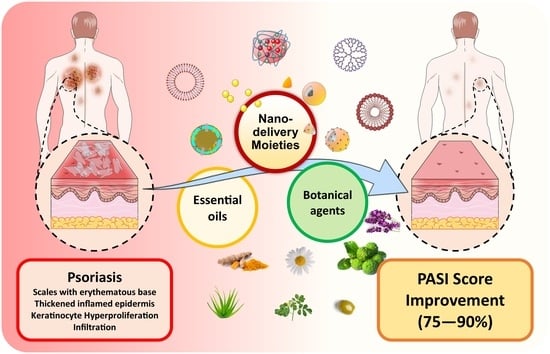
Can Essential Oils/Botanical Agents Smart-Nanoformulations Be the Winning Cards against Psoriasis?
Abstract
:1. Introduction
2. Pathogenesis of Psoriasis
3. Phenotypes of Psoriasis
4. Therapeutic Approaches
4.1. Topical Therapy
4.2. UV Therapy
4.3. Systemic Treatments
4.4. Biologics
4.5. Botanical Treatment
Limitations of Botanical Treatment
5. Nano-Based Topical Drug Delivery Systems
6. Essential Oils/Botanical Agents-Based Advanced Nano-Delivery Systems with a High Potential for the Topical Management of Psoriasis
6.1. Nigella sativa (Black Cumin) (NS)
6.2. Bergamot
6.3. Capsaicin
6.4. Cassia tora
6.5. Aloe Vera
6.6. Avocado Oil
6.7. Chamomile
6.8. Coconut Oil
6.9. Curcumin
6.10. Fish Oil
6.11. Lavender Oil
6.12. Olive Oil
6.13. Thymol
6.14. Tea Tree Oil
6.15. Eucalyptus Oil
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bergboer, J.G.M.; Zeeuwen, P.L.J.M.; Schalkwijk, J. Genetics of Psoriasis: Evidence for Epistatic Interaction between Skin Barrier Abnormalities and Immune Deviation. J. Investig. Dermatol. 2012, 132, 2320–2331. [Google Scholar] [CrossRef] [Green Version]
- Milavec-Puretić, V.; Mance, M.; Ceović, R.; Lipozenčić, J. Drug Induced Psoriasis. Acta Dermatovenerol. Croat. ADC 2011, 19, 39–42. [Google Scholar]
- De Oliveira, M.F.S.P.; de Rocha, B.O.; Duarte, G.V. Psoriasis: Classical and Emerging Comorbidities. An. Bras. Dermatol. 2015, 90, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Parisi, R.; Symmons, D.P.M.; Griffiths, C.E.M.; Ashcroft, D.M. Global Epidemiology of Psoriasis: A Systematic Review of Incidence and Prevalence. J. Investig. Dermatol. 2013, 133, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Springate, D.A.; Parisi, R.; Kontopantelis, E.; Reeves, D.; Griffiths, C.E.M.; Ashcroft, D.M. Incidence, Prevalence and Mortality of Patients with Psoriasis: A U.K. Population-Based Cohort Study. Br. J. Dermatol. 2017, 176, 650–658. [Google Scholar] [CrossRef]
- Bronckers, I.M.G.J.; Paller, A.S.; van Geel, M.J.; van de Kerkhof, P.C.M.; Seyger, M.M.B. Psoriasis in Children and Adolescents: Diagnosis, Management and Comorbidities. Paediatr. Drugs 2015, 17, 373–384. [Google Scholar] [CrossRef] [Green Version]
- Fereig, S.; Mamdouh, G.; Arafa, M.; Abdel-Mottaleb, M. Self-Assembled Tacrolimus-Loaded Lecithin-Chitosan Hybrid Nanoparticles for In Vivo Management of Psoriasis. Int. J. Pharm. 2021, 608, 121114. [Google Scholar] [CrossRef]
- Saleem, S.; Iqubal, M.K.; Garg, S.; Ali, J.; Baboota, S. Trends in Nanotechnology-Based Delivery Systems for Dermal Targeting of Drugs: An Enticing Approach to Offset Psoriasis. Expert Opin. Drug Deliv. 2020, 17, 817–838. [Google Scholar] [CrossRef]
- Lowes, M.; Krueger, J.; Lowes, M.A.; Bowcock, A.M.; Krueger, J.G. Pathogenesis and Therapy of Psoriasis. Nature 2007, 445, 866–873. [Google Scholar] [CrossRef]
- Aggarwal, S.; Ghilardi, N.; Xie, M.-H.; de Sauvage, F.J.; Gurney, A.L. Interleukin-23 Promotes a Distinct CD4 T Cell Activation State Characterized by the Production of Interleukin-17. J. Biol. Chem. 2003, 278, 1910–1914. [Google Scholar] [CrossRef] [Green Version]
- Hijnen, D.; Knol, E.F.; Gent, Y.Y.; Giovannone, B.; Beijn, S.J.P.; Kupper, T.S.; Bruijnzeel-Koomen, C.A.F.M.; Clark, R.A. CD8(+) T Cells in the Lesional Skin of Atopic Dermatitis and Psoriasis Patients Are an Important Source of IFN-γ, IL-13, IL-17, and IL-22. J. Investig. Dermatol. 2013, 133, 973–979. [Google Scholar] [CrossRef] [Green Version]
- Albanesi, C.; Scarponi, C.; Cavani, A.; Federici, M.; Nasorri, F.; Girolomoni, G. Interleukin-17 Is Produced by Both Th1 and Th2 Lymphocytes, and Modulates Interferon-γ- and Interleukin-4-Induced Activation of Human Keratinocytes. J. Investig. Dermatol. 2000, 115, 81–87. [Google Scholar] [CrossRef]
- Cai, Y.; Fleming, C.; Yan, J. New Insights of T Cells in the Pathogenesis of Psoriasis. Cell. Mol. Immunol. 2012, 9, 302–309. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, C.E.; Barker, J.N. Pathogenesis and Clinical Features of Psoriasis. Lancet Lond. Engl. 2007, 370, 263–271. [Google Scholar] [CrossRef]
- Goodfield, M.; Hull, S.M.; Holland, D.; Roberts, G.; Wood, E.; Reid, S.; Cunliffe, W. Investigations of the “active” Edge of Plaque Psoriasis: Vascular Proliferation Precedes Changes in Epidermal Keratin. Br. J. Dermatol. 1994, 131, 808–813. [Google Scholar] [CrossRef]
- Baran, R. The Burden of Nail Psoriasis: An Introduction. Dermatology 2010, 221 (Suppl. S1), 1–5. [Google Scholar] [CrossRef]
- Jiaravuthisan, M.M.; Sasseville, D.; Vender, R.B.; Murphy, F.; Muhn, C.Y. Psoriasis of the Nail: Anatomy, Pathology, Clinical Presentation, and a Review of the Literature on Therapy. J. Am. Acad. Dermatol. 2007, 57, 1–27. [Google Scholar] [CrossRef]
- Stern, R.S. Psoriasis. Lancet Lond. Engl. 1997, 350, 349–353. [Google Scholar] [CrossRef]
- Ogdie, A.; Weiss, P. The Epidemiology Psoriatic Arthritis. Rheum. Dis. Clin. N. Am. 2015, 41, 545–568. [Google Scholar] [CrossRef] [Green Version]
- FitzGerald, O.; Haroon, M.; Giles, J.T.; Winchester, R. Concepts of Pathogenesis in Psoriatic Arthritis: Genotype Determines Clinical Phenotype. Arthritis Res. Ther. 2015, 17, 115. [Google Scholar] [CrossRef] [Green Version]
