Early Detection of Vitality Changes of Multi-Temporal Norway Spruce Laboratory Needle Measurements—The Ring-Barking Experiment
Abstract
:1. Introduction
- Is it possible to detect change of vitality shortly after the trees are affected by an unspecified stress while the trees still appear green and healthy?
- Which spectral wavelength regions are highlighted using different methods for identifying various stages of development—and, subsequently, is a time relation detectable?
- How can ring-barking effects be explained with plant physiology in relation to spectroscopy?
2. Study Area
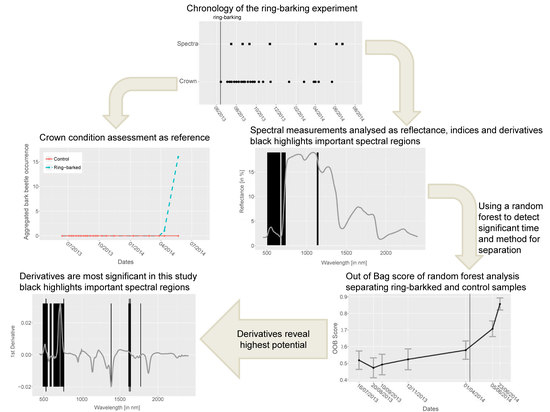
3. Methods
3.1. Ring-Barking Experiment
3.2. Spectral Measurements
3.3. Data Analysis
- The spectral data were analysed for their separability into ring-barked and control group using a Mann–Whitney-U test.
- Nine known stress related indices, see Table 2, were calculated for their mean and standard deviation and analysed regarding their separability of ring-barked and control measurements using Mann-Whitney-U test.
- The 1st derivative with prior Savitzky–Golay filter of all spectra were derived using the R function ‘savitzkyGolay’ in package ‘prospectr’ with polynomial order = 3 and moving window = 5. Derivatives emphasise the spectral shape, suppress albedo differences and can enhance subtle changes. Inflection points in spectra cause positive or negative peaks in 1st derivative data. Therefore, the 1st derivative is commonly used for extracting the red-edge inflection point. The separability was analysed using a Mann–Whitney-U test.
- A non-parametric tree based random forest classifier [25] was set up with the database of all spectra, indices and derivatives to discriminate ring-barked and control measurements using package ‘scikit-learn’ for . Even though the index and derivative data are calculated out of the spectral data, this approach was selected due to extracting important features for separation and extracting the internal out of bag (OOB) score. This OOB score is 1-the average OOB error calculated at each node using features that are not contained in this bootstrap sample. This allows testing of the random forest during training. A test of bias was performed using the most important method resulting from random forest classification (here derivative data, see Section 4) and running the random forest only on these data. This test did result in similar OOB values and important features. This concludes that the random forest classification of spectral, index and derivative data is neither dependent nor biased. At each node sqrt(n_variables) were randomly sampled for 500 trees. This number was determined by subsequently testing the amount between 10 and 1000 trees and comparing the OOB score that is an inverse internal calculation of misclassification. The benefits of random forest classifiers are that they do not overfit with a large amount of classification trees, they return an internal error estimate (OOB error) and importance of variables as well as estimate strength and correlation [26]. The OOB score here is used for stating the accuracy for separating control and ring-barked measurements as two groups. No split into test and train set is applied here for cross-validation since the accuracy results were not comparable with OOB score due to the limited number of samples. Additionally, the important features for classifying control and ring-barked samples were extracted. This was used for detecting the most appropriate method and spectral range that contribute to a high separability of ring-barked and control samples.
