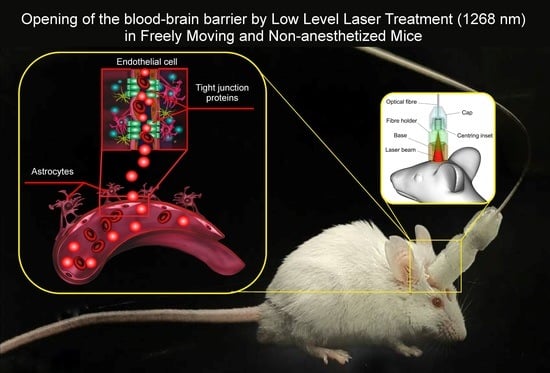
Low-Level Laser Treatment Induces the Blood-Brain Barrier Opening and the Brain Drainage System Activation: Delivery of Liposomes into Mouse Glioblastoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Laser 1122 nm and 1268 nm Irradiation
2.3. Spectrofluorometric Assay of the Evans Blue Extravasation
2.4. In Vivo Real-Time Two-Photon Laser Scanning Microscopy (2PLSM)
2.5. Magnetic Resonance Imaging of the BBB Permeability
2.6. MRI Data Analysis and Processing
2.7. The In Vitro BBB Model
2.8. Measurement of Lactate Levels in the Culture Medium
2.9. Implantation of EPNT-5-TagRFP GBM
2.10. Synthesis of GM1-Liposomes
2.11. Analysis of the ROS Production
2.12. Optical Monitoring of the Brain Drainage System and Liposomes Delivery into GBM
2.13. Immunohistochemical (IHC) Assay
2.14. Histological Analysis of the Brain Tissues
2.15. Statistical Analysis
3. Results
3.1. Selection of Optimal Dose of Laser Irradiation for Effective BBBO
3.2. 1268 nm Laser Irradiation Induces Singlet Oxygen Generation in the In Vitro BBB Model
3.3. Laser Irradiation with 1268 nm but Not with 1122 nm Induces BBBO
3.4. Mechanisms of Underlying 1268 nm-Induced BBBO and Recovery
3.5. The 1268 nm Laser-Induced BBBO as an Effective Technology for Brain drug Delivery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Cote, D.J.; Ascha, M.; Kruchko, C.; Barnholtz-Sloan, J.S. Adult glioma incidence and survival by race or ethnicity in the United States from 2000 to 2014. JAMA Oncol. 2018, 4, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Fangusaro, J. Pediatric high grade glioma: A review and update on tumor clinical characteristics and biology. Front. Oncol. 2012, 2, 105. [Google Scholar] [PubMed]
- Jones, C.; Perryman, L.; Hargrave, D. Paediatric and adult malignant glioma: Close relatives or distant cousins? Nat. Rev. Clin. Oncol. 2012, 9, 400–413. [Google Scholar] [PubMed]
- Minniti, G.; Lombardi, G.; Paolini, S. Glioblastoma in elderly patients: Current Management and Future perspectives. Cancers 2019, 11, 336. [Google Scholar]
- Kramer, E.D.; Donaldson, M.; Geyer, J.R. Survival of infants with malignant astrocytomas: A report from the childrens cancer group. Cancer 1995, 76, 1685–1686. [Google Scholar]
- Sun, R.; Cuthbert, H.; Watts, C. Fluorescence-guided surgery in the surgical treatment of gliomas: Past, present and future. Cancers 2021, 13, 3508. [Google Scholar]
- Wang, L.M.; Banu, M.A.; Canoll, P.; Bruce, J.N. Rationale and clinical implications of fluorescein-guided supramarginal resection in newly diagnosed high-grade glioma. Front. Oncol. 2021, 11, 666734. [Google Scholar] [CrossRef]
- Davis, M.E. Glioblastoma: Overview of disease and treatment. Clin. J. Oncol. Nurs. 2016, 20, 2–8. [Google Scholar]
- Neira, J.A.; Ung, T.H.; Sims, J.S.; Malone, H.R.; Chow, D.S.; Samanamud, J.L.; Zanazzi, G.J.; Guo, X.; Bowden, S.G.; Zhao, B.; et al. Aggressive resection at the infiltrative margins of glioblastoma facilitated by intraoperative fluorescein guidance. J. Neurosurg. 2017, 127, 111–122. [Google Scholar]
- Thon, N.; Tonn, J.C.; Kreth, F.W. The surgical perspective in precision treatment of diffuse gliomas. Onco. Targets Ther. 2019, 12, 1497–1508. [Google Scholar] [CrossRef]