- Martínez-García, E.; Arias-Santiago, S.; Valenzuela-Salas, I.; Garrido-Colmenero, C.; García-Mellado, V.; Buendía-Eisman, A. Quality of Life in Persons Living with Psoriasis Patients. J. Am. Acad. Dermatol. 2014, 71, 302–307. [Google Scholar] [CrossRef]
- Hébert, H.L.; Ali, F.R.; Bowes, J.; Griffiths, C.E.M.; Barton, A.; Warren, R.B. Genetic Susceptibility to Psoriasis and Psoriatic Arthritis: Implications for Therapy. Br. J. Dermatol. 2012, 166, 474–482. [Google Scholar] [CrossRef]
- Menter, A.; Griffiths, C.E. Current and Future Management of Psoriasis. Lancet Lond. Engl. 2007, 370, 272–284. [Google Scholar] [CrossRef]
- Mason, J.; Mason, A.R.; Cork, M.J. Topical Preparations for the Treatment of Psoriasis: A Systematic Review. Br. J. Dermatol. 2002, 146, 351–364. [Google Scholar] [CrossRef]
- Morita, A. Current Developments in Phototherapy for Psoriasis. J. Dermatol. 2018, 45, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Wong, T.; Hsu, L.; Liao, W. Phototherapy in Psoriasis: A Review of Mechanisms of Action. J. Cutan. Med. Surg. 2013, 17, 6–12. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, M.X. A Clinical Review of Phototherapy for Psoriasis. Lasers Med. Sci. 2018, 33, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Valkova, S. UVB Phototherapeutic Modalities. Comparison of Two Treatments for Chronic Plaque Psoriasis. Acta Dermatovenerol. Alp. Pannonica Adriat. 2007, 16, 26–30. [Google Scholar]
- Bökkerink, J.P.; De Abreu, R.A.; Bakker, M.A.; Hulscher, T.W.; van Baal, J.M.; Schretlen, E.D.; De Bruijn, C.H. Effects of Methotrexate on Purine and Pyrimidine Metabolism and Cell-Kinetic Parameters in Human Malignant Lymphoblasts of Different Lineages. Biochem. Pharmacol. 1988, 37, 2329–2338. [Google Scholar] [CrossRef]
- Markham, T.; Watson, A.; Rogers, S. Adverse Effects with Long-Term Cyclosporin for Severe Psoriasis. Clin. Exp. Dermatol. 2002, 27, 111–114. [Google Scholar] [CrossRef]
- Yamauchi, P.S.; Rizk, D.; Kormeili, T.; Patnaik, R.; Lowe, N.J. Current Systemic Therapies for Psoriasis: Where Are We Now? J. Am. Acad. Dermatol. 2003, 49, 66–77. [Google Scholar] [CrossRef]
- Hsu, L.; Armstrong, A.W. Anti-Drug Antibodies in Psoriasis: A Critical Evaluation of Clinical Significance and Impact on Treatment Response. Expert Rev. Clin. Immunol. 2013, 9, 949–958. [Google Scholar] [CrossRef]
- Alwawi, E.A.; Krulig, E.; Gordon, K.B. Long-Term Efficacy of Biologics in the Treatment of Psoriasis: What Do We Really Know? Dermatol. Ther. 2009, 22, 431–440. [Google Scholar] [CrossRef]
- Lebwohl, M.G.; Bachelez, H.; Barker, J.; Girolomoni, G.; Kavanaugh, A.; Langley, R.G.; Paul, C.F.; Puig, L.; Reich, K.; van de Kerkhof, P.C.M. Patient Perspectives in the Management of Psoriasis: Results from the Population-Based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J. Am. Acad. Dermatol. 2014, 70, 871–881.e1-30. [Google Scholar] [CrossRef]
- Farahnik, B.; Sharma, D.; Alban, J.; Sivamani, R.K. Topical Botanical Agents for the Treatment of Psoriasis: A Systematic Review. Am. J. Clin. Dermatol. 2017, 18, 451–468. [Google Scholar] [CrossRef]
- Gendrisch, F.; Haarhaus, B.; Krieger, N.; Quirin, K.-W.; Schempp, C.M.; Wölfle, U. The Effect of Herbal Medicinal Products on Psoriasis-Like Keratinocytes. Biomolecules 2021, 11, 371. [Google Scholar] [CrossRef]
- Nguyen, L.T.H. Signaling Pathways and Targets of Natural Products in Psoriasis Treatment. Explor. Med. 2022, 3, 345–367. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Topically Used Herbal Products for the Treatment of Psoriasis—Mechanism of Action, Drug Delivery, Clinical Studies. Planta Med. 2016, 82, 1447–1455. [Google Scholar] [CrossRef] [Green Version]
- Dabholkar, N.; Rapalli, V.K.; Singhvi, G. Potential Herbal Constituents for Psoriasis Treatment as Protective and Effective Therapy. Phytother. Res. 2021, 35, 2429–2444. [Google Scholar] [CrossRef]
- Abuelella, K.E.; Abd-Allah, H.; Soliman, S.M.; Abdel-Mottaleb, M.M.A. Skin Targeting by Chitosan/Hyaluronate Hybrid Nanoparticles for the Management of Irritant Contact Dermatitis: In Vivo Therapeutic Efficiency in Mouse-Ear Dermatitis Model. Int. J. Biol. Macromol. 2023, 232, 123458. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.M.A. Chapter 13—Nanoparticles for Treatment of Atopic Dermatitis. In Nanoscience in Dermatology; Hamblin, M.R., Avci, P., Prow, T.W., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 167–175. ISBN 978-0-12-802926-8. [Google Scholar]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of Nanoparticles as Permeation Enhancers and Targeted Delivery Options for Skin: Advantages and Disadvantages. Drug Des. Devel. Ther. 2020, 14, 3271–3289. [Google Scholar] [CrossRef]
- Qadir, A.; Aqil, M.; Ali, A.; Warsi, M.H.; Mujeeb, M.; Ahmad, F.J.; Ahmad, S.; Beg, S. Nanostructured Lipidic Carriers for Dual Drug Delivery in the Management of Psoriasis: Systematic Optimization, Dermatokinetic and Preclinical Evaluation. J. Drug Deliv. Sci. Technol. 2020, 57, 101775. [Google Scholar] [CrossRef]
