Application of Electrocoagulation for the Removal of Transition Metals in Water
Abstract
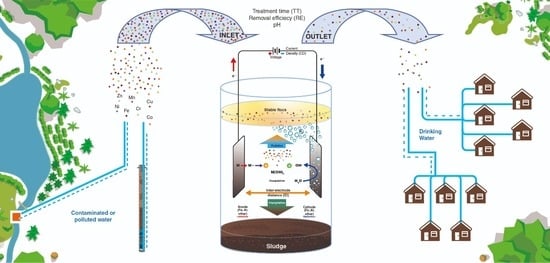
:1. Introduction
2. Materials and Methods
2.1. Bibliometric Search and Analysis
2.2. Statistical Analysis
- The coefficient has a value within the range −1 to 1, that is .
- The coefficient = 1 only when an event coincides with an event , that is, they are equivalent.
- = −1 when an event A coincides with an event .
- = 0 if, and only if, events and are independent. .
- The signs of and are equal, that is, if an event has positive dependence in relation to an event , then an event also has positive dependence in relations to an event . The same property is true if is negative.
- 6.
- .
- 7.
- = 1 only when events and are equivalent.
- 8.
- = −1 when an event coincides with an event .
- 9.
- = 0 if, and only if, events and are independent.
- 10.
- : almost independent;
- : weak dependence;
- : moderate dependence;
- : medium dependence;
- : strong dependence
- when R = 0 and R = 1 variables are independent and totally dependent, respectively.
3. Results and Discussion
- 0.02 Co 100 mg/L
- 0.04 Cr 23 mg/L
- 0.04 Cu 27.8 mg/L
- 10 Fe 25 mg/L
- 0.02 Mn 360 mg/L
- 0.05 Ni 41 mg/L
- Zn = 20 mg/L
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Il’ina, O.; Kolobov, M.; Il’inskii, V. Plastic pollution of the coastal surface water in the middle and southern Baikal. Water Resour. 2021, 48, 56–64. [Google Scholar] [CrossRef]
- Batista, E.; Garcia, L.; Albuquerque, A.; Ballaminut, N.; Scalize, P.; Gil, E. Application of a voltammetric enzymatic biosensor based on crude extract of marasmiellus colocasiae for the detection of phenolic compounds in drinking water. Rev. Ambiente Agua 2020, 15, e2610. [Google Scholar] [CrossRef]
- Kumar, M.; Borah, P.; Devi, P. Priority and emerging pollutants in water. In Inorganic Pollutants in Water; Devi, P., Singh, P., Kumar Kansal, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Rogowska, J.; Cieszynska-Semenowicz, M.; Ratajczyk, W.; Wolska, L. Micropollutants in treated wastewater. AMBIO 2020, 49, 487–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, F. Emerging poly- and perfluoroalkyl substances in the aquatic environment: A review of current literature. Water Res. 2017, 124, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Ternes, T. Water analysis: Emerging contaminants and current issues. Anal. Chem. 2014, 86, 2813–2848. [Google Scholar] [CrossRef] [PubMed]
- Mir, I.; SreePrabash, M.; Sridhar, V.; Maruthi, K. Distribution, sources and toxicity of heavy metals in surface sediments of north western Karnataka, south India. Sci. Rep. 2022, 12, 15782. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Ren, B. Pollution and risk assessment of heavy metals in rivers in the antimony capital of Xikuangshan. Sci. Rep. 2022, 12, 14393. [Google Scholar] [CrossRef] [PubMed]
- Neto, W.M.; Matosinhos, C.C.; Baggio Filho, H. Effect of soil use and occupation on the concentration of metals in the waters of the paraúna Sub-Basin. Braz. J. Anim. Environ. Res. 2021, 4, 749–759. [Google Scholar] [CrossRef]
- Howarth, R.; Chan, F.; Swaney, D.; Marino, R.; Hayn, M. Role of external inputs of nutrients to aquatic ecosystems in determining prevalence of nitrogen vs. phosphorus limitation of net primary productivity. Biogeochemistry 2021, 154, 293–306. [Google Scholar] [CrossRef]
- Penuelas, J.; Janssens, I.; Ciais, P.; Obersteiner, M.; Sardans, J. Anthropogenic global shifts in biospheric N and P concentrations and ratios and their impacts on biodiversity, ecosystem productivity, food security, and human health. Glob. Change Biol. 2020, 26, 1962–1985. [Google Scholar] [CrossRef] [Green Version]
- Wasewar, K.; Singh, S.; Kansal, S. Process intensification of treatment of inorganic water pollutants. In Inorganic Pollutants in Water; Devi, P., Singh, P., Kumar Kansal, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Chapter 13; pp. 245–271. [Google Scholar] [CrossRef]
- Qin, B.; Zhou, J.; Elser, J.; Gardner, W.; Deng, J.; Brookes, J. Water Depth Underpins the Relative Roles and Fates of Nitrogen and Phosphorus in Lakes. Environ. Sci. Technol. 2020, 54, 3191–3198. [Google Scholar] [CrossRef] [PubMed]
- EPA. Water Quality Topics: Pathogens. United States Environmental Protection Agency (EPA), USA. 2022. Available online: https://www.epa.gov/wqclr/water-quality-topics-pathogens (accessed on 22 October 2022).