4. Results
4.1. Spectra Analysis
4.2. Index Analysis
4.3. Derivative Analysis
4.4. Random Forest
5. Discussion
5.1. Methods
5.1.1. Spectra Analysis
5.1.2. Index Analysis
5.1.3. Derivative Analysis
5.1.4. Random Forest
5.2. Plant Physiology
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kölling, C.; Knoke, T.; Schall, P.; Ammer, C. Überlegungen zum Risiko des Fichtenanbaus in Deutschland vor dem Hintergrund des Klimawandels. Forstarchiv 2009, 80, 42–54. [Google Scholar]
- Levitt, J. Water, Radiation, Salt, and Other Stresses, Band 2; Academic Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Lichtenthaler, H.K. Vegetation Stress: An Introduction to the Stress Concept in Plants. J. Plant Physiol. 1996, 148, 4–14. [Google Scholar] [CrossRef]
- Rock, B.; Hoshizaki, T.; Miller, J. Comparison of in situ and airborne spectral measurements of the blue shift associated with forest decline. Remote Sens. Environ. 1988, 24, 109–127. [Google Scholar] [CrossRef]
- Carter, G.A.; Knapp, A.K. Leaf optical properties in higher plants: Linking spectral characteristics to stress and chlorophyll concentration. Am. J. Bot. 2001, 88, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.A. Responses of Leaf Spectral Reflectance to Plant Stress. Am. J. Bot. 1993, 80, 239. [Google Scholar] [CrossRef]
- Carter, G.A. Ratios of leaf reflectances in narrow wavebands as indicators of plant stress. Int. J. Remote Sens. 1994, 15, 697–703. [Google Scholar] [CrossRef]
- Hoque, E.; Hutzler, P.J. Spectral blue-shift of red edge minitors damage class of beech trees. Remote Sens. Environ. 1992, 39, 81–84. [Google Scholar] [CrossRef]
- Hoque, E.; Hutzler, P.; Hiendl, H. Studies on reflective features of Norway spruce and their possible applications in remote sensing of forest damage. Toxicol. Environ. Chem. 1990, 27, 209–215. [Google Scholar] [CrossRef]
- Cheng, T.; Rivard, B.; Sánchez-Azofeifa, G.; Feng, J.; Calvo-Polanco, M. Continuous wavelet analysis for the detection of green attack damage due to mountain pine beetle infestation. Remote Sens. Environ. 2010, 114, 899–910. [Google Scholar] [CrossRef]
- Niemann, K.O.; Quinn, G.; Stephen, R.; Visintini, F.; Parton, D. Hyperspectral Remote Sensing of Mountain Pine Beetle with an Emphasis on Previsual Assessment. Can. J. Remote Sens. 2015, 41, 191–202. [Google Scholar] [CrossRef]
- Fassnacht, F.E.; Latifi, H.; Koch, B. An angular vegetation index for imaging spectroscopy data—Preliminary results on forest damage detection in the Bavarian National Park, Germany. Int. J. Appl. Earth Obs. Geoinf. 2012, 19, 308–321. [Google Scholar] [CrossRef]
- Fassnacht, F.E.; Latifi, H.; Ghosh, A.; Joshi, P.K.; Koch, B. Assessing the potential of hyperspectral imagery to map bark beetle-induced tree mortality. Remote Sens. Environ. 2014, 140, 533–548. [Google Scholar] [CrossRef]
- Lausch, A.; Heurich, M.; Gordalla, D.; Dobner, H.J.; Gwillym-Margianto, S.; Salbach, C. Forecasting potential bark beetle outbreaks based on spruce forest vitality using hyperspectral remote-sensing techniques at different scales. For. Ecol. Manag. 2013, 308, 76–89. [Google Scholar] [CrossRef]
- Dawson, T.P.; Curran, P.J. Technical note A new technique for interpolating the reflectance red edge position. Int. J. Remote Sens. 1998, 19, 2133–2139. [Google Scholar] [CrossRef]
- Cho, M.A.; Skidmore, A.K. A new technique for extracting the red edge position from hyperspectral data: The linear extrapolation method. Remote Sens. Environ. 2006, 101, 181–193. [Google Scholar] [CrossRef]
- Mutanga, O.; Skidmore, A.K. Red edge shift and biochemical content in grass canopies. J. Photogramm. Remote Sens. 2007, 62, 34–42. [Google Scholar] [CrossRef]
