. Delivery of antiseptic solutions by a bacterial cellulose wound dressing: Uptake, release and antibacterial efficacy of octenidine and povidone-iodine. Burns 2020, 46, 918–927. [Google Scholar] [CrossRef]
- Zhang, C.; Chung, J.W.; Priestley, R.D. Dialysis Nanoprecipitation of Polystyrene Nanoparticles. Macromol. Rapid Commun. 2012, 33, 1798–1803. [Google Scholar] [CrossRef]
- Barud, H.S.; Souza, J.L.; Santos, D.B.; Crespi, M.S.; Ribeiro, C.A.; Messaddeq, Y.; Ribeiro, S.J.L. Bacterial cellulose/poly(3-hydroxybutyrate) composite membranes. Carbohydr. Polym. 2011, 83, 1279–1284. [Google Scholar] [CrossRef]
- Sampaio, L.M.P.; Padrão, J.; Faria, J.; Silva, J.P.; Silva, C.J.; Dourado, F.; Zille, A. Laccase immobilization on bacterial nanocellulose membranes: Antimicrobial, kinetic and stability properties. Carbohydr. Polym. 2016, 145, 1–12. [Google Scholar] [CrossRef]
- Siepmann, J.; Elkharraz, K.; Siepmann, F.; Klose, D. How Autocatalysis Accelerates Drug Release from PLGA-Based Microparticles: A Quantitative Treatment. Biomacromolecules 2005, 6, 2312–2319. [Google Scholar] [CrossRef]
- Gosau, M.; Müller, B.W. Release of gentamicin sulphate from biodegradable PLGA-implants produced by hot melt extrusion. Die Pharm.—Int. J. Pharm. Sci. 2010, 65, 487–492. [Google Scholar] [CrossRef]
- Lee, J.C.; Kandula, S.; Sherber, N.S. Beyond wet-to-dry: A rational approach to treating chronic wounds. Eplasty 2009, 9, e14. [Google Scholar] [PubMed]
- Beekmann, U.; Zahel, P.; Karl, B.; Schmölz, L.; Börner, F.; Gerstmeier, J.; Werz, O.; Lorkowski, S.; Wiegand, C.; Fischer, D.; et al. Modified Bacterial Cellulose Dressings to Treat Inflammatory Wounds. Nanomaterials 2020, 10, 2508. [Google Scholar] [CrossRef]
- Czaja, W.; Krystynowicz, A.; Kawecki, M.; Wysota, K.; Sakiel, S.; Wróblewski, P.; Glik, J.; Nowak, M.; Bielecki, S. Biomedical applications of microbial cellulose in burn wound recovery. In Cellulose: Molecular and Structural Biology; Springer: Berlin/Heidelberg, Germany, 2007; pp. 307–321. [Google Scholar]
- Hammell, D.C.; Zhang, L.P.; Ma, F.; Abshire, S.M.; McIlwrath, S.L.; Stinchcomb, A.L.; Westlund, K.N. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur. J. Pain. 2016, 20, 936–948. [Google Scholar] [CrossRef]
- Pötzinger, Y.; Rahnfeld, L.; Kralisch, D.; Fischer, D. Immobilization of plasmids in bacterial nanocellulose as gene activated matrix. Carbohydr. Polym. 2019, 209, 62–73. [Google Scholar] [CrossRef]
- Yoo, J.; Won, Y.-Y. Phenomenology of the Initial Burst Release of Drugs from PLGA Microparticles. ACS Biomater. Sci. Eng. 2020, 6, 6053–6062. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I.; Cohen, M.; Delie, F.; Bastida-Ruiz, D.; Yart, L.; Martin-Sabroso, C.; Fernández-Carballido, A. PLGA Nanoparticles for the Intraperitoneal Administration of CBD in the Treatment of Ovarian Cancer: In Vitro and In Ovo Assessment. Pharmaceutics 2020, 12, 439. [Google Scholar] [CrossRef]
- Bairwa, K.; Jachak, S.M. Development and optimisation of 3-Acetyl-11-keto-β-boswellic acid loaded poly-lactic-co-glycolic acid-nanoparticles with enhanced oral bioavailability and in-vivo anti-inflammatory activity in rats. J. Pharm. Pharmacol. 2015, 67, 1188–1197. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, F.; Zhu, L.; Jiang, J. An in-situ fabrication of bamboo bacterial cellulose/sodium alginate nanocomposite hydrogels as carrier materials for controlled protein drug delivery. Int. J. Biol. Macromol. 2021, 170, 459–468. [Google Scholar] [CrossRef]
- Adepu, S.; Khandelwal, M. Ex-situ modification of bacterial cellulose for immediate and sustained drug release with insights into release mechanism. Carbohydr. Polym. 2020, 249, 116816. [Google Scholar] [CrossRef]
- Pavaloiu, R.-D.; Stoica-Guzun, A.; Stroescu, M.; Jinga, S.I.; Dobre, T. Composite films of poly(vinyl alcohol)–chitosan–bacterial cellulose for drug controlled release. Int. J. Biol. Macromol. 2014, 68, 117–124. [Google Scholar] [CrossRef]