- Pradhan, M.; Singh, D.; Singh, M.R. Novel Colloidal Carriers for Psoriasis: Current Issues, Mechanistic Insight and Novel Delivery Approaches. J. Control Release Off. J. Control Release Soc. 2013, 170, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Gungor, S.; Rezigue, M. Nanocarriers Mediated Topical Drug Delivery for Psoriasis Treatment. Curr. Drug Metab. 2017, 18, 454–468. [Google Scholar] [CrossRef]
- Ramanunny, A.K.; Wadhwa, S.; Singh, S.K.; Sharma, D.S.; Khursheed, R.; Awasthi, A. Treatment Strategies Against Psoriasis: Principle, Perspectives and Practices. Curr. Drug Deliv. 2020, 17, 52–73. [Google Scholar] [CrossRef]
- El Zaafarany, G.M.; Awad, G.A.S.; Holayel, S.M.; Mortada, N.D. Role of Edge Activators and Surface Charge in Developing Ultradeformable Vesicles with Enhanced Skin Delivery. Int. J. Pharm. 2010, 397, 164–172. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.; Try, C.; Pellequer, Y.; Lamprecht, A. Nanomedicine Strategies for Targeting Skin Inflammation. Nanomedicine 2014, 9, 1727–1743. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.M.A.; Moulari, B.; Beduneau, A.; Pellequer, Y.; Lamprecht, A. Nanoparticles Enhance Therapeutic Outcome in Inflamed Skin Therapy. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2012, 82, 151–157. [Google Scholar] [CrossRef]
- Yadav, K.; Soni, A.; Singh, D.; Singh, M.R. Polymers in Topical Delivery of Anti-Psoriatic Medications and Other Topical Agents in Overcoming the Barriers of Conventional Treatment Strategies. Prog. Biomater. 2021, 10, 1–17. [Google Scholar] [CrossRef]
- Svenson, S. Dendrimers as Versatile Platform in Drug Delivery Applications. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2009, 71, 445–462. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.M.A.; Lamprecht, A. In Vivo Skin Penetration of Macromolecules in Irritant Contact Dermatitis. Int. J. Pharm. 2016, 515, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Ascenso, A.; Ribeiro, H.M.; Marto, J. Current and Future Therapies for Psoriasis with a Focus on Serotonergic Drugs. Mol. Neurobiol. 2020, 57, 2391–2419. [Google Scholar] [CrossRef] [PubMed]
- Fereig, S.A.; El-Zaafarany, G.M.; Arafa, M.; Abdel-Mottaleb, M.M.A. Tackling the Various Classes of Nano-Therapeutics Employed in Topical Therapy of Psoriasis. Drug Deliv. 2020, 27, 662–680. [Google Scholar] [CrossRef] [PubMed]
- Damiani, G.; Pacifico, A.; Linder, D.M.; Pigatto, P.D.M.; Conic, R.; Grada, A.; Bragazzi, N.L. Nanodermatology-Based Solutions for Psoriasis: State-of-the Art and Future Prospects. Dermatol. Ther. 2019, 32, e13113. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, G.; Hejmady, S.; Rapalli, V.K.; Dubey, S.K.; Dubey, S. Chapter 4—Nanocarriers for Topical Delivery in Psoriasis. In Delivery of Drugs; Shegokar, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 75–96. ISBN 978-0-12-817776-1. [Google Scholar]
- Zhang, M.; Cheng, J.; Hu, J.; Luo, J.; Zhang, Y.; Lu, F.; Kong, H.; Qu, H.; Zhao, Y. Green Phellodendri Chinensis Cortex-Based Carbon Dots for Ameliorating Imiquimod-Induced Psoriasis-like Inflammation in Mice. J. Nanobiotechnol. 2021, 19, 105. [Google Scholar] [CrossRef]
- Ernst, E. Adverse Effects of Herbal Drugs in Dermatology. Br. J. Dermatol. 2000, 143, 923–929. [Google Scholar] [CrossRef]
- Bassim Atta, M. Some Characteristics of Nigella (Nigella sativa L.) Seed Cultivated in Egypt and Its Lipid Profile. Food Chem. 2003, 83, 63–68. [Google Scholar] [CrossRef]
- Babazadeh, B.; Sadeghnia, H.R.; Safarpour Kapurchal, E.; Parsaee, H.; Nasri, S.; Tayarani-Najaran, Z. Protective Effect of Nigella sativa and Thymoquinone on Serum/Glucose Deprivation-Induced DNA Damage in PC12 Cells. Avicenna J. Phytomed. 2012, 2, 125–132. [Google Scholar]
- Sayeed, S.; Imam, S.S.; Najmi, A.K.; Aqil, M.; Akhtar, M. Nonionic Surfactant Based Thymoquinone Loaded Nanoproniosomal Formulation: In Vitro Physicochemical Evaluation and in Vivo Hepatoprotective Efficacy. Drug Dev. Ind. Pharm. 2017, 43, 1413–1420. [Google Scholar] [CrossRef]
- Yimer, E.M.; Tuem, K.B.; Karim, A.; Ur-Rehman, N.; Anwar, F. Nigella sativa L. (Black Cumin): A Promising Natural Remedy for Wide Range of Illnesses. Evid. Based Complement. Alternat. Med. 2019, 2019, e1528635. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.; Imam, S.S.; Afroz Ahmad, M.; Najmi, A.K.; Mujeeb, M.; Aqil, M. Neuroprotective Study of Nigella sativa-Loaded Oral Provesicular Lipid Formulation: In Vitro and Ex Vivo Study. Drug Deliv. 2014, 21, 487–494. [Google Scholar] [CrossRef]
- Okasha, E.F.; Bayomy, N.A.; Abdelaziz, E.Z. Effect of Topical Application of Black Seed Oil on Imiquimod-Induced Psoriasis-like Lesions in the Thin Skin of Adult Male Albino Rats. Anat. Rec. 2018, 301, 166–174. [Google Scholar] [CrossRef] [Green Version]
- Negi, P.; Sharma, I.; Hemrajani, C.; Rathore, C.; Bisht, A.; Raza, K.; Katare, O.P. Thymoquinone-Loaded Lipid Vesicles: A Promising Nanomedicine for Psoriasis. BMC Complement. Altern. Med. 2019, 19, 334. [Google Scholar] [CrossRef] [Green Version]