- Grossman, Y.; Ustin, S.; Jacquemoud, S.; Sanderson, E.; Schmuck, G.; Verdebout, J. Critique of stepwise multiple linear regression for the extraction of leaf biochemistry information from leaf reflectance data. Remote Sens. Environ. 1996, 56, 182–193. [Google Scholar] [CrossRef]
- Yao, H.; Huang, Y.; Hruska, Z.; Thomson, S.J.; Reddy, K.N. Using vegetation index and modified derivative for early detection of soybean plant injury from glyphosate. Comput. Electron. Agric. 2012, 89, 145–157. [Google Scholar] [CrossRef]
- Smith, K.; Steven, M.; Colls, J. Use of hyperspectral derivative ratios in the red-edge region to identify plant stress responses to gas leaks. Remote Sens. Environ. 2004, 92, 207–217. [Google Scholar] [CrossRef]
- Bayerisches Landesamt für Umwelt. GeoFachdatenAtlas (Boden Informations System Bayern); Bayerisches Landesamt für Umwelt: Augsburg, Germany, 2016. [Google Scholar]
- Bayer, A. Methodological Developments for Mapping Soil Constituents Using Imaging Spectroscopy. Ph.D. Thesis, University Potsdam, Potsdam, Germany, February 2013. [Google Scholar]
- Dorigo, W.; Bachmann, M.; Heldens, W. AS Toolbox and Processing of Field Spectra—User’s Manual; Technical Report; German Aerospace Center: Wessling, Germany, 2006. [Google Scholar]
- Einzmann, K.; Ng, W.T.; Immitzer, M.; Pinnel, N.; Atzberger, C.; Atzberger, C. Method Analysis for Collecting and Processing in-situ Hyperspectral Needle Reflectance Data for Monitoring Norway Spruce<BR> Methodenanalyse zur Erfassung und Prozessierung hyperspektraler in-situ Nadelreflexionsdaten zum Monitoring von Fichten. Photogramm. Fernerkund. Geoinf. 2014, 2014, 423–434. [Google Scholar]
- Breiman, L. Classification and Regression Trees; Chapman & Hall: London, UK, 1984; p. 358. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-band model for noninvasive estimation of chlorophyll, carotenoids, and anthocyanin contents in higher plant leaves. Geophys. Res. Lett. 2006, 33, L11402. [Google Scholar] [CrossRef]
- Daughtry, C. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Gao, B.C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1996, 58, 257–266. [Google Scholar] [CrossRef]
- Hunt, E.R.; Rock, B. Detection of changes in leaf water content using Near- and Middle-Infrared reflectances. Remote Sens. Environ. 1989, 30, 43–54. [Google Scholar]
- Serrano, L.; Peñuelas, J.; Ustin, S.L. Remote sensing of nitrogen and lignin in Mediterranean vegetation from AVIRIS data: Decomposing biochemical from structural signals. Remote Sens. Environ. 2002, 81, 355–364. [Google Scholar] [CrossRef]
- Delegido, J.; Verrelst, J.; Rivera, J.P.; Ruiz-Verdú, A.; Moreno, J. Brown and green LAI mapping through spectral indices. Int. J. Appl. Earth Obs. Geoinf. 2015, 35, 350–358. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Blackburn, G.A. Quantifying Chlorophylls and Caroteniods at Leaf and Canopy Scales: An Evaluation of Some Hyperspectral Approaches. Remote Sens. Environ. 1998, 66, 273–285. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Signature Analysis of Leaf Reflectance Spectra: Algorithm Development for Remote Sensing of Chlorophyll. J. Plant Physiol. 1996, 148, 494–500. [Google Scholar] [CrossRef]
- Gates, D.M.; Keegan, H.J.; Schleter, J.C.; Weidner, V.R. Spectral Properties of Plants. Appl. Opt. 1965, 4, 11. [Google Scholar] [CrossRef]
- Curran, P.J. Remote-Sensing of Foliar Chemistry. Remote Sens. Environ. 1989, 30, 271–278. [Google Scholar] [CrossRef]
- Kokaly, R.F.; Skidmore, A.K. Plant phenolics and absorption features in vegetation reflectance spectra near 1.66 μm. Int. J. Appl. Earth Obs. Geoinf. 2015, 43, 55–83. [Google Scholar] [CrossRef]