- Nichols, G.; Lake, I.; Heaviside, C. Climate change and water-related infectious diseases. Atmosphere 2018, 9, 385. [Google Scholar] [CrossRef] [Green Version]
- Jin, D.; Kong, X.; Cui, B.; Jin, S.; Xie, Y.; Wang, X.; Deng, Y. Bacterial communities and potential waterborne pathogens within the typical urban surface waters. Sci. Rep. 2018, 8, 13368. [Google Scholar] [CrossRef] [Green Version]
- Bain, R.; Cronk, R.; Wright, J.; Yang, H.; Slaymaker, T.; Bartram, J. Fecal Contamination of drinking-water in low- and middle-income countries: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001644. [Google Scholar] [CrossRef] [Green Version]
- Arman, N.; Salmiati, S.; Aris, A.; Salim, M.; Nazifa, T.; Muhamad, M.; Marpongahtun, M. A Review on Emerging Pollutants in the Water Environment: Existences, Health Effects and Treatment Processes. Water 2021, 13, 3258. [Google Scholar] [CrossRef]
- Su, C.; Cui, Y.; Liu, D.; Zhang, H.; Baninla, Y. Endocrine Disrupting Compounds, Pharmaceuticals and Personal Care Products in the Aquatic Environment of China: Which Chemicals Are the Prioritized Ones? Sci. Total Environ. 2020, 720, 137652. [Google Scholar] [CrossRef]
- Lin, T.; Yu, S.; Chen, W. Occurrence, Removal and Risk Assessment of Pharmaceutical and Personal Care Products (PPCPs) in an Advanced Drinking Water Treatment Plant (ADWTP) around Taihu Lake in China. Chemosphere 2016, 152, 1–9. [Google Scholar] [CrossRef]
- Puljko, A.; Milakovic, M.; Krizanovic, S.; Kosic-Vuksic, J.; Babic, I.; Petric, I.; Maravic, A.; Jelic, M.; Udikovic-Kolic, N. Prevalence of enteric opportunistic pathogens and extended-spectrum cephalosporin- and carbapenem-resistant coliforms and genes in wastewater from municipal wastewater treatment plants in Croatia. J. Hazard. Mater. 2022, 427, 128155. [Google Scholar] [CrossRef]
- Stec, J.; Kosikowska, U.; Mendrycka, M.; Stepien-Pysniak, D.; Niedzwiedzka-Rystwej, P.; Bebnowska, D.; Hrynkiewicz, R.; Zietara-Wysocka, J.; Grywalska, E. Opportunistic Pathogens of Recreational Waters with Emphasis on Antimicrobial Resistance—A Possible Subject of Human Health Concern. Int. J. Environ. Res. Public Health 2022, 19, 7308. [Google Scholar] [CrossRef]
- Cui, B.; Liang, S. Monitoring Opportunistic Pathogens in Domestic Wastewater from a Pilot-Scale Anaerobic Biofilm Reactor to Reuse in Agricultural Irrigation. Water 2019, 11, 1283. [Google Scholar] [CrossRef] [Green Version]
- Peña-Guzmán, C.; Ulloa-Sánchez, S.; Mora, K.; Helena-Bustos, R.; Lopez-Barrera, E.; Alvarez, J.; Rodriguez-Pinzón, M. Emerging Pollutants in the Urban Water Cycle in Latin America: A Review of the Current Literature. J. Environ. Manag. 2019, 237, 408–423. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Thakur, A.K.; Mazumder, P.; Kuroda, K.; Mohapatra, S.; Rinklebe, J.; Ramanathan, A.; Cetecioglu, Z.; Jain, S.; Tyagi, V.K.; et al. Frontier review on the propensity and repercussion of SARS-CoV-2 migration to aquatic environment. J. Hazard. Mater. Lett. 2020, 1, 100001. [Google Scholar] [CrossRef] [PubMed]
- Naddeo, V.; Liu, H. Editorial Perspectives: 2019 novel coronavirus (SARS-CoV-2): What is its fate in urban water cycle and how can the water research community respond? Environ. Sci. Water Res. Technol. 2020, 6, 1213–1216. [Google Scholar] [CrossRef]
- Rimoldi, S.G.; Stefani, F.; Gigantiello, A.; Polesello, S.; Comandatore, F.; Mileto, D.; Maresca, M.; Longobardi, C.; Mancon, A.; Romeri, F.; et al. Presence and infectivity of SARS-CoV-2 virus in wastewaters and rivers. Sci. Total Environ. 2020, 744, 140911. [Google Scholar] [CrossRef]
- Yeo, C.; Kaushal, S.; Yeo, D. Enteric involvement of coronaviruses: Is faecal–oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol. Hepatol. 2020, 5, 335–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.; Chen, Z.; Gong, C.; Liu, H.; Li, B.; Li, K.; Chen, X.; Xu, C.; Jing, Q.; Liu, G.; et al. Sewage as a possible transmission vehicle during a coronavirus disease 2019 outbreak in a densely populated community: Guangzhou, China, April 2020. Clin. Infect. Dis. 2020, 73, e1795–e1802. [Google Scholar] [CrossRef]
- Pashaei, R.; Dzingelevičienė, R.; Bradauskaitė, A.; Lajevardipour, A.; Mlynska-Szultka, M.; Dzingelevičius, N.; Raugelė, S.; Razbadauskas, A.; Abbasi, S.; Rees, R.M.; et al. Pharmaceutical and Microplastic Pollution before and during the COVID-19 Pandemic in Surface Water, Wastewater, and Groundwater. Water 2022, 14, 3082. [Google Scholar] [CrossRef]
- United Nations (UN). United Nations World Water Development Report 2021: Valuing Water. Available online: https://www.unwater.org/news/un-world-water-development-report-2021-%E2%80%98valuing-water%E2%80%99 (accessed on 22 October 2022).