- Williams, N.L.; Rotondo, R.L.; Bradley, J.A. Late Effects After Radiotherapy for Childhood Low-grade Glioma. Am. J. Clin. Oncol. 2018, 41, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Fallai, C.; Olmi, P. Hyperfractionated and accelerated radiation therapy in central nervous system tumors (malignant gliomas, pediatric tumors, and brain metastases). Radiother. Oncol. 1997, 43, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Geyer, J.R.; Finlay, J.L.; Boyett, J.M. Survival of infants with malignant astrocytomas. A report from the Childrens Cancer Group. Cancer 1995, 75, 1045–1050. [Google Scholar] [PubMed]
- Desouky, O. Targeted and non-targeted effects of ionizing radiation. J. Radiat. Res. Appl. Sci. 2015, 8, 247–254. [Google Scholar] [CrossRef]
- Guruangan, S.; Dunkel, I.J.; Goldman, S. Myeloablative chemotherapy with autologous bone marrow rescue in young children with recurrent malignant brain tumors. J. Clin. Oncol. 1998, 16, 2486–2493. [Google Scholar]
- Finlay, J.L.; Dhall, G.; Boyett, J.M. Myeloablative chemotherapy with autologous bone marrow rescue in children and adolescents with recurrent malignant astrocytoma: Outcome compared with conventional chemotherapy: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2008, 51, 806–811. [Google Scholar]
- Omidi, Y.; Kianinejad, N.; Kwon, Y.; Omidian, H. Drug delivery and targeting to brain tumors: Considerations for crossing the blood-brain barrier. Expert Rev. Clin. Pharmacol. 2021, 14, 357–381. [Google Scholar]
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K.; et al. 2018 Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol. 2020, 20, 184–191. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar]
- Wang, D.; Wang, C.; Wang, L.; Chen, Y. Comprehensive review in improving delivery of small-molecule chemotherapeutic agents overcoming the blood–brain/brain tumor barriers for glioblastoma treatment. Drug Deliv. 2019, 26, 551–565. [Google Scholar]
- Agarwal, S.; Sane, R.; Oberoi, R.; Ohlfest, J.R.; Elmquist, W.F. Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev. Mol. Med. 2011, 13, 17. [Google Scholar]
- Schipmann, S.; Müther, M.; Stögbauer, L. Combination of ALA-induced fluorescence-guided resection and intraoperative open photodynamic therapy for recurrent glioblastoma: Case series on a promising dual strategy for local tumor control. J. Neurosurg. 2020, 24, 426–436. [Google Scholar] [CrossRef]
- Whelan, H.T. Photodynamic Therapy (PDT) for Recurrent Pediatric Brain Tumors; Clinical Trial Report; U.S. National Laboratory of Medicine: Bethesda, MD, USA, 2019. [Google Scholar]
- Wickremasinghe, A.C.; Kuzniewicz, M.W.; Grimes, B.A.; Mc Culloch, C.E.; Newman, T.B. Neonatal Phototherapy and Infantile Cancer. Pediatrics 2016, 137, 20151353. [Google Scholar] [CrossRef]
- Cramer, S.W.; Chen, C.C. Photodynamic Therapy for the Treatment of Glioblastoma. Front. Surg. 2020, 6, 81. [Google Scholar] [CrossRef]
- Kaneko, S.; Fujimoto, S.; Yamaguchi, H.; Yamauchi, T.; Yoshimoto, T.; Tokuda, K. Photodynamic therapy of malignant gliomas. Prog. Neurol. Surg. 2018, 32, 1–13. [Google Scholar]
- Vermandel, M.; Dupont, C.; Lecomte, F.; Leroy, H.-A.; Tuleasca, C.; Mordon, S.; Hadjipanayis, C.G.; Reyns, N. Standardized intraoperative 5-ALA photodynamic therapy for newly diagnosed glioblastoma patients: A preliminary analysis of the INDYGO clinical trial. J. Neuro.-Oncol. 2021, 152, 501–514. [Google Scholar]
- Leroy, H.-A.; Baert, G.; Guerin, L.; Delhem, N.; Mordon, S.; Reyns, N.; Vignion-Dewalle, A.-S. Interstitial photodynamic therapy for glioblastomas: A standardized procedure for clinical use. Cancers 2021, 13, 5754. [Google Scholar]