- Palaniswamy, D.; Nithyanantham, M.; Raghu, P.; Dwarampudi, L. Antipsoriatic Activity and Cytotoxicity of Ethanolic Extract of Nigella sativa Seeds. Pharmacogn. Mag. 2012, 8, 268. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, J.H.; Ibraheem, A.Y.; Al-Hamdi, K.I. Evaluation of Efficacy, Safety and Antioxidant Effect of Nigella sativa in Patients with Psoriasis: A Randomized Clinical Trial. J. Clin. Exp. Investig. 2014, 5, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Khatoon, K.; Ali, A.; Ahmad, F.J.; Hafeez, Z.; Rizvi, M.M.A.; Akhter, S.; Beg, S. Novel Nanoemulsion Gel Containing Triple Natural Bio-Actives Combination of Curcumin, Thymoquinone, and Resveratrol Improves Psoriasis Therapy: In Vitro and In Vivo Studies. Drug Deliv. Transl. Res. 2021, 11, 1245–1260. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.; Katiyar, S.S.; Kushwah, V.; Jain, S. Active Natural Oil-Based Nanoemulsion Containing Tacrolimus for Synergistic Antipsoriatic Efficacy. Nanomedicine 2018, 13, 1985–1998. [Google Scholar] [CrossRef] [PubMed]
- Navarra, M.; Mannucci, C.; Delbò, M.; Calapai, G. Citrus Bergamia Essential Oil: From Basic Research to Clinical Application. Front. Pharmacol. 2015, 6, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarra, M.; Ferlazzo, N.; Cirmi, S.; Trapasso, E.; Bramanti, P.; Lombardo, G.E.; Minciullo, P.L.; Calapai, G.; Gangemi, S. Effects of Bergamot Essential Oil and Its Extractive Fractions on SH-SY5Y Human Neuroblastoma Cell Growth. J. Pharm. Pharmacol. 2015, 67, 1042–1053. [Google Scholar] [CrossRef]
- Risitano, R.; Currò, M.; Cirmi, S.; Ferlazzo, N.; Campiglia, P.; Caccamo, D.; Ientile, R.; Navarra, M. Flavonoid Fraction of Bergamot Juice Reduces LPS-Induced Inflammatory Response through SIRT1-Mediated NF-ΚB Inhibition in THP-1 Monocytes. PLoS ONE 2014, 9, e107431. [Google Scholar] [CrossRef] [Green Version]
- Impellizzeri, D.; Cordaro, M.; Campolo, M.; Gugliandolo, E.; Esposito, E.; Benedetto, F.; Cuzzocrea, S.; Navarra, M. Anti-Inflammatory and Antioxidant Effects of Flavonoid-Rich Fraction of Bergamot Juice (BJe) in a Mouse Model of Intestinal Ischemia/Reperfusion Injury. Front. Pharmacol. 2016, 7, 203. [Google Scholar] [CrossRef] [Green Version]
- Costa, R.; Dugo, P.; Navarra, M.; Raymo, V.; Dugo, G.; Mondello, L. Study on the Chemical Composition Variability of Some Processed Bergamot (Citrus Bergamia) Essential Oils. Flavour Fragr. J. 2010, 25, 4–12. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Cirmi, S.; Calapai, G.; Ventura-Spagnolo, E.; Gangemi, S.; Navarra, M. Anti-Inflammatory Activity of Citrus Bergamia Derivatives: Where Do We Stand? Molecules 2016, 21, 1273. [Google Scholar] [CrossRef] [Green Version]
- Graziano, A.C.E.; Cardile, V.; Crascì, L.; Caggia, S.; Dugo, P.; Bonina, F.; Panico, A. Protective Effects of an Extract from Citrus Bergamia against Inflammatory Injury in Interferon-γ and Histamine Exposed Human Keratinocytes. Life Sci. 2012, 90, 968–974. [Google Scholar] [CrossRef]
- Xiao, S.; Yu, H.; Xie, Y.; Guo, Y.; Fan, J.; Yao, W. The Anti-Inflammatory Potential of Cinnamomum Camphora (L.) J. Presl Essential Oil in Vitro and in Vivo. J. Ethnopharmacol. 2021, 267, 113516. [Google Scholar] [CrossRef]
- Mannucci, C.; Navarra, M.; Calapai, F.; Squeri, R.; Gangemi, S.; Calapai, G. Clinical Pharmacology of Citrus Bergamia: A Systematic Review. Phytother. Res. 2017, 31, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Wesołowska, A.; Jadczak, D.; Grzeszczuk, M. Chemical Composition of the Pepper Fruit Extracts of Hot Cultivars Capsicum Annuum L. Acta Sci. Pol. Hortorum Cultus 2011, 10, 171–184. [Google Scholar]
- Jessell, T.M.; Iversen, L.L.; Cuello, A.C. Capsaicin-Induced Depletion of Substance P from Primary Sensory Neurones. Brain Res. 1978, 152, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Farber, E.M.; Nickoloff, B.J.; Recht, B.; Fraki, J.E. Stress, Symmetry, and Psoriasis: Possible Role of Neuropeptides. J. Am. Acad. Dermatol. 1986, 14, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Yu, C. Study on HIF-1α Gene Translation in Psoriatic Epidermis with the Topical Treatment of Capsaicin Ointment. Int. Sch. Res. Not. 2011, 2011, 821874. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, U.; Gupta, M.; Vyas, S.P. Capsaicin Delivery into the Skin with Lipidic Nanoparticles for the Treatment of Psoriasis. Artif. Cells Nanomed. Biotechnol. 2015, 43, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Somagoni, J.; Boakye, C.H.A.; Godugu, C.; Patel, A.R.; Mendonca Faria, H.A.; Zucolotto, V.; Singh, M. Nanomiemgel—A Novel Drug Delivery System for Topical Application—In Vitro and In Vivo Evaluation. PLoS ONE 2014, 9, e115952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, P.R.; Marepally, S.; Patel, A.R.; Voshavar, C.; Chaudhuri, A.; Singh, M. Topical Delivery of Anti-TNFα SiRNA and Capsaicin via Novel Lipid-Polymer Hybrid Nanoparticles Efficiently Inhibits Skin Inflammation in Vivo. J. Control Release Off. J. Control Release Soc. 2013, 170, 51–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.; Gupta, M.; Mangal, S.; Agrawal, U.; Vyas, S.P. Capsaicin-Loaded Vesicular Systems Designed for Enhancing Localized Delivery for Psoriasis Therapy. Artif. Cells Nanomed. Biotechnol. 2016, 44, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Ramankutty, C.; Warrier, P.K.; Nambiar, V.P.K. Indian Medicinal Plants: A Compendium of 500 Species; Orient Black Swan: Hyderabad, India, 1993; ISBN 978-81-7371-702-4. [Google Scholar]