- Briffa, J.; Sinagra, E.; Renald Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Tchounwou, P.; Yedjou, C.; Patlolla, A.; Sutton, D. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology. Experientia Supplementum; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; Volume 101. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Aris, A.; Puad, N.H.A.; Shafie, N.; Juen, L.; Mangala, S. The Chemometric Approach as a Useful Tool in the Identification of Metal Pollution Sources of Riverine-Mangrove Sediment of Kota Marudu, Sabah, Malaysia. Environ. Asia 2014, 7, 70–78. [Google Scholar]
- Petrucci, R.; Harwood, W.; Herring, F. General Chemistry: Principles and Modern Applications, 8th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2002; pp. 341–342. [Google Scholar]
- Salonem, J.T.; Nyysönem, K.; Korpela, H.; Tuomilehto, J.; Seppänem, R.; Salonem, R. High stored iron levels are associated with excess risk of myocardial infarction in estern finnish man. Circulation 1992, 86, 803–811. [Google Scholar] [CrossRef] [Green Version]
- Papanikolau, G.; Pantopoulos, K. Iron metabolism and toxicity. Toxicol. Appl. Pharmacol. 2005, 202, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Gibb, H.; Lees, P.; Pinsk, P.; Rooney, B. Lung cancer among workers in chromium chemical production. Am. J. Ind. Med. 2000, 38, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R. The carcinogenicity of metals in humans. Cancer Causes Control 1997, 8, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Al-Rashdi, B.; Johnson, D.; Hilal, N. Removal of heavy metal ions by nanofiltration. Desalination 2013, 315, 2–17. [Google Scholar] [CrossRef]
- Ammami, M.; Benamar, A.; Wang, H.; Bailleul, C.; Legras, M.; Le Derf, F.; Portet-Koltalo, F. Simultaneous electrokinetic removal of polycyclic aromatic hydrocarbons and metals from a sediment using mixed enhancing agents. Int. J. Environ. Sci. 2014, 11, 1801–1816. [Google Scholar] [CrossRef] [Green Version]
- Brazil. Portaria Ministério da Saúde/Gabinete do Ministro GM/MS n. 888, 4 May 2021. Para Dispor Sobre os Procedimentos de Controle e de Vigilância da Qualidade da Água para Consumo Humano e Seu Padrão de Potabilidade. Diário Oficial da União. Brasília, Brazil, n. 85, p. 127, 7 May 2021, Section 1. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/gm/2021/prt0888_07_05_2021.html (accessed on 12 October 2022).
- Çelebi, H.; Gök, G.; Gök, O. Adsorption capability of brewed tea waste in waters containing toxic lead(II), cadmium (II), nickel (II), and zinc(II) heavy metal ions. Sci. Rep. 2020, 10, 17570. [Google Scholar] [CrossRef]
- Guedes, J.A.; Lima, R.F.S.; Souza, L.C. Metais pesados em água do rio Jundiaí Macaíba/RN. Rev. Geol. 2005, 18, 131–142. [Google Scholar]
- Alves, R.I.S.; Tonani, K.A.A.; Nikaido, M.; Cardoso, O.O.; Trevilato, T.M.B.; Segura-Muňoz, S.I. Avaliação das concentrações de metais pesados em águas superficiais e sedimentos do Córrego Monte Alegre e afluentes, Ribeirão Preto, SP, Brazil, Ambiente e Água—An interdisciplinary. J. Appl. Sci. 2010, 5, 122–132. [Google Scholar] [CrossRef]
- Ferraz, L.L.; Dourado, A.A.; Rodrigues, A.; Rocha, F.A. Análise da presença de metais pesados na água em diferentes reservatórios subterrâneos no município de Vitória da Conquista—BA. Agrar. Acad. 2018, 5, 1–10. Available online: https://www.conhecer.org.br/Agrarian%20Academy/2018a/analise%20da%20presenca.pdf (accessed on 14 October 2022). [CrossRef]
- Pan, Z.; An, L. Removal of Heavy Metal from Wastewater Using Ion Exchange Membranes. In Applications of Ion Exchange Materials in the Environment; Inamuddin, A.M., Asiri, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 25–46. [Google Scholar] [CrossRef]
- Farooq, U.; Kozinski, J.; Khan, M.; Athar, M. Biosorption of heavy metal ions using wheat based biosorbents—A review of the recent literature. Bioresour. Technol. 2010, 101, 5043–5053. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, H.; Mustafa, M.; Isa, M. Adsorption of chromium, copper, lead and mercury ions from aqueous solution using bio and nano adsorbents: A review of recent trends in the application of AC, BC, nZVI and MXene. Environ. Res. 2022, 212, 113138. [Google Scholar] [CrossRef] [PubMed]
- Anastopoulos, I.; Pashalidis, I.; Hosseini-Bandegharaei, A.; Giannakoudakis, D.; Robalds, A.; Usman, M.; Escudero, L.; Zhou, Y.; Colmenares, J.; Núnez-Delgado, A.; et al. Agricultural biomass/waste as adsorbents for toxic metal decontamination of aqueous solutions. J. Mol. Liq. 2019, 295, 111684. [Google Scholar] [CrossRef]
- Czikkely, M.; Neubauer, E.; Fekete, I.; Ymeri, P.; Fogarassy, C. Review of heavy metal adsorption processes by several organic matters from wastewaters. Water 2018, 10, 1377. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, C.; Scheufele, F.; Espinoza-Quiñones, F.; Módenes, A.; Vieira, M.; Kroumov, D.; Borba, C. A comprehensive evaluation of heavy metals removal from battery industry wastewaters by applying bio-residue, mineral and commercial adsorbent materials. J. Mater. Sci. 2018, 53, 7976–7995. [Google Scholar] [CrossRef]