- Muragaki, Y.; Akimoto, J.; Maruyama, T.; Iseki, H.; Ikuta, S.; Nitta, M.; Maebayashi, K.; Saito, T.; Okada, Y.; Kaneko, S.; et al. Phase II clinical study on intraoperative photodynamic therapy with Talaporfin sodium and semiconductor laser in patients with malignant brain tumors: Clinical article. J. Neurosurg. 2013, 119, 845–852. [Google Scholar] [CrossRef]
- Hadjipanayis, C.G.; Stummer, W. 5-ALA and FDA approval for glioma surgery. J. Neurooncol. 2019, 141, 479–486. [Google Scholar]
- Vasilev, A.; Sofi, R.; Rahman, R.; Smith, S.J.; Teschemacher, A.G.; Kasparov, S. Using Light for Therapy of Glioblastoma Multiforme (GBM). Brain Sci. 2020, 10, 75. [Google Scholar] [CrossRef]
- Hirschberg, H. Disruption of the blood–brain barrier following ALA-mediated photodynamic therapy. Lasers Surg. Med. 2008, 40, 535–542. [Google Scholar] [PubMed]
- Madsen, S.J. Site-specific opening of the blood-brain barrier. J. Biophoton. 2010, 3, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.J. Increased nanoparticle-loaded exogenous macrophage migration into the brain following PDT-induced blood-brain barrier disruption. Lasers Surg. Med. 2013, 45, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.J. Nanoparticle-loaded macrophage mediated photothermal therapy: Potential for glioma treatment. Lasers Med. Sci. 2015, 4, 1357–1365. [Google Scholar]
- Zhang, C.; Feng, W.; Li, Y.; Kürths, J.; Yu, T.; Semyachkina-Glushkovskaya, O.; Zhu, D. Age differences in photodynamic opening of blood-brain barrier through optical clearing skull window in mice. Lasers Surg. Med. 2019, 51, 625–633. [Google Scholar]
- Zhang, C.; Feng, W.; Vodovosova, E.; Tretiakova, D.; Boldyrev, I.; Li, Y.; Kurths, J.; Yu, T.; Semyachkina-Glushkovskaya, O.; Zhu, D. Photodynamic opening of the blood-brain barrier to high weight molecules and liposomes through an optical clearing skull window. Biomed. Opt. Express 2018, 9, 4850–4862. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Kurths, J.; Borisova, E.; Sokolovsky, S.; Mantareva, N.; Angelov, I.; Shirokov, A.; Navolokin, N.; Shushunova, N.; Khorovodov, A.; et al. Photodynamic opening of blood-brain barrier. Biomed. Opt. Express 2017, 8, 5040–5048. [Google Scholar]
- Semyachkina-Glushkovskaya, O.; Chehonin, V.; Borisova, E.; Fedosov, I.; Namykin, A.; Abdurashitov, A.; Shirokov, A.; Khlebtsov, B.; Lyubun, Y.; Navolokin, N.; et al. Photodynamic opening of the blood-brain barrier and pathways of brain clearing pathways. J. Biophotonics 2018, 11, 201700287. [Google Scholar]
- Feng, W.; Zhang, C.; Yu, T.; Semyachkina-Glushkovskaya, O.; Zhu, D. In vivo monitoring blood-brain barrier permeability using spectral imaging through optical clearing skull window. J. Biophotonics 2019, 12, 201800330. [Google Scholar]
- Semyachkina-Glushkovskaya, O.; Terskov, A.; Khorovodov, A.; Telnova, V.; Blokhina, I.; Saranceva, E.; Kurths, J. Photodynamic Opening of the Blood–Brain Barrier and the Meningeal Lymphatic System: The New Niche in Immunotherapy for Brain Tumors. Pharmaceutics 2022, 14, 2612. [Google Scholar] [CrossRef]
- Hofmann, G.A.; Weber, B. Drug-induced photosensitivity: Culprit drugs, potential mechanisms and clinical consequences. J. Ger. Soc. Dermatol. 2021, 19, 19–29. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, Z.; Zhao, Z.; Tang, B.Z. Type I AIE photosensitizers: Mechanism and application. View 2023, 3, 20200121. [Google Scholar] [CrossRef]
- Kowalska, J.; Rok, J.; Rzepka, Z.; Wrześniok, D. Drug-Induced Photosensitivity—From Light and Chemistry to Biological Reactions and Clinical Symptoms. Pharmaceuticals 2021, 14, 723. [Google Scholar] [CrossRef]