- Saeedi Garakani, S.; Davachi, S.M.; Bagher, Z.; Heraji Esfahani, A.; Jenabi, N.; Atoufi, Z.; Khanmohammadi, M.; Abbaspourrad, A.; Rashedi, H.; Jalessi, M. Fabrication of chitosan/polyvinylpyrrolidone hydrogel scaffolds containing PLGA microparticles loaded with dexamethasone for biomedical applications. Int. J. Biol. Macromol. 2020, 164, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Galeska, I.; Kim, T.-K.; Patil, S.D.; Bhardwaj, U.; Chatttopadhyay, D.; Papadimitrakopoulos, F.; Burgess, D.J. Controlled release of dexamethasone from PLGA microspheres embedded within polyacid-containing PVA hydrogels. AAPS J. 2005, 7, E231–E240. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, S.H.; Kee, I.; Krantz, W.B.; Chow, P.K.-H.; Wang, C.-H. Hydrogel Matrix Entrapping PLGA-Paclitaxel Microspheres: Drug Delivery with Near Zero-Order Release and Implantability Advantages for Malignant Brain Tumour Chemotherapy. Pharm. Res. 2009, 26, 2101–2114. [Google Scholar] [CrossRef] [PubMed]
- Kamali, A.; Oryan, A.; Hosseini, S.; Ghanian, M.H.; Alizadeh, M.; Baghaban Eslaminejad, M.; Baharvand, H. Cannabidiol-loaded microspheres incorporated into osteoconductive scaffold enhance mesenchymal stem cell recruitment and regeneration of critical-sized bone defects. Mater. Sci. Eng. C 2019, 101, 64–75. [Google Scholar] [CrossRef]
- Bai, F.; Chen, X.; Yang, H.; Xu, H.-G. Acetyl-11-Keto-β-Boswellic Acid Promotes Osteoblast Differentiation by Inhibiting Tumor Necrosis Factor-α and Nuclear Factor-κB Activity. J. Craniofacial Surg. 2018, 29, 1996–2002. [Google Scholar] [CrossRef]
- Kralisch, D.; Hessler, N.; Klemm, D.; Erdmann, R.; Schmidt, W. White biotechnology for cellulose manufacturing--the HoLiR concept. Biotechnol. Bioeng. 2010, 105, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Xi, T.; Zheng, Y.; Guo, T.; Hou, J.; Wan, Y.; Gao, C. In Vitro Cytotoxicity of Bacterial Cellulose Scaffolds Used for Tissue-engineered Bone. J. Bioact. Compat. Polym. 2009, 24 (Suppl. S1), 137–145. [Google Scholar] [CrossRef]
- Gupta, A.; Low, W.L.; Britland, S.T.; Radecka, I.; Martin, C. Physicochemical characterisation of biosynthetic bacterial cellulose as a potential wound dressing material. Br. J. Pharm. 2018, 2, S37–S38. [Google Scholar] [CrossRef]
- Pita, P.C.d.C.; Pinto, F.C.M.; Lira, M.M.d.M.; Melo, F.d.A.D.; Ferreira, L.M.; Aguiar, J.L.d.A. Biocompatibility of the bacterial cellulose hydrogel in subcutaneous tissue of rabbits. Acta Cir. Bras. 2015, 30, 296–300. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Muddineti, O.S.; Omri, A. Current trends in PLGA based long-acting injectable products: The industry perspective. Expert Opin. Drug Deliv. 2022, 19, 559–576. [Google Scholar] [CrossRef] [PubMed]






| Solvent | NMP | PEG 400 | Cyr | EL | |
|---|---|---|---|---|---|
| Structure |  |  |  |  | |
| Molar mass [g/mol] | 99 | 400 | 128 | 118 | |
| Viscosity at 25 °C [mPa s] | 1.7 [66] | 94.4 [67] | 14.5 [68] | 2.4 [69] | |
| Critical water concentration for phase inversion [% v/v] * | 10 mg mL−1 PLGA | 15 | 7 | 17 | 2 |
| 20 mg mL−1 PLGA | 14 | 6 | 16 | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellmann, T.; Thamm, J.; Beekmann, U.; Kralisch, D.; Fischer, D. In situ Formation of Polymer Microparticles in Bacterial Nanocellulose Using Alternative and Sustainable Solvents to Incorporate Lipophilic Drugs. Pharmaceutics 2023, 15, 559. https://doi.org/10.3390/pharmaceutics15020559
Bellmann T, Thamm J, Beekmann U, Kralisch D, Fischer D. In situ Formation of Polymer Microparticles in Bacterial Nanocellulose Using Alternative and Sustainable Solvents to Incorporate Lipophilic Drugs. Pharmaceutics. 2023; 15(2):559. https://doi.org/10.3390/pharmaceutics15020559
Chicago/Turabian StyleBellmann, Tom, Jana Thamm, Uwe Beekmann, Dana Kralisch, and Dagmar Fischer. 2023. "In situ Formation of Polymer Microparticles in Bacterial Nanocellulose Using Alternative and Sustainable Solvents to Incorporate Lipophilic Drugs" Pharmaceutics 15, no. 2: 559. https://doi.org/10.3390/pharmaceutics15020559
APA StyleBellmann, T., Thamm, J., Beekmann, U., Kralisch, D., & Fischer, D. (2023). In situ Formation of Polymer Microparticles in Bacterial Nanocellulose Using Alternative and Sustainable Solvents to Incorporate Lipophilic Drugs. Pharmaceutics, 15(2), 559. https://doi.org/10.3390/pharmaceutics15020559