- Wierzba, S.; Makuchowska-Fryc, J.; Kłos, A.; Ziembik, Z.; Ochędzan-Siodłak, Z. Role of calcium carbonate in the process of heavy metal biosorption from solutions: Synergy of metal removal mechanisms. Sci. Rep. 2022, 12, 17668. [Google Scholar] [CrossRef]
- Serrano, J.; Leiva, E. Removal of Arsenic Using Acid/Metal-Tolerant Sulfate Reducing Bacteria: A New Approach for Bioremediation of High-Arsenic Acid Mine Waters. Water 2017, 9, 994. [Google Scholar] [CrossRef] [Green Version]
- Serrano, L.; Lara, N.; Vera, R.; Cholico-González, D. Removal of Fe(III), Cd(II), and Zn(II) as Hydroxides by Precipitation–Flotation System. Sustainability 2021, 13, 11913. [Google Scholar] [CrossRef]
- Belizario, P.; Scalize, P.; Albuquerque, A. Heavy Metal Removal in a Detention Basin for Road Runoff. Open Eng. 2016, 6, 412–417. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Ahmaruzzaman, M. A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. J. Water Process Eng. 2016, 10, 39–47. [Google Scholar] [CrossRef]
- Adetokun, A.; Uba, S.; Garba, Z. Optimization of adsorption of metal ions from a ternary aqueous solution with activated carbon from Acacia Senegal (L.) Willd pods using Central Composite Design. J. King Saud Univ. Sci. 2019, 31, 1452–1462. [Google Scholar] [CrossRef]
- Ankit; Bauddh, K.; Korstad, J. Phycoremediation: Use of Algae to Sequester Heavy Metals. Hydrobiology 2022, 1, 288–303. [Google Scholar] [CrossRef]
- Hansen, D.; Horne, A. The Effect of Drying/Re-Flooding on Trace Metal, As and Se Fluxes in a Treatment Wetland: Addressing Growing Environmental Concerns. Biology 2022, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Sladkovska, T.; Wolski, K.; Bujak, H.; Radkowski, A.; Sobol, Ł. A Review of Research on the Use of Selected Grass Species in Removal of Heavy Metals. Agronomy 2022, 12, 2587. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental Remediation and Application of Nanoscale Zero-Valent Iron and Its Composites for the Removal of Heavy Metal Ions: A Review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Torres, I.; Ruggeri, H.; Scalize, P.; Albuquerque, A.; Gil, E. Application of electrocoagulation with a new steel-swarf-based electrode for the removal of heavy metals and total coliforms from sanitary landfill leachate. Appl. Sci. 2021, 11, 5009. [Google Scholar] [CrossRef]
- Das, D.; Nandi, B.K. Removal of Fe (II) ions from drinking water using Electrocoagulation (EC) process: Parametric optimization and kinetic study. J. Environ. Chem. Eng. 2019, 7, 103–116. [Google Scholar] [CrossRef]
- Mousazadeh, M.; Naghdali, Z.; Al-Qodah, Z.; Alizadeh, S.M.; Niaragh, E.K.; Malekmohammadi, S.; Nidheesh, P.V.; Roberts, E.P.L.; Sillanpää, M.; Emamjomeh, M.M. A systematic diagnosis of state of the art in the use of electrocoagulation as a sustainable technology for pollutant treatment: An updated review. Sustain. Energy Technol. Assess. 2021, 47, 101353. [Google Scholar] [CrossRef]
- Mollah, M.Y.A.; Schennach, R.; Parga, J.R.; Cocke, D.L. Electrocoagulation (EC)—Science and applications. J. Hazard. Mater. 2001, B84, 29–41. [Google Scholar] [CrossRef]
- Ebba, M.; Asaithambi, P.; Alemayehu, E. Investigation on operating parameters and cost using an electrocoagulation process for wastewater treatment. Appl. Water Sci. 2021, 11, 175. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Shannag, M. Heavy metal ions removal from wastewater using electrocoagulation processes: A comprehensive review. Sep. Sci. Technol. 2017, 52, 2649–2676. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Mohammadi, L.; Ansari-MOGHADDAM, A.; Mahvi, A.H. Heavy metals removal from aqueous environments by electrocoagulation process—A systematic review. J. Environ. Health Sci. Eng. 2015, 13, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alavijeh, H.; Sadeghi, M.; Kashani, M.; Moheb, A. Efficient chemical coagulation-electrocoagulation-membrane filtration integrated systems for baker’s yeast wastewater treatment: Experimental and economic evaluation. Clean. Chem. Eng. 2022, 3, 100032. [Google Scholar] [CrossRef]
- Vik, E.; Carlson, D.; Eikum, A.; Gjessing, E. Electrocoagulation of potable water. Water Res. 1984, 18, 1355–1360. [Google Scholar] [CrossRef]
- Sahu, O.; Mazumdar, B.; Chaudhari, P.K. Treatment of wastewater by electrocoagulation: A review. Environ. Sci. Pollut. Res. 2014, 21, 2397–2413. [Google Scholar] [CrossRef] [PubMed]
- Khor, C.; Wang, J.; Li, M.; Oettel, B.; Kaner, R.; Jassby, D.; Hoek, E. Performance, Energy and Cost of Produced Water Treatment by Chemical and Electrochemical Coagulation. Water 2020, 12, 3426. [Google Scholar] [CrossRef]
- Holt, P.K.; Barton, G.W.; Wark, M.; Mitchell, C.A. A quantitative comparison between chemical dosing and electrocoagulation. Colloids Surf. 2002, 211, 233–248. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization (WHO): Geneva, Switzerland, 2011. Available online: https://apps.who.int/iris/handle/10665/44584 (accessed on 9 October 2022).
- EPA. National Secondary Drinking Water Regulations; United States Environmental Protection Agency (U.S. EPA): Washington, DC, USA, 1991. Available online: https://www.govinfo.gov/content/pkg/CFR-2007-title40-vol22/pdf/CFR-2007-title40-vol22-part143.pdf (accessed on 10 October 2022).