- Lozzi, F.; Di Raimondo, C.; Lanna, C.; Diluvio, L.; Mazzilli, S.; Garofalo, V.; Dika, E.; Dellambra, E.; Coniglione, F.; Bianchi, L.; et al. Latest Evidence Regarding the Effects of Photosensitive Drugs on the Skin: Pathogenetic Mechanisms and Clinical Manifestations. Pharmaceutics 2020, 12, 1104. [Google Scholar]
- Sokolovski, S.; Edik, R.; Abramov, A.; Angelova, P. Singlet oxygen stimulates mitochondrial bioenergetics in brain cells. Free Radic. Biol. Med. 2021, 163, 306–313. [Google Scholar]
- Kim, M.M.; Darafsheh, A. Light sources and dosimetry techniques for photodynamic therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar]
- Salehpour, F.; Cassano, P.; Rouhi, N.; Hamblin, M.R.; De Taboada, L.; Farajdokht, F.; Mahmoudi, J. Penetration profiles of visible and near-infrared lasers and light-emitting diode light through the head tissues in animal and human species: A review of literature. Photobiomodul. Photomed. Laser Surg. 2019, 37, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Genina, E.A.; Bashkatov, A.N.; Tuchina, D.K.; Dyachenko-Timoshina, P.A.; Navolokin, N.; Shirokov, A.; Khorovodov, A.; Terskov, A.; Klimova, M.; Mamedova, A.; et al. Optical properties of brain tissues at the different stages of glioma development in rats: Pilot study. Biomed. Opt. Express 2019, 10, 5182–5197. [Google Scholar] [CrossRef]
- Sokolovski, S.G.; Zolotovskaya, S.A.; Goltsov, A.; Pourreyron, C.; South, A.P.; Rafailov, E.U. Infrared laser pulse triggers in-creased singlet oxygen production in tumour cells. Sci. Rep. 2013, 3, 3484. [Google Scholar]
- Wang, H.L.; Lai, T. Optimization of Evans blue quantitation in limited rat tissue samples. Sci. Rep. 2014, 4, 6588. [Google Scholar]
- Qi, Y.; Yu, T.; Xu, J.; Wan, P.; Ma, Y.; Zhu, J.; Li, Y.; Gong, H.; Luo, Q.; Dan Zhu, D. FDISCO: Advanced solvent-based clearing method for imaging whole organs. Sci. Adv. 2019, 5, 8355. [Google Scholar]
- Bragin, D.; Kameneva, M.; Bragina, O.; Thomson, S.; Statom, G.; Lara, D.; Yang, Y.; Nemoto, E. 2017 Rheological effects of drug-reducing polymers improve cerebral blood flow and oxygenation after traumatic brain injury in rats. J. Cereb. Blood Flow Metab. 2017, 37, 762–775. [Google Scholar] [PubMed]
- Patlak, S.C.; Blasberg, R.G.; Fenstermacher, J.D. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J. Cereb. Blood Flow Metab. 1983, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xue, Q.; Tang, Q.; Hou, M.; Qi, H.; Chen, G.; Chen, W.; Zhang, J.; Chen, Y.; Xu, X. A simple method for isolating and culturing the rat brain microvascular endothelial cells. Microvasc. Res. 2013, 90, 199–205. [Google Scholar]
- Khilazheva, E.D.; Boytsova, E.B.; Pozhilenkova, E.A.; Solonchuk, Y.R.; Salmina, A.B. Obtaining a three-cell model of a neurovascular unit in vitro. Cell Tissue Biol. 2015, 9, 447–451. [Google Scholar]
- Semyachkina-Glushkovskaya, O.; Shirokov, A.; Blokhina, I.; Telnova, V.; Vodovozova, E.; Alekseeva, A.; Boldyrev, I.; Fedosov, I.; Dubrovsky, A.; Khorovodov, A.; et al. Intranasal Delivery of Liposomes to Glioblastoma by Photostimulation of the Lymphatic System. Pharmaceutics 2023, 15, 36. [Google Scholar]
- Semyachkina-Glushkovskaya, O.; Diduk, S.; Elina, A.; Artem, K.; Khorovodov, A.; Shirokov, A.; Fedosov, I.; Dubrovsky, A.; Blokhina, I.; Terskov, A.; et al. Music improves the therapeutic effects of bevacizumab in rats with glioblastoma: Modulation of drug distribution to the brain. Front. Oncol. 2022, 12, 1010188. [Google Scholar]