- Singhal, M.; Kansara, N. Cassia Tora Linn Cream Inhibits Ultraviolet-B-Induced Psoriasis in Rats. ISRN Dermatol. 2012, 2012, 346510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibezim, A.; Amara, E.E. In-Silico Study of Flavonoids from Cassia Tora as Potential Anti-Psoriatic Agent. J. Appl. Pharm. Sci. 2019, 9, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Gaikwad, R.; Shinde, A.; Hajare, A. Herbal Treatment for Management of Psoriasis: An Overview. Res. J. Pharm. Technol. 2022, 15, 1385–1392. [Google Scholar] [CrossRef]
- Shelton, R.M. Aloe Vera. Its Chemical and Therapeutic Properties. Int. J. Dermatol. 1991, 30, 679–683. [Google Scholar] [CrossRef]
- Vázquez, B.; Avila, G.; Segura, D.; Escalante, B. Antiinflammatory Activity of Extracts from Aloe Vera Gel. J. Ethnopharmacol. 1996, 55, 69–75. [Google Scholar] [CrossRef]
- Choonhakarn, C.; Busaracome, P.; Sripanidkulchai, B.; Sarakarn, P. A Prospective, Randomized Clinical Trial Comparing Topical Aloe Vera with 0.1% Triamcinolone Acetonide in Mild to Moderate Plaque Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 168–172. [Google Scholar] [CrossRef]
- El-Gammal, A.; Nardo, V.D.; Daaboul, F.; Tchernev, G.; Wollina, U.; Lotti, J.; Lotti, T. Is There a Place for Local Natural Treatment of Psoriasis? Open Access Maced. J. Med. Sci. 2018, 6, 839–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Singhal, P.; Singh, A.; Chauhan, R.; Kumar, B. Nutritional and pharmceutical benifits of avocado plant. J. Adv. Res. 2018, 9, 4–11. [Google Scholar]
- Flores, M.; Saravia, C.; Vergara, C.E.; Avila, F.; Valdés, H.; Ortiz-Viedma, J. Avocado Oil: Characteristics, Properties, and Applications. Molecules 2019, 24, 2172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stücker, M.; Memmel, U.; Hoffmann, M.; Hartung, J.; Altmeyer, P. Vitamin B12 Cream Containing Avocado Oil in the Therapy of Plaque Psoriasis. Dermatology 2001, 203, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.K.; Pandey, M.; Gupta, S. Chamomile, a Novel and Selective COX-2 Inhibitor with Anti-Inflammatory Activity. Life Sci. 2009, 85, 663–669. [Google Scholar] [CrossRef] [Green Version]
- Bhaskaran, N.; Shukla, S.; Srivastava, J.K.; Gupta, S. Chamomile, an Anti-Inflammatory Agent Inhibits Inducible Nitric Oxide Synthase Expression by Blocking RelA/P65 Activity. Int. J. Mol. Med. 2010, 26, 935–940. [Google Scholar]
- Kolahdooz, S.; Karimi, M.; Esmaili, N.; Zargaran, A.; Kordafshari, G.; Mozafari, N.; Ayati, M.H. Evaluation of the Efficacy of a Topical Chamomile-Pumpkin Oleogel for the Treatment of Plaque Psoriasis: An Intra-Patient, Double-Blind, Randomized Clinical Trial. Biomed. Res. Ther. 2018, 5, 2811–2819. [Google Scholar] [CrossRef]
- Aertgeerts, P.; Albring, M.; Klaschka, F.; Nasemann, T.; Patzelt-Wenczler, R.; Rauhut, K.; Weigl, B. Comparative testing of Kamillosan cream and steroidal (0.25% hydrocortisone, 0.75% fluocortin butyl ester) and non-steroidal (5% bufexamac) dermatologic agents in maintenance therapy of eczematous diseases. Z. Hautkr. 1985, 60, 270–277. [Google Scholar]
- Villarino, B.J.; Dy, L.M.; Lizada, M.C.C. Descriptive Sensory Evaluation of Virgin Coconut Oil and Refined, Bleached and Deodorized Coconut Oil. LWT—Food Sci. Technol. 2007, 40, 193–199. [Google Scholar] [CrossRef]
- Varma, S.R.; Sivaprakasam, T.O.; Arumugam, I.; Dilip, N.; Raghuraman, M.; Pavan, K.B.; Rafiq, M.; Paramesh, R. In vitro anti-inflammatory and skin protective properties of Virgin coconut oil. J. Tradit. Complement. Med. 2019, 9, 5–14. [Google Scholar] [CrossRef]
- Evangelista, M.T.P.; Abad-Casintahan, F.; Lopez-Villafuerte, L. The Effect of Topical Virgin Coconut Oil on SCORAD Index, Transepidermal Water Loss, and Skin Capacitance in Mild to Moderate Pediatric Atopic Dermatitis: A Randomized, Double-Blind, Clinical Trial. Int. J. Dermatol. 2014, 53, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Verallo-Rowell, V.M.; Dillague, K.M.; Syah-Tjundawan, B.S. Novel Antibacterial and Emollient Effects of Coconut and Virgin Olive Oils in Adult Atopic Dermatitis. Dermat. Contact Atopic Occup. Drug 2008, 19, 308–315. [Google Scholar] [CrossRef]
- Chainani-Wu, N. Safety and Anti-Inflammatory Activity of Curcumin: A Component of Tumeric (Curcuma longa). J. Altern. Complement. Med. N. Y. N 2003, 9, 161–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Zhao, Y.; Hu, J. Curcumin Inhibits Imiquimod-Induced Psoriasis-Like Inflammation by Inhibiting IL-1beta and IL-6 Production in Mice. PLoS ONE 2013, 8, e67078. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Alam, M.; Imam, F.I.; Siddiqui, M.R. Topical Nanoemulsion of Turmeric Oil for Psoriasis: Characterization, Ex Vivo and in Vivo Assessment. Int. J. Drug Deliv. 2012, 4, 184. [Google Scholar] [CrossRef]
- Varma, S.R.; Sivaprakasam, T.O.; Mishra, A.; Prabhu, S.; Rafiq, M.; Rangesh, P. Imiquimod-Induced Psoriasis-like Inflammation in Differentiated Human Keratinocytes: Its Evaluation Using Curcumin. Eur. J. Pharmacol. 2017, 813, 33–41. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Nourein, I.H.; Ahmad, J. Co-Delivery of Imiquimod and Curcumin by Nanoemugel for Improved Topical Delivery and Reduced Psoriasis-Like Skin Lesions. Biomolecules 2020, 10, 968. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulsion Loaded Polymeric Hydrogel for Topical Delivery of Curcumin in Psoriasis. J. Drug Deliv. Sci. Technol. 2020, 59, 101847. [Google Scholar] [CrossRef]