- EPA. National Primary Drinking Water Regulations; United States Environmental Protection Agency (U.S. EPA): Washington, DC, USA, 2009. Available online: https://www.epa.gov/sites/default/files/2016-06/documents/npwdr_complete_table.pdf (accessed on 10 October 2022).
- EU. Council Directive 2020/2184/EU of 16 December 2020, on the quality of water intended for human consumption. Off. J. Eur. Union 2020, L435, 1–62. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020L2184 (accessed on 10 October 2022).
- Khan, K.; Lu, Y.; Khan, H.; Zakir, S.; Ihsanullah; Khan, S.; Khan, A.A.; Wei, L.; Wang, T. Health risks associated with heavy metals in the drinking water of Swat, northern Pakistan. J. Environ. Sci. 2013, 25, 2003–2013. [Google Scholar] [CrossRef]
- Mughal, A.; Sultan, K.; Ashraf, K.; Hassan, A.; Zaman, Q.U.; Haider, F.U.; Shahzad, B. Risk Analysis of Heavy Metals and Groundwater Quality Indices in Residential Areas: A Case Study in the Rajanpur District, Pakistan. Water 2022, 14, 3551. [Google Scholar] [CrossRef]
- Ullah, H.; Naz, I.; Alhodaib, A.; Abdullah, M.; Muddassar, M. Coastal Groundwater Quality Evaluation and Hydrogeochemical Characterization Using Chemometric Techniques. Water 2022, 14, 3583. [Google Scholar] [CrossRef]
- Pan, H.; Zhou, G.; Yang, R.; Cheng, Z.; Sun, B. Heavy Metals and As in Ground Water, Surface Water, and Sediments of Dexing Giant Cu-Polymetallic Ore Cluster, East China. Water 2022, 14, 352. [Google Scholar] [CrossRef]
- Mokarram, M.; Saber, A.; Sheykhi, V. Effects of heavy metal contamination on river water quality due to release of industrial effluents. J. Clean. Prod. 2020, 277, 123380. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, D.; Wang, X.; Huang, W.; Lawless, D.; Feng, X. Removal of heavy metals from water using polyvinylamine by polymer-enhanced ultrafiltration and flocculation. Sep. Purif. Technol. 2016, 158, 124–136. [Google Scholar] [CrossRef]
- Ashoori, R.; Samaei, M.R.; Yousefinejad, S.; Azhdarpoor, A.; Emadi, Z.; Mohammadpour, A.; Lari, A.R.; Khaneghah. Simultaneous removal of fluoride and nitrate from synthetic aqueous solution and groundwater by the electrochemical process using non-coated and coated anode electrodes: A human healt risk study. Environ. Res. 2022, 214, 113938. [Google Scholar] [CrossRef]
- Nanseu-Njiki, C.P.; Tchamango, S.R.; Ngom, P.C.; Darchen, A.; Ngameni, E. Mercury (II) removal from water by electrocoagulation using aluminium and iron electrodes. J. Hazard. Mater. 2009, 168, 1430–1436. [Google Scholar] [CrossRef]
- Daneshvar, N.; Sorkhabi, A.; Kasiri, M.B. Decolorization of dye solution containing Acid Red 14 by electrocoagulation with a comparative investigation of different electrode connections. J. Hazard. Mater. 2004, 112, 55–62. [Google Scholar] [CrossRef]
- Samaka, I.S.; Naje, A.S.; Al-Zubaidi, A.M. Treatment of saline water using electrocoagulation process with monopolar connection of electrodes. Nat. Environ. Pollut. Technol. 2022, 21, 795–802. [Google Scholar] [CrossRef]
- Dutta, N.; Gupta, A. Development of arsenic removal unit with electrocoagulation and activated alumina sorption: Field trial at rural West Bengal, India. J. Water Process Eng. 2022, 49, 103013. [Google Scholar] [CrossRef]
- Naje, A.S.; Naser, G.F.; Samaka, I.S.; Al-Zubaide, H.A.M. Environmental assessment of kinetics behavior of electrocoagulation process for industrial wastewater treatment. Mater. Today Proc. 2021, 68. [Google Scholar] [CrossRef]
- Vasudevan, S.; Lakshimi, J.; Sozhan, G. Studies on the Al-Zn-In-alloy as anode material for the removal of chromium from drinking water in electrocoagulation process. Desalination 2011, 275, 260–268. [Google Scholar] [CrossRef]
- Moussa, D.T.; El-Naas, M.H.; Nasser, M.; Al-Marri, M.J. A comprehensive review of electrocoagulation for water treatment: Potentials and Challenges. J. Environ. Manag. 2017, 186, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Obreshkov, N. Probability Theory; Nauka i Izkustvo: Sofia, Bulgaria, 1963. (In Bulgarian) [Google Scholar]
- Dimitrov, B. Some Obreshkov measures of dependence and their use, Compte Rendus de l’Academie Bulgare des Sciences, V. Sciences 2010, 63, 15–18. Available online: https://digitalcommons.kettering.edu/mathematics_facultypubs/22 (accessed on 5 October 2022).