- Tretiakova, D.S.; Alekseeva, A.S.; Galimzyanov, T.R.; Boldyrev, A.M.; Chernyadyev, A.Y.; Ermakov, Y.A.; Batishchev, O.V.; Vodovozova, E.L.; Boldyrev, I.A. Lateral stress profile and fluorescent lipid probes. FRET pair of probes that introduces minimal distortions into lipid packing. Biochim. Biophys. Acta Biomembr. 2018, 1860, 2337–2347. [Google Scholar]
- Wang, Q.; Zou, M.H. Measurement of reactive oxygen species (ROS) and mitochondrial ROS in AMPK knockout mice blood vessels. Methods Mol. Biol. 2018, 1732, 507–517. [Google Scholar] [PubMed]
- Devos, S.L.; Miller, T.M. Direct intraventricular delivery of drugs to the rodent central nervous system. J. Vis. Exp. 2013, 12, 50326. [Google Scholar]
- Bergersen, L.; Rafiki, A.; Ottersen, O.P. Immunogold cytochemistry identifies specialized membrane domains for monocarboxylate transport in the central nervous system. Neurochem. Res. 2002, 27, 89–96. [Google Scholar] [CrossRef]
- Pierre, K.; Pellerin, L.; Debernardi, R.; Riederer, B.M.; Magistretti, P.J. Cell-specific localization of monocarboxylate transporters, MCT1 and MCT2, in the adult mouse brain revealed by double immunohistochemical labeling and confocal microscopy. Neuroscience 2000, 100, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Mitusova, K.; Peltek, O.O.; Karpov, T.E.; Muslimov, A.R.; Zyuzin, M.V.; Timin, A.S. Overcoming the blood–brain barrier for the therapy of malignant brain tumor: Current status and prospects of drug delivery approaches. J Nanobiotechnol. 2022, 20, 412. [Google Scholar] [CrossRef] [PubMed]
- Dubois, L.G.; Campanat, L.; Righy, C.; D’Andrea-Meira, I.; Leite de Sampaio e Spohr, T.C.; Porto-Carreiro, I.; Pereira, C.M.; Balça-Silva, J.; Assad Kahn, S.; DosSantos, M.F.; et al. Gliomas and the vascular fragility of the blood brain barrier. Front. Cell Neurosci. 2014, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Karmur, B.S.; Philteos, J.; Abbasian, A.; Zacharia, B.E.; Lipsman, N.; Levin, V.; Grossman, S.; Mansouri, A. Blood-Brain Barrier Disruption in Neuro-Oncology: Strategies, Failures, and Challenges to Overcome. Front. Oncol. 2020, 10, 563840. [Google Scholar] [CrossRef]
- Pafundi, D.H.; Laack, N.N.; Youland, R.S.; Parney, I.F.; Lowe, V.J.; Giannini, C.; Kemp, B.J.; Grams, M.P.; Morris, J.M.; Hoover, J.M.; et al. Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: Results of a prospective pilot study. Neuro Oncol. 2013, 15, 1058–1067. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Fedosov, I.; Shirokov, A.; Vodovozov, E.; Alekseev, A.; Khorovodov, A.; Blokhina, I.; Terskov, A.; Mamedova, A.; Klimova, M.; et al. Photomodulation of lymphatic delivery of liposomes to the brain bypassing the blood-brain barrier: New perspectives for glioma therapy. Nanophoton 2021, 10, 3215–3227. [Google Scholar] [CrossRef]
- Ortega-Berlanga, B.; Gonzalez, C.; Navarro-Tovar, G. Recent advances in the use of lipid-based nanoparticles against glioblastoma multiforme. Arch. Immunol. Ther. Exp. 2021, 69, 8. [Google Scholar] [CrossRef]
- Li, J.; Zeng, H.; You, Y. Active targeting of orthotopic glioma using biomimetic liposomes co-loaded elemene and cabazitaxel modified by transferritin. J. Nanobiotechnol. 2021, 19, 289. [Google Scholar] [CrossRef]
- Juhairiyah, F.; de Lange, E.C.M. Understanding drug delivery to the brain using liposome-based strategies: Studies that provide mechanistic insights are essential. AAPS J. 2021, 23, 114. [Google Scholar] [CrossRef]