- Elvidge, C.D. Visible and near infrared reflectance characteristics of dry plant materials. Int. J. Remote Sens. 1990, 11, 1775–1795. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Sood, P.; Citovsky, V. The roles of plant phenolics in defence and communication during Agrobacterium and Rhizobium infection. Mol. Plant Pathol. 2015, 11, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Noel, A.R.A. The girdled tree. Bot. Rev. 1970, 36, 162–195. [Google Scholar] [CrossRef]
- Casas, A.; Riaño, D.; Ustin, S.; Dennison, P.; Salas, J. Estimation of water-related biochemical and biophysical vegetation properties using multitemporal airborne hyperspectral data and its comparison to MODIS spectral response. Remote Sens. Environ. 2014, 148, 28–41. [Google Scholar] [CrossRef]
- Zarco-Tejada, P.; Pushnik, J.; Dobrowski, S.; Ustin, S. Steady-state chlorophyll a fluorescence detection from canopy derivative reflectance and double-peak red-edge effects. Remote Sens. Environ. 2003, 84, 283–294. [Google Scholar] [CrossRef]
- Curran, P.J.; Dungan, J.L.; Macler, B.A.; Plummer, S.E. The effect of a red leaf pigment on the relationship between red edge and chlorophyll concentration. Remote Sens. Environ. 1991, 35, 69–76. [Google Scholar] [CrossRef]
- Nultsch, W. Allgemeine Botanik; Thieme: Stuttgart, Germany, 1996. [Google Scholar]
- Resa, F. Ueber Die Periode der Wurzelbildung; Carthouse: Bonn, Germany, 1877. [Google Scholar]
- Raven, P.H.; Evert, R.F.; Eichhorn, S.E. Biologie der Pflanzen; Gruyter: Berlin, Germany, 2006. [Google Scholar]
- Puhe, J. Growth and development of the root system of Norway spruce (Picea abies) in forest stands—A review. For. Ecol. Manag. 2003, 175, 253–273. [Google Scholar] [CrossRef]
- Steyn, W.J.; Wand, S.J.E.; Holcroft, D.M.; Jacobs, G. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef]
- Gitelson, A.; Jacquemoud, S.; Schaepman, M.; Asner, G.P.; Gamon, J.A.; Zarco-Tejada, P. Retrieval of foliar information about plant pigment systems from high resolution spectroscopy. Remote Sens. Environ. 2009, 113, S67–S77. [Google Scholar]
- Chalker-Scott, L. Environmental Significance of Anthocyanins in Plant Stress Responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Burger, J.; Edwards, G.E. Photosynthetic Efficiency, and Photodamage by UV and Visible Radiation, in Red versus Green Leaf Coleus Varieties. Plant Cell Physiol. 1996, 37, 395–399. [Google Scholar] [CrossRef]
- Blackburn, G.A. Hyperspectral remote sensing of plant pigments. J. Exp. Bot. 2007, 58, 855–867. [Google Scholar] [CrossRef] [PubMed]
- McClure, J.W. Physiology and Functions of Flavonoids. In The Flavonoids; Springer: Boston, MA, USA, 1975; pp. 970–1055. [Google Scholar]
- Gitelson, A.; Merzlyak, M. Non-Destructive Assessment of Chlorophyll Carotenoid and Anthocyanin Content in Higher Plant Leaves: Principles and Algorithms. Pap. Nat. Resour. 2004, 263, 78–94. [Google Scholar]
- Jeannette, E.; Reyss, A.; Gregory, N.; Gantet, P.; Prioul, J.L. Carbohydrate metabolism in a heat-girdled maize source leaf. Plant Cell Environ. 2000, 23, 61–69. [Google Scholar] [CrossRef]
- Barker, D.H.; Seaton, G.G.R.; Robinson, S.A. Internal and external photoprotection in developing leaves of the CAM plant Cotyledon orbiculata. Plant Cell Environ. 1997, 20, 617–624. [Google Scholar] [CrossRef]
- Lauer, M.J.; Pallardy, S.G.; Blevins, D.G.; Randall, D.D. Whole Leaf Carbon Exchange Characteristics of Phosphate Deficient Soybeans (Glycine max L.). Plant Physiol. 1989, 91, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.J.; Driscoll, S.P. Sugar repression of photosynthesis: the role of carbohydrates in signalling nitrogen deficiency through source:sink imbalance. Plant Cell Environ. 1997, 20, 110–116. [Google Scholar] [CrossRef]