- Ghosh, D.; Solanki, H.; Purkait, M.K. Removal of Fe (II) from tap water by electrocoagulation technique. J. Hazard. Mater. 2008, 155, 135–143. [Google Scholar] [CrossRef]
- Abdulhadi, B.; Kot, P.; Hashim, K.; Shaw, A.; Muradov, M.; Al-Khaddar, R. Continuous-flow electrocoagulation (EC) process for iron removal from water: Experimental, statistical and economic study. Sci. Total Environ. 2021, 760, 143417. [Google Scholar] [CrossRef]
- Das, D.; Nandi, B.K. Simultaneous removal of fluoride and FE (II) ions from drinking water by electrocoagulation. J. Environ. Chem. Eng. 2020, 8, 103643. [Google Scholar] [CrossRef]
- Doggaz, A.; Attour, A.; Mostefa, M.L.P.; Mohamed, T.; Lapicque, F. Iron removal from waters by electrocoagulation: Investigations of the various physicochemical phenomena involved. Sep. Purif. Technol. 2018, 203, 217–225. [Google Scholar] [CrossRef]
- Silva, J.F.A.; Graça, N.S.; Ribeiro, A.M.; Rodrigues, A.E. Electrocoagulation process for the removal of co-existent fluoride, arsenic and iron from contaminated drinking water. Sep. Purif. Technol. 2017, 197, 237–243. [Google Scholar] [CrossRef]
- Pan, C.; Troyer, L.D.; Catalano, J.G.; Giammar, D.E. Dynamics of Chromium (VI) removal from drinking water by iron electrocoagulation. Environ. Sci. Technol. 2016, 50, 13502–13510. [Google Scholar] [CrossRef]
- Thella, K.; Verma, B.; Srivastava, V.C.; Srivastava, K.K. Electrocoagulation study for the removal of arsenic and chromium from aqueous solution. J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2008, 43, 554–562. [Google Scholar] [CrossRef]
- Hamdan, S.S.; El-Naas, M.H. An electrocoagulation column (ECC) for groundwater purification. J. Water Process Eng. 2014, 4, 25–30. [Google Scholar] [CrossRef]
- Martín-Domínguez, A.; Rivera-Huerta, M.; Pérez-Castrejón, S.; Garrido-Hoyos, S.; Villegas-Medoza, I.; Gelover-Santiago, S.; Drogui, P.; Buelna, G. Chromium removal from drinking water by redox-assisted coagulation: Chemical versus electrocoagulation. Sep. Purif. Technol. 2018, 200, 266–272. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.M.; Marchesiello, M.; Thivel, P.X. Removal of copper, zinc and nickel present in natural water containing Ca2+ and HCO3 ions by electrocoagulation. Sep. Purif. Technol. 2013, 107, 109–117. [Google Scholar] [CrossRef]
- Shafaei, A.; Pajootan, E.; Nikazar, M.; Arami, M. Removal of Co (II) from aqueous solution by electrocoagulation process using aluminium electrodes. Desalination 2011, 279, 121–126. [Google Scholar] [CrossRef]
- Mateen, Q.S.; Khan, S.U.; Islam, D.T.; Khan, N.A.; Farooqi, I.H. Copper (II) removal in a column reactor using electrocoagulation: Parametric optimization by response surface methodology using central composite design. Water Environ. Res. 2020, 92, 1350–1362. [Google Scholar] [CrossRef]
- Hernandéz, M.C.; Barletta, L.; Dogliotti, M.B.; Russo, N.; Fino, D.; Spinelli, P. Heavy metal removal by means of electrocoagulation using aluminium electrodes for drinking water purification. J. Appl. Electrochem. 2012, 42, 809–817. [Google Scholar] [CrossRef]
- Ganesan, P.; Lakshmi, J.; Sozhan, G.; Vasudevan, S. Removal of manganese from water by electrocoagulation: Adsorption, kinects and thermodynamic studies. Can. J. Chem. Eng. 2013, 91, 448–458. [Google Scholar] [CrossRef]
- Gomes, J.A.; Islam, M.K.; Islam, M.R.; Irwin, G.; Bernazzani, P.; Cocke, D.L. Utilization of electrochemical techniques for copper removal, speciation and analysis in aqueous systems. Electrochem. Soc. 2010, 18, 59–68. [Google Scholar] [CrossRef]
- Hussain, M.; Syed, Q.; Bashir, R.; Adnan, A. Electrochemical process for simultaneous removal of chemical and biological contaminants from drinking water. Environ. Sci. Pollut. Res. 2021, 28, 45780–45792. [Google Scholar] [CrossRef]
- Shahreza, S.O.; Mokhtarian, N.; Behnam, S. Optimization of Mn removal from aqueous solutions through electrocoagulation. Environ. Technol. 2018, 41, 890–900. [Google Scholar] [CrossRef]
- Pan, C.; Toyer, L.D.; Liao, P.; Catalano, J.G.; Li, W.; Giammar, D.E. Effect of humic acid on the removal of chromium (VI) and the production of solids in iron electrocoagulation. Environ. Sci. Technol. 2017, 51, 6308–6318. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Lakshimi, J.; Sozhan, G. Studies on the removal of Iron from drinking water by electrocoagulation—A clean process. Clean J. 2009, 37, 45–51. [Google Scholar] [CrossRef]