- Shetty, A.K.; Zanirati, G. The Interstitial System of the Brain in Health and Disease. Aging Dis. 2020, 11, 200–211. [Google Scholar] [PubMed]
- Ma, Q.; Schlegel, F.; Bachmann, S.B.; Schneider, H.; Decker, Y.; Rudin, M.; Weller, M.; Proulx, S.T.; Detmar, M. Lymphatic outflow of cerebrospinal fluid is reduced in glioma. Sci. Rep. 2019, 9, 14815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hablitz, L.M.; Vinitsky, H.; Sun, Q.; Stæger, F.F.; Sigurdsson, B.; Mortensen, K.N.; Lilius, T.O.; Nedergaard, M. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv. 2019, 5, 5447. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R. Photobiomodulation for traumatic brain injury and stroke. J. Neurosci. Res. 2018, 96, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, F.; Mahmoudi, J.; Kamari, F.; Sadigh-Eteghad, S.; Rasta, S.H.; Hamblin, M.R. Brain photobiomodulation therapy: A narrative review. Mol. Neurobiol. 2018, 55, 6601–6636. [Google Scholar] [CrossRef] [PubMed]
- Salehpour, F.; Khademi, M.; Bragin, D.E.; DiDuro, J.O. Photobiomodulation Therapy and the Glymphatic System: Promising Applications for Augmenting the Brain Lymphatic Drainage System. Int. J. Mol. Sci. 2022, 23, 2975. [Google Scholar] [CrossRef]
- Koepsell, H. Glucose transporters in brain in health and disease. Eur. J. Physiol. 2020, 472, 1299–1343. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.R.; Kovacs, J.J.; Whalen, E.J.; Rajagopal, S.; Strachan, R.T.; Grant, W.; Towers, A.J.; Williams, B.; Lam, C.M.; Xiao, K.; et al. A stress response pathway regulates DNA damage through β2-adrenoreceptors and β-arrestin-1. Nature 2011, 477, 349–353. [Google Scholar] [CrossRef]
- Hebda, J.K.; Leclair, H.M.; Azzi, S.; Roussel, C.; Scott, M.G.; Bidère, N.; Gavard, J. The C-terminus region of β-arrestin1 modulates VE-cadherin expression and endothelial cell permeability. Cell Commun. Signal 2013, 11, 37. [Google Scholar] [CrossRef]
- Hu, S.S.; Cheng, H.B.; Zheng, Y.R.; Zhang, R.Y.; Yue, W.; Zhang, H. Effects of photodynamic therapy on the ultrastructure of glioma cells. Biomed. Environ. Sci. 2007, 20, 269–273. [Google Scholar]
- Buzza, H.; de Fraitas, L.C.F.; Moriayama, L.T.; Rosa, R.; Bagnato, F.; Kurachi, C. Vascular effects of photodynamic therapy with circumin in a chlorioallantoic membrane model. Int. J. Mol. Sci. 2019, 20, 1084. [Google Scholar] [CrossRef] [PubMed]
- Dharmajaya, R.; Sari, D.K. Malondialdehyde value as radical oxidative marker and endogenous antioxidant value analysis in brain tumor. Ann. Med. Surg. 2022, 77, 103231. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Karavaev, A.; Prokhorov, M.; Runnova, A.E.; Borovkova, E.I.; Hramkov, A.N.; Kulminskiy, D.D.; Semenova, N.I.; Sergeev, K.S.; Slepnev, A.V.; et al. EEG biomarkers of activation of the lymphatic drainage system of the brain during sleep and opening of the blood-brain barrier. Comput. Struct. Biotechnol. J. 2023, 21, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Esmat, A.; Bragin, D.; Bragina, O.; Shirokov, A.A.; Navolokin, N.; Yang, Y.; Abdurashitov, A.; Khorovodov, A.; Terskov, A. Phenomenon of music-induced opening of the blood-brain barrier in healthy mice. Proc. R Soc. B 2020, 287, 20202337. [Google Scholar] [CrossRef]
- Batuk, P.; Fuxe, J.; Hashizume, H.; Romano, T.; Lashnits, E.; Butz, S.; Vestweber, D.; Corada, M.; Molendinin, C.; Dejana, E. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 2004, 204, 2349–2362. [Google Scholar]