- Larcher, W.W. Physiological Plant Ecology: Ecophysiology and Stress Physiology of Functional Groups; Springer: Berlin/Heidelberg, Germany, 1995; p. 506. [Google Scholar]
- Horler, D.; Dockray, M.; Barber, J.; Barringer, A. Red edge measurements for remotely sensing plant chlorophyll content. Adv. Space Res. 1983, 3, 273–277. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Lichtenthaler, H.K. Detection of Red Edge Position and Chlorophyll Content by Reflectance Measurements Near 700 nm. J. Plant Physiol. 1996, 148, 501–508. [Google Scholar] [CrossRef]
- Carter, G.A. Primary and Secondary Effects of Water Content on the Spectral Reflectance of Leaves. Am. J. Bot. 1991, 78, 916. [Google Scholar] [CrossRef]









| Sample | Date | Measurements | Age Classes | Trees |
|---|---|---|---|---|
| 1 | 16th July 2013 | 16 | 4 | 32 |
| 2 | 20th August 2013 | 16 | 4 | 32 |
| 3 | 10th September 2013 | 16 | 4 | 32 |
| 4 | 12th November 2013 | 16 | 4 | 32 |
| 5 | 1st April 2014 | 16 | 4 | 32 |
| 6 | 5th June 2014 | 16 | 4 | 32 |
| 7 | 23rd June 2014 | 16 | 4 | 32 |
| Indices | Formular | Reference |
|---|---|---|
| CRI | [27] | |
| mARI | [27] | |
| MCARI | [28] | |
| NDVI705 | [29] | |
| REP | [16] m and c represent slope and intercept of two lines extrapolated from derivative data, their intersection is REP | |
| NDWI | [30] | |
| MSI | [31] | |
| NDLI | [32] | |
| GBVI | [33] |
| CRI | mARI | MCARI | NDVI705 | REP | NDWI | MSI | NDLI | GBVI | |
|---|---|---|---|---|---|---|---|---|---|
| Date | p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | p-Value | p-Value |
| 16th July 2013 | 0.645 | 0.492 | 0.509 | 0.674 | 0.509 | 0.367 | 0.617 | 0.535 | 0.412 |
| 20th August 2013 | 0.626 | 0.396 | 0.984 | 0.889 | 0.785 | 0.553 | 0.598 | 0.404 | 0.553 |
| 10th September 2013 | 0.475 | 0.589 | 0.419 | 0.684 | 0.404 | 0.346 | 0.427 | 0.236 | 0.132 |
| 12th November 2013 | 0.475 | 0.774 | 0.150 | 0.580 | 0.492 | 0.571 | 0.847 | 0.889 | 0.544 |
| 1st April 2014 | 0.868 | 0.942 | 0.931 | 0.645 | |||||
| 5th June 2014 | 0.216 | 0.282 | 0.282 | 0.589 | |||||
| 23rd June 2014 | 0.082 | 0.103 | 0.077 | 0.360 |
| Date | OOB Score | StDev |
|---|---|---|
| 16th July 2013 | 0.52 | 0.055 |
| 20th August 2013 | 0.47 | 0.060 |
| 10th September 2013 | 0.49 | 0.062 |
| 12th November 2013 | 0.52 | 0.063 |
| 1st April 2014 | 0.58 | 0.055 |
| 5th June 2014 | 0.71 | 0.047 |
| 23rd June 2014 | 0.86 | 0.036 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reichmuth, A.; Henning, L.; Pinnel, N.; Bachmann, M.; Rogge, D. Early Detection of Vitality Changes of Multi-Temporal Norway Spruce Laboratory Needle Measurements—The Ring-Barking Experiment. Remote Sens. 2018, 10, 57. https://doi.org/10.3390/rs10010057
Reichmuth A, Henning L, Pinnel N, Bachmann M, Rogge D. Early Detection of Vitality Changes of Multi-Temporal Norway Spruce Laboratory Needle Measurements—The Ring-Barking Experiment. Remote Sensing. 2018; 10(1):57. https://doi.org/10.3390/rs10010057
Chicago/Turabian StyleReichmuth, Anne, Lea Henning, Nicole Pinnel, Martin Bachmann, and Derek Rogge. 2018. "Early Detection of Vitality Changes of Multi-Temporal Norway Spruce Laboratory Needle Measurements—The Ring-Barking Experiment" Remote Sensing 10, no. 1: 57. https://doi.org/10.3390/rs10010057
APA StyleReichmuth, A., Henning, L., Pinnel, N., Bachmann, M., & Rogge, D. (2018). Early Detection of Vitality Changes of Multi-Temporal Norway Spruce Laboratory Needle Measurements—The Ring-Barking Experiment. Remote Sensing, 10(1), 57. https://doi.org/10.3390/rs10010057