- Vasudevan, S.; Lakshmi, J.; Sozhan, G. Optimization of electrocoagulation process for the simultaneous removal of mercury, lead, and nickel from contaminated water. Environ. Sci. Pollut. Res. 2012, 19, 2734–2744. [Google Scholar] [CrossRef] [PubMed]
- Hashim, K.S.; Shaw, A.; Al Khaddar, R.; Pedrola, M.O.; Phipps, D. Iron removal, energy consumption and operating cost of electrocoagulation of drinking water using a new flow column reactor. J. Environ. Manag. 2017, 189, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Zaied, B.K.; Rashid, M.; Nasrullah, M.; Zularisam, A.W.; Pant, D.; Singh, L. A comprehensive review on contaminants removal from pharmaceutical wastewater by electrocoagulation process. Sci. Total Environ. 2020, 726, 138095. [Google Scholar] [CrossRef]
- Zeboudji, B.; Drouiche, N.; Lounici, H.; Mameri, N.; Ghaffour, N. The Influence of Parameters Affecting Boron Removal by Electrocoagulation Process. Sep. Sci. Technol. 2013, 48, 37–41. [Google Scholar] [CrossRef]
- Mansoorian, H.J.; Mahvi, A.H.; Jafari, A.J. Removal of lead and zinc from battery industry wastewater using electrocoagulation process: Influence of direct and alternating current by using iron and stainless steel rod electrodes. Sep. Purif. Technol. 2014, 135, 165–175. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Kim, S.D.; Yoon, Y.S. Enhanced phosphorus and COD removals for retrofit of existing sewage treatment by electrocoagulation process with cylindrical aluminum electrodes. Desalination Water Treat. 2014, 52, 2388–2399. [Google Scholar] [CrossRef]
- Lu, J.; Li, Y.; Yin, M.; Ma, X.; Lin, S. Removing heavy metal ions with continuous aluminum electrocoagulation: A study on back mixing and utilization rate of electro-generated Al ions. Chem. Eng. J. 2015, 267, 86–92. [Google Scholar] [CrossRef]
- Vepsäläinem, M.; Sillanpää, M. Electrocoagulation in the treatment of industrial waters and wastewaters. Adv. Water Treat. Electrochem. Methods 2020, 1, 1–78. [Google Scholar] [CrossRef]




| Metal | WHO [76] | EU [79] | Brazil [43] | EPA [77,78] |
|---|---|---|---|---|
| Cr | 0.05 | 0.025 | 0.05 | 0.1 |
| Co | NL | NL | NL | NL |
| Cu | 2.0 | 0.002 | 2.0 | 1.0 |
| Fe | 0.3 | 0.2 | 0.3 | 0.3 |
| Mn | 0.5 | 0.05 | 0.1 | 0.05 |
| Ni | 0.07 | 0.02 | 0.07 | 0.02 |
| Zn | 3.0 | NL | 5.0 | 5.0 |
| Variable | Category | Abbreviation |
|---|---|---|
| Metal (P) | Cobalt (Co) | P. Co |
| Copper (Cu) | P. Cu | |
| Chromium (Cr) | P. Cr | |
| Iron (Fe) | P. Fe | |
| Manganese (Mn) | P. Mn | |
| Nickel (Ni) | P. Ni | |
| Zinc (Zn) | P. Zn | |
| Experimental arrangement (EA) | Parallel plates (PP) | EA. PP |
| Different arrangement (D) | EA.D. | |
| Initial concentration (Ci) | ≤1 mg/L | Ci ≤ 1 |
| 1 to 20 mg/L | 1 < Ci ≤ 20 | |
| >20 mg/L | Ci > 20 | |
| Inter-electrode distance (Id) | ≥1 cm | Id ≥ 1 |
| <1 cm | Id < 1 | |
| Current density (CD) | ≥10 A/m² | CD ≥ 10 |
| <10 A/m² | CD < 10 | |
| pH | Acid | pH < 7 |
| Neutral | pH = 7 | |
| Basic | pH > 7 | |
| Removal efficiency (RE) | ≥95% | RE ≥ 95% |
| <95% | RE < 95% | |
| Cathode material (CM) | Aluminum (Al) | CM Al |
| Iron (Fe) | CM Fe | |
| Other | CM Other | |
| Anode material (AM) | Aluminum (Al) | AM Al |
| Iron (Fe) | AM Fe | |
| Other | AM Other | |
| Treatment time (TT) | ≥60 min | TT ≥ 60 |
| <60 min | TT < 60 |
| Author | Water Type | Metal | EA | Ci (mg/L) | Id (cm) | CD (A/m²) | pHo (pHi–pHf) | RE (%) | CM | AM | TT (min) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ghosh et al. (2008) [96] | Manipulated | Fe | PP | 20.00 | 0.5 | 0.40 | 7.5 (7.5–7.8) | 100.00 | Aluminum | Aluminum | 35 |
| Abdulhadi et al. (2020) [97] | Manipulated | Fe | PP | 10.00 | 0.5 | 30.00 | 7.0 (4.0–10.0) | 99.90 | Aluminum | Aluminum | 50 |
| Das e Nandi (2020) [98] | Manipulated | Fe | PP | 20.00 | 1.0 | 43.10 | 7.0 (5.0–9.0) | 99.96 | Aluminum | Aluminum | 60 |
| Das e Nandi (2019) [65] | Manipulated | Fe | PP | 20.00 | 1.0 | 20.00 | 6.3 (6.3–6.6) | 98.60 | Aluminum | Aluminum | 45 |
| Doggaz et al. (2018) [99] | Manipulated | Fe | PP | 20.00 | 1.0 | 25.00 | 8 (5.0–8.0) | 97.00 | Aluminum | Aluminum | 60 |
| Silva et al. (2017) [100] | Manipulated | Fe | PP | 20.00 | 0.8 | 12.50 | 6.3 (6.0–8.0) | 100.00 | Aluminum | Aluminum | 80 |