- Kesler, C.; Kiao, S.; Munn, L.; Padera, T. Lymphatic vessels in health and diseases. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 111–124. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Abdurashitov, A.; Klimova, M.; Dubrovsky, A.; Shirokov, A.; Fomin, A.; Terskov, A.; Agranovich, I.; Mamedova, M.; Khorovodov, A.; et al. Photostimulation of cerebral and peripheral lymphatic functions. Translat. Biophot. 2020, 2, 201900036. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Borisova, E.; Mantareva, V.; Angelov, I.; Eneva, I.; Terskov, A.; Mamedova, A.; Shirokov, A.; Khorovodov, A.; Klimova, M.; et al. Photodynamic Opening of the Blood–Brain Barrier Using Different Photosensitizers in Mice. Appl. Sci. 2020, 10, 33. [Google Scholar] [CrossRef]
- Karu, T.I.; Pyatibrat, L.V.; Afanasyeva, N.I. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg. Med. 2005, 36, 307–314. [Google Scholar] [CrossRef]
- Li, G.Y.; Liu, S.J.; Yu, T.T.; Liu, Z.; Sun, S.L.; Bragin, D.; Navolokin, N.; Kurths, J.; Glushkovskaya-Semyachkina, O.; Zhu, D. Photostimulation of lymphatic clearance of red blood cells from the mouse brain after intraventricular hemorrhage. bioRxiv 2020. [Google Scholar]
- Stanley, C.P.; Maghzal, G.J.; Ayer, A.; Talib, J.; Giltrap, A.M.; Shengule, S.; Wolhuter, K.; Wang, Y.; Chadha, P.; Suarna, C.; et al. Singlet molecular oxygen regulates vascular tone and blood pressure in inflammation. Nature 2019, 566, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Liu, L.; Lu, Y.; Zhang, Y.; He, X.; Chen, X.; Zhang, Y.; Chen, Q.; Guo, Q.; Sun, T.; et al. Substance P-modified human serum albumin nanoparticles loaded with paclitaxel for targeted therapy of glioma. Acta Pharm. Sin. B 2018, 8, 85–96. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semyachkina-Glushkovskaya, O.; Bragin, D.; Bragina, O.; Socolovski, S.; Shirokov, A.; Fedosov, I.; Ageev, V.; Blokhina, I.; Dubrovsky, A.; Telnova, V.; et al. Low-Level Laser Treatment Induces the Blood-Brain Barrier Opening and the Brain Drainage System Activation: Delivery of Liposomes into Mouse Glioblastoma. Pharmaceutics 2023, 15, 567. https://doi.org/10.3390/pharmaceutics15020567
Semyachkina-Glushkovskaya O, Bragin D, Bragina O, Socolovski S, Shirokov A, Fedosov I, Ageev V, Blokhina I, Dubrovsky A, Telnova V, et al. Low-Level Laser Treatment Induces the Blood-Brain Barrier Opening and the Brain Drainage System Activation: Delivery of Liposomes into Mouse Glioblastoma. Pharmaceutics. 2023; 15(2):567. https://doi.org/10.3390/pharmaceutics15020567
Chicago/Turabian StyleSemyachkina-Glushkovskaya, Oxana, Denis Bragin, Olga Bragina, Sergey Socolovski, Alexander Shirokov, Ivan Fedosov, Vasily Ageev, Inna Blokhina, Alexander Dubrovsky, Valeria Telnova, and et al. 2023. "Low-Level Laser Treatment Induces the Blood-Brain Barrier Opening and the Brain Drainage System Activation: Delivery of Liposomes into Mouse Glioblastoma" Pharmaceutics 15, no. 2: 567. https://doi.org/10.3390/pharmaceutics15020567
APA StyleSemyachkina-Glushkovskaya, O., Bragin, D., Bragina, O., Socolovski, S., Shirokov, A., Fedosov, I., Ageev, V., Blokhina, I., Dubrovsky, A., Telnova, V., Terskov, A., Khorovodov, A., Elovenko, D., Evsukova, A., Zhoy, M., Agranovich, I., Vodovozova, E., Alekseeva, A., Kurths, J., & Rafailov, E. (2023). Low-Level Laser Treatment Induces the Blood-Brain Barrier Opening and the Brain Drainage System Activation: Delivery of Liposomes into Mouse Glioblastoma. Pharmaceutics, 15(2), 567. https://doi.org/10.3390/pharmaceutics15020567