- Mao, K.-L.; Fan, Z.-L.; Yuan, J.-D.; Chen, P.-P.; Yang, J.-J.; Xu, J.; ZhuGe, D.-L.; Jin, B.-H.; Zhu, Q.-Y.; Shen, B.-X.; et al. Skin-Penetrating Polymeric Nanoparticles Incorporated in Silk Fibroin Hydrogel for Topical Delivery of Curcumin to Improve Its Therapeutic Effect on Psoriasis Mouse Model. Colloids Surf. B Biointerfaces 2017, 160, 704–714. [Google Scholar] [CrossRef]
- Iriventi, P.; Gupta, N.V.; Osmani, R.A.M.; Balamuralidhara, V. Design & Development of Nanosponge Loaded Topical Gel of Curcumin and Caffeine Mixture for Augmented Treatment of Psoriasis. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2020, 28, 489–506. [Google Scholar] [CrossRef]
- Rahman, M.; Beg, S.; Ahmad, M.Z.; Kazmi, I.; Ahmed, A.; Rahman, Z.; Ahmad, F.J.; Akhter, S. Omega-3 Fatty Acids as Pharmacotherapeutics in Psoriasis: Current Status and Scope of Nanomedicine in Its Effective Delivery. Curr. Drug Targets 2013, 14, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.M.; Chiang, N.; Arita, M.; Serhan, C.N. Resolvin E1 and Protectin D1 Activate Inflammation-Resolution Programmes. Nature 2007, 447, 869–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Kumar, A.; Sahni, J.K.; Ali, J.; Baboota, S. Nanoemulsion Based Hydrogel Containing Omega 3 Fatty Acids as a Surrogate of Betamethasone Dipropionate for Topical Delivery. Adv. Sci. Lett. 2012, 6, 221–231. [Google Scholar] [CrossRef]
- Zulfakar, M.H.; Abdelouahab, N.; Heard, C.M. Enhanced Topical Delivery and Ex Vivo Anti-Inflammatory Activity from a Betamethasone Dipropionate Formulation Containing Fish Oil. Inflamm. Res. 2010, 59, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Aluwi, M.F.F.M.; Rullah, K.; Wai, L.K.; Mohd Amin, M.C.I.; Zulfakar, M.H. Probing the Effects of Fish Oil on the Delivery and Inflammation-Inducing Potential of Imiquimod. Int. J. Pharm. 2015, 490, 131–141. [Google Scholar] [CrossRef]
- Huo, M.; Cui, X.; Xue, J.; Chi, G.; Gao, R.; Deng, X.; Guan, S.; Wei, J.; Soromou, L.W.; Feng, H.; et al. Anti-Inflammatory Effects of Linalool in RAW 264.7 Macrophages and Lipopolysaccharide-Induced Lung Injury Model. J. Surg. Res. 2013, 180, e47–e54. [Google Scholar] [CrossRef]
- Rai, V.K.; Sinha, P.; Yadav, K.S.; Shukla, A.; Saxena, A.; Bawankule, D.U.; Tandon, S.; Khan, F.; Chanotiya, C.S.; Yadav, N.P. Anti-Psoriatic Effect of Lavandula Angustifolia Essential Oil and Its Major Components Linalool and Linalyl Acetate. J. Ethnopharmacol. 2020, 261, 113127. [Google Scholar] [CrossRef]
- Martín-Peláez, S.; Covas, M.I.; Fitó, M.; Kušar, A.; Pravst, I. Health Effects of Olive Oil Polyphenols: Recent Advances and Possibilities for the Use of Health Claims. Mol. Nutr. Food Res. 2013, 57, 760–771. [Google Scholar] [CrossRef]
- Palombo, R.; Savini, I.; Avigliano, L.; Madonna, S.; Cavani, A.; Albanesi, C.; Mauriello, A.; Melino, G.; Terrinoni, A. Luteolin-7-Glucoside Inhibits IL-22/STAT3 Pathway, Reducing Proliferation, Acanthosis, and Inflammation in Keratinocytes and in Mouse Psoriatic Model. Cell Death Dis. 2016, 7, e2344. [Google Scholar] [CrossRef]
- Michalsen, A.; Eddin, O.; Salama, A. A Case Series of the Effects of a Novel Composition of a Traditional Natural Preparation for the Treatment of Psoriasis. J. Tradit. Complement. Med. 2016, 6, 395–398. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, M.; Alexander, A.; Singh, M.R.; Singh, D.; Saraf, S.; Saraf, S.; Yadav, K. Ajazuddin Statistically Optimized Calcipotriol Fused Nanostructured Lipid Carriers for Effectual Topical Treatment of Psoriasis. J. Drug Deliv. Sci. Technol. 2021, 61, 102168. [Google Scholar] [CrossRef]
- Rashid, S.A.; Bashir, S.; Naseem, F.; Farid, A.; Rather, I.A.; Hakeem, K.R. Olive Oil Based Methotrexate Loaded Topical Nanoemulsion Gel for the Treatment of Imiquimod Induced Psoriasis-like Skin Inflammation in an Animal Model. Biology 2021, 10, 1121. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, J.B.; Hsu, C.-L.; Bryce, P.J. IgE-Mediated Mast Cell Responses Are Inhibited by Thymol-Mediated, Activation-Induced Cell Death in Skin Inflammation. J. Allergy Clin. Immunol. 2014, 133, 1735–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pivetta, T.P.; Simões, S.; Araújo, M.M.; Carvalho, T.; Arruda, C.; Marcato, P.D. Development of Nanoparticles from Natural Lipids for Topical Delivery of Thymol: Investigation of Its Anti-Inflammatory Properties. Colloids Surf. B Biointerfaces 2018, 164, 281–290. [Google Scholar] [CrossRef]
- Hammer, K.A.; Carson, C.F.; Riley, T.V.; Nielsen, J.B. A Review of the Toxicity of Melaleuca alternifolia (Tea Tree) Oil. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2006, 44, 616–625. [Google Scholar] [CrossRef]
- Finlay-Jones, J.; Hart, P.H. Rural Industries Research and Development Corporation (Australia). In Regulation of Immune Responses in Human Skin by Tea Tree Oil; Tea Tree Oil Research Program, Ed.; RIRDC: Barton, Australia, 2004; ISBN 978-0-642-58747-3. [Google Scholar]
- Sonia, K.; Anupama, D. Microemulsion Based Transdermal Drug Delivery of Tea Tree Oil. Int. J. Drug Dev. Res. 2011, 3, 191–198. [Google Scholar]
- Alam, M.S.; Saraswat, R.; Sharma, P.; Ansari, M.S. In-Vivo Assessment of Glucocorticoid Loaded Tea Tree Oil Nanoemulsion Gel. J. Drug Deliv. Ther. 2019, 9, 108–113. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.S.; Ali, M.D.; Ansari, M.S.; Sharma, P. Inhibitory effects on tumor necrosis factor alpha and interleukin 12 using clobetasol propionate loaded tea tree oil nanoemulsion gel on animal model. Asian J. Pharm. Clin. Res. 2018, 11, 182–185. [Google Scholar] [CrossRef] [Green Version]
- Zrira, S.S.; Benjilali, B.B. Seasonal Changes in the Volatile Oil and Cineole Contents of Five Eucalyptus Species Growing in Morocco. J. Essent. Oil Res. 1996, 8, 19–24. [Google Scholar] [CrossRef]
- Nakamura, T.; Yoshida, N.; Yamanoi, Y.; Honryo, A.; Tomita, H.; Kuwabara, H.; Kojima, Y. Eucalyptus Oil Reduces Allergic Reactions and Suppresses Mast Cell Degranulation by Downregulating IgE-FcεRI Signalling. Sci. Rep. 2020, 10, 20940. [Google Scholar] [CrossRef]
- Hussein, A.; Abdel-Mottaleb, M.; El-assal, M.; Sammour, O. Novel biocompatible essential oil-based lipid nanocapsules with antifungal properties. Drug Del. Sci. Technol. 2020, 56, 101605. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.M.A.; Moulari, B.; Beduneau, A.; Pellequer, Y.; Lamprecht, A. Surface-charge-dependent nanoparticles accumulation in inflamed skin. J. Pharm. Sci. 2012, 101, 4231–4239. [Google Scholar] [CrossRef] [PubMed]
- Fereig, S.; Mamdouh, G.; Arafa, M.; Abdel-Mottaleb, M. Tacrolimus-loaded chitosan nanoparticles for enhanced skin deposition and management of plaque psoriasis. Carbohydr. Polym. 2021, 268, 118238. [Google Scholar] [CrossRef]
- Fereig, S.; Mamdouh, G.; Arafa, M.; Abdel-Mottaleb, M. Boosting the anti-inflammatory effect of self-assembled hybrid lecithin–chitosan nanoparticles via hybridization with gold nanoparticles for the treatment of psoriasis. Drug Deliv. 2022, 29, 1726–1742. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mottaleb, M.M.A.; Neumann, D.; Lamprecht, A. Lipid nanocapsules for dermal application: A comparative study of lipid-based versus polymer-based nanocarriers. Eur. J. Pharm. Biophar. 2011, 79, 36–42. [Google Scholar] [CrossRef] [PubMed]
- El-Tokhy, F.; Abdel-Mottaleb, M.M.A.; Abdel-Mageed, S.; Mahmoud, A.; El-ghany, E.; Geneidi, A. Boosting the In Vivo Transdermal Bioavailability of Asenapine Maleate Using Novel Lavender Oil-Based Lipid Nanocapsules for Management of Schizophrenia. Pharmaceutics 2023, 15, 490. [Google Scholar] [CrossRef]



| Formulation | Botanical Agent (Active Constituents) | Animal Used | Results | Reference |
|---|---|---|---|---|
| Nanoemulsion | Tea tree oil Tepinen-4-ol | Swiss albino mice | Study showed enhancement of terpinen-4-ol permeation through skin layers with high tolerability to the formulation regarding irritation and safety | [130] |
| Nanoemulsion | Turmeric oil | Carrageenan induced paw edema method | Superior activity over the control group with higher stability and lack of adverse effects or irritation | [108] |
| Lipid-polymer hybrid nanoparticles | Capsaicin anti-TNFα siRNA | C57BL/6 mice | Improvement of psoriatic lesions and reduction of inflammatory markers compared to capsaicin solution or anti-TNFα siRNA | [85] |
| Mixture of nanomicelles and nanoemulsion | Capsaicin | C57BL/6 mice. | Significant reduction in PASI score and decline in spleen weight compared to free drug gel, nano-micelles, nanoemulsion | [84] |
| NLCs and SLNs | Capsaicin | Albino rat model | Improved drug accumulation and skin permeation with lack of irritation upon application compared to capsaicin solution | [83] |
| Vesicular system (liposomes, niosomes, emulsomes) | Capsaicin | Albino rat | Enhancement of skin permeation and deposition in viable skin layers for effective psoriasis treatment with minimal skin irritation compared to capsaicin plain gel | [86] |
| Polymeric nanoparticles incorporated in RRR-α-tocopheryl succinate-grafted-ε-polylysine conjugate (VES-g-ε-PLL) hydrogel | Curcumin | Male BALB/c mice | Enhanced permeation through skin layers and reduction of inflammatory cytokine mediators compared to curcumin | [112] |
| Nanoemulsion In Carbopol 840 gel | Combination of Nigella sativa oil and tacrolimus | BALB/c mice model | Marked decrease in skin inflammatory markers (spleen weight and cytokine level) compared to free drug in gel | [69] |
| NLCs incorporated in carbopol 940 gel | Thymol | Mouse model | Symptoms of psoriasis were relieved including erythema and scaling with no infiltration of neutrophils in comparison to thymol gel | [127] |
| Ethosomal vesicles in carbopol 934 hydrogel | Nigella sativa Thymoquinone | Mouse tail model | Substantial decrease in psoriatic lesions compared to Nigella sativa extract and plain thymoquinone suspension | [65] |
| Nanoemulsion | Tea tree oil (Tepinen-4-ol) and clobetasol | Carrageenan induced rat paw edema | Reduction in edema score, ear thickness and inflammatory mediators compared to placebo nanoemulsion and marketed formulation | [131] |
| Nanoemulsion | Curcumin | Imiquimod induced psoriasis in BALB/c mice | Significant reduction in psoriatic lesions compared to conventional imiiquimod nanogel | [110] |
| Nanoemulsion in carbopol 934 hydrogel | Curcumin | BALB/c mice | Improvement of symptoms and complete healing by the tenth day of topical application compared to betamethasone gel or curcumin gel | [111] |
| Nanosponge | Curcumin and caffeine | BALB/c mice | Improvement of PASI score and enhancement of therapeutic efficacy compared to curcumin marketed formulation | [113] |
| Nanoemulsion | Nigella sativa (NS) Thymoquinone | BALB/c mice model | Significant reduction in PASI score with reduced scaling and inflammation compared to free drug solution | [68] |
| NLCs enriched nanogel | Olive oil and calcipotriol | Mouse model | Curative effect and enhanced drug permeation and deposition through skin layers compared to calcipotriol enriched plain gel | [124] |
| Nanoemulsion | Olive oil and methotrexate | Mouse model | Better drug targeting and retention in skin layers with an enhanced efficacy against psoriasis compared to methotrexate plain gel | [125] |
| Active Agent/Dosage Form | Study Type and Duration | Method | Result | Reference |
|---|---|---|---|---|
| Nigella sativa ointment and capsules | Randomized clinical trial for 12 weeks | Three groups were examined, 20 patients each: the ointment was applied to the first group, crude powder and oral capsules were given to the second group and a combination therapy of oral capsules (250 mg) and the ointment were given to the third group. | Good responses were achieved in both group 1 (65%) and group 2 (50%) with lower incidence of psoriasis relapses in more than half of the population. Results were excellent in group 3 (85%) with no observed side effects | [67] |
| Aloe vera cream | Prospective, randomized clinical trial for 8 weeks | 80 patients were divided into two groups: the first group received aloe vera cream and the second group received triamcinolone acetonide cream. Assessment of PASI score was conducted | Significant decrease in PASI score was observed in AV cream group (7.7) compared to triamcinolone group (6.6), indicating alleviation symptoms of psoriasis | [93] |
| Propolis and aloe vera cream | Double-blind control study for 12 weeks | 2248 patients with mild to moderate psoriasis were divided into two groups: one group received a mixture of propolis and aloe vera while the other group received placebo treatment | Symptoms of psoriasis were completely relieved/excellent response in 65% of population of propolis and aloe vera-treated group, and good response in 22% of this group, while no observed improvement in another placebo group | [94] |
| Avocado oil and vitamin B12 cream | Randomized, prospective clinical trial for 12 weeks | The study was conducted on 13 patients with chronic plaque psoriasis. Vitamin D3 analog (calcipotriol), avocado oil cream and vitamin B12 were applied on intraindividual right/left sides. PASI score was determined | Avocado oil cream achieved results similar to calcipotriol as PASI decreased from approximately 9 to 0.8 in both groups after 12 weeks of treatment | [97] |
| Chamomile oil oleogel | Double-blind, randomized clinical trial for 4 weeks | A total of 40 patients with mild-to-moderate symmetrical plaque psoriasis were treated with both placebo and chamomile oleogel. PASI score was used for clinical assessment | The mean PASI scores of the patients treated with chamomile oleogel decreased by more than 4 and were significantly lower than the placebo group | [100] |
| Virgin coconut oil | Randomized, double-blind, clinical trial for 8 weeks | 117 patients were divided into two groups: one group received virgin coconut oil and other received mineral oil | Significant improvement was achieved in virgin coconut oil group (49%), compared to mineral oil-treated group (19%) | [104] |
| A mixture of black cumin, olive oil, tea tree oil, cocoa butter, vitamin A, and vitamin B12 | Case series study for 12 weeks | 12 patients with moderate-to-severe psoriasis were treated using the combination of the oils. PASI score was determined | Significant improvement of psoriatic symptoms in most of the cases in more than 80% of patients | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashraf, M.; El-Sawy, H.S.; El Zaafarany, G.M.; Abdel-Mottaleb, M.M.A. Can Essential Oils/Botanical Agents Smart-Nanoformulations Be the Winning Cards against Psoriasis? Pharmaceutics 2023, 15, 750. https://doi.org/10.3390/pharmaceutics15030750
Ashraf M, El-Sawy HS, El Zaafarany GM, Abdel-Mottaleb MMA. Can Essential Oils/Botanical Agents Smart-Nanoformulations Be the Winning Cards against Psoriasis? Pharmaceutics. 2023; 15(3):750. https://doi.org/10.3390/pharmaceutics15030750
Chicago/Turabian StyleAshraf, Mohamed, Hossam S. El-Sawy, Ghada M. El Zaafarany, and Mona M. A. Abdel-Mottaleb. 2023. "Can Essential Oils/Botanical Agents Smart-Nanoformulations Be the Winning Cards against Psoriasis?" Pharmaceutics 15, no. 3: 750. https://doi.org/10.3390/pharmaceutics15030750
APA StyleAshraf, M., El-Sawy, H. S., El Zaafarany, G. M., & Abdel-Mottaleb, M. M. A. (2023). Can Essential Oils/Botanical Agents Smart-Nanoformulations Be the Winning Cards against Psoriasis? Pharmaceutics, 15(3), 750. https://doi.org/10.3390/pharmaceutics15030750