| Pan et al. (2016) [101] | Manipulated | Cr | PP | 0.50 | 2.0 | 10.00 | 6.0 (4.0–8.0) | 99.00 | Iron | Iron | 30 |
| Thella et al. (2008) [102] | Manipulated | Cr | PP | 15.00 | 1.0 | 50.00 | 2.0 (2.0–8.0) | 100.00 | Steel | Iron | 40 |
| Hamdan and El-Naas (2014) [103] | Groundwater | Cr | D | 5.00 | - | 76.10 | 8.0 (5.0–8.0) | 100.00 | Iron | Iron | 30 |
| Martín-Dominguez et al. (2018) [104] | Groundwater | Cr | D | 19.00 | 0.3 | 30.00 | 6.2 (6.2–7.0) | 99.70 | Carbon Steel | Carbon Steel | 0.5 |
| Ferreira et al. (2013) [105] | Groundwater | Cu | PP | 12.00 | 1.5 | 42.00 | 7.7 (7.7–7.9) | 90.00 | Aluminum | Aluminum | 180 |
| Ferreira et al. (2013) [105] | Groundwater | Zn | PP | 20.00 | 1.5 | 42.00 | 7.7 (7.7–7.9) | 90.00 | Aluminum | Aluminum | 180 |
| Ferreira et al. (2013) [105] | Groundwater | Ni | PP | 20.00 | 1.5 | 42.00 | 7.7 (7.7–7.9) | 90.00 | Aluminum | Aluminum | 180 |
| Shafaei et al. (2011) [106] | Manipulated | Co | PP | 100.00 | 1.0 | 62.50 | 7.0 (2.0–8.0) | 99.00 | Aluminum | Aluminum | 30 |
| Mateen et al. (2020) [107] | Manipulated | Cu | D | 27.80 | 2.0 | 46.40 | 7.0 (6.0–8.0) | 95.29 | Iron | Iron | 5.4 |
| Hernández et al. (2012) [108] | Groundwater | Ni | PP | 41.00 | 0.5 | 16.00 | 7.4 (7.4–8.4) | 93.90 | Aluminum | Aluminum | 120 |
| Hernández et al. (2012) [108] | Groundwater | Cr | PP | 23.00 | 0.5 | 16.00 | 7.3 (7.3–8.0) | 82.60 | Aluminum | Aluminum | 120 |
| Ganesan et al. (2013) [109] | Manipulated | Mn | PP | 2.00 | 0.3 | 20.00 | 7.0 (3.0–11.0) | 98.00 | Stainless Steel | Magnesium | 110 |
| Gomes et al. (2010) [110] | Manipulated | Cu | PP | 10.00 | - | 30.00 | 6.0 (6.0–9.1) | 99.90 | Iron | Iron | 40 |
| Hussain et al. (2021) [111] | Groundwater | Co | PP | 0.02 | 1.5 | 1000.00 | 7.8 (7.8–8.4) | 75.70 | Aluminum | Iron | 5 |
| Hussain et al. (2021) [111] | Groundwater | Cr | PP | 0.04 | 1.5 | 1000.00 | 7.8 (7.8–8.4) | 100.00 | Aluminum | Iron | 5 |
| Hussain et al. (2021) [111] | Groundwater | Ni | PP | 0.05 | 1.5 | 1000.00 | 7.8 (7.8–8.4) | 100.00 | Aluminum | Iron | 5 |
| Hussain et al. (2021) [111] | Groundwater | Mn | PP | 0.02 | 1.5 | 1000.00 | 7.8 (7.8–8.4) | 100.00 | Aluminum | Iron | 5 |
| Hussain et al. (2021) [111] | Groundwater | Cu | PP | 0.04 | 1.5 | 1000.00 | 7.8 (7.8–8.4) | 100.00 | Aluminum | Iron | 5 |
| Shahreza et al. (2018) [112] | Manipulated | Mn | PP | 360.00 | 2.0 | 10.00 | 9.0 (3.0–10.0) | 92.00 | Aluminum | Aluminum | 190 |
| Pan et al. (2017) [113] | Manipulated | Cr | PP | 2.00 | 2.0 | 10.00 | 6.0 (6.0–9.0) | 100.00 | Iron | Iron | 40 |
| Vasudevan et al. (2009) [114] | Manipulated | Fe | PP | 25.00 | 0.5 | 12.00 | 6.0 (4.0–9.0) | 98.40 | Galvanized Iron | Magnesium | 60 |
| Vasudevan et al. (2011) [92] | Manipulated | Cr | PP | 5.00 | 0.5 | 20.00 | 7.0 (2.0–12.0) | 99.60 | Aluminum | Aluminum | 240 |
| Vasudevan et al. (2012) [115] | Shallow water | Cu | PP | 10.00 | 0.5 | 2.50 | 7.0 (4.0–12.0) | 98.50 | Aluminum | Aluminum | 35 |
| Vasudevan et al. (2012) [115] | Manipulated | Co | PP | 10.00 | 0.5 | 2.50 | 7.0 (2.0–10.0) | 100.00 | Galvanized Iron | Magnesium | 30 |
| Vasudevan et al. (2012) [115] | Manipulated | Cu | PP | 10.00 | 0.5 | 2.50 | 7.0 (2.0–10.0) | 99.00 | Galvanized Iron | Magnesium | 30 |
| Vasudevan et al. (2012) [115] | Manipulated | Cr | PP | 10.00 | 0.5 | 2.50 | 7.0 (2.0–10.0) | 97.00 | Galvanized Iron | Magnesium | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguiar, T.; Baumann, L.; Albuquerque, A.; Teixeira, L.; de Souza Gil, E.; Scalize, P. Application of Electrocoagulation for the Removal of Transition Metals in Water. Sustainability 2023, 15, 1492. https://doi.org/10.3390/su15021492
Aguiar T, Baumann L, Albuquerque A, Teixeira L, de Souza Gil E, Scalize P. Application of Electrocoagulation for the Removal of Transition Metals in Water. Sustainability. 2023; 15(2):1492. https://doi.org/10.3390/su15021492
Chicago/Turabian StyleAguiar, Tales, Luis Baumann, Antonio Albuquerque, Luiza Teixeira, Eric de Souza Gil, and Paulo Scalize. 2023. "Application of Electrocoagulation for the Removal of Transition Metals in Water" Sustainability 15, no. 2: 1492. https://doi.org/10.3390/su15021492
APA StyleAguiar, T., Baumann, L., Albuquerque, A., Teixeira, L., de Souza Gil, E., & Scalize, P. (2023). Application of Electrocoagulation for the Removal of Transition Metals in Water. Sustainability, 15(2), 1492. https://doi.org/10.3390/